To Treat or Not to Treat: A Scoping Review of Speech Treatment for Dysarthria in Amyotrophic Lateral Sclerosis (ALS)
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Concept and Context
2.3. Search Strategy and Study Selection Criteria
2.4. Data Extraction, Analysis, and Reporting
3. Results
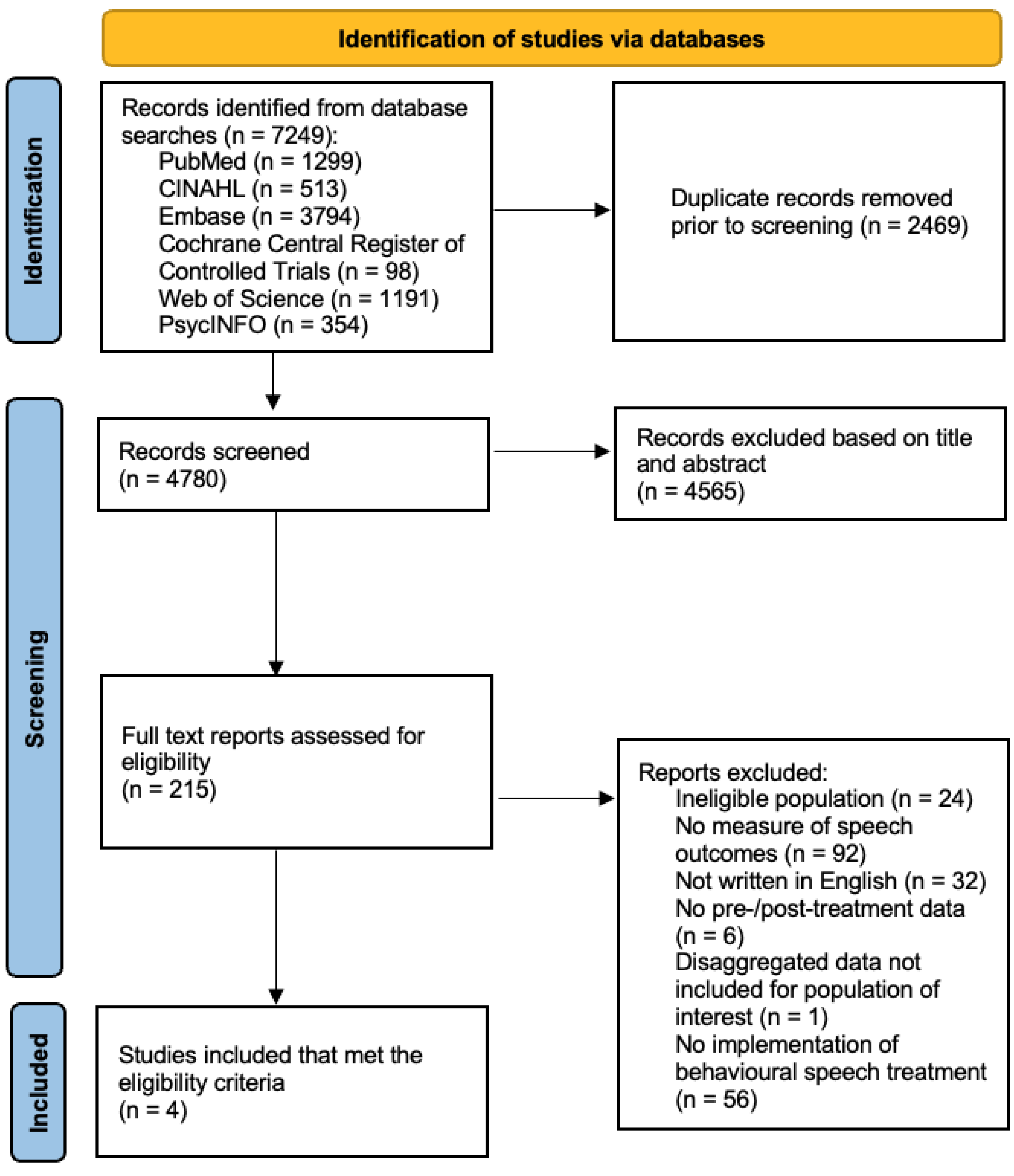
3.1. Screening and Identification
3.2. Types of Studies, Levels of Evidence and Context
3.3. Speech Treatments, Aims, and Outcomes
3.3.1. Music-Based Speech Therapy
3.3.2. Integrated Multisubsystem Rehabilitation Program
3.3.3. Lee Silverman Voice Treatment (LSVT)-LOUD®, Vocal Deconstriction, Isometric Vocal Fold Exercises, and Articulation Therapy
3.3.4. Tongue Strengthening and Articulation Training
4. Discussion
4.1. Deficit-Specific Versus Integrated Subsystems Approaches
4.2. Speech Treatment Hierarchy for Mixed Spastic-Flaccid Dysarthria
4.3. Heterogeneity of Bulbar Deterioration in Treatment Selection
5. Conclusions
5.1. Limitations
5.2. Directions for Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| LSVT | Lee Silverman Voice Treatment |
| AAC | Augmentative and Alternative Communication |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| NHMRC | National Health and Medical Research Council |
| JBI | Joanna Briggs Institute |
| MT | Music Therapy |
| ALSFRS-R | ALS Functional Rating Scale- Revised |
| FFR | Frequency Following Response |
| ICF | International Classification of Functioning, Disability and Health |
References
- Makkonen, T.; Korpijaakko-Huuhka, A.-M.; Ruottinen, H.; Puhto, R.; Hollo, K.; Ylinen, A.; Palmio, J. Oral motor functions, speech and communication before a definitive diagnosis of amyotrophic lateral sclerosis. J. Commun. Disord. 2016, 61, 97–105. [Google Scholar] [CrossRef]
- Turner, M.R.; Scaber, J.; Goodfellow, J.A.; Lord, M.E.; Marsden, R.; Talbot, K. The diagnostic pathway and prognosis in bulbar-onset amyotrophic lateral sclerosis. J. Neurol. Sci. 2010, 294, 81–85. [Google Scholar] [CrossRef]
- Langmore, S.E.; Lehman, M.E. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. J. Speech Hear. Res. 1994, 37, 28–37. [Google Scholar] [CrossRef]
- Chen, A.; Garrett, C.G. Otolaryngologic presentations of amyotrophic lateralsclerosis. Otolaryngol. Head. Neck Surg. 2005, 132, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, L.J.; Appel, V.; Appel, S.H. Natural history of amyotrophic lateral sclerosis in a database population validation of a scoring system and a model for survival prediction. Brain 1995, 118, 707–719. [Google Scholar] [CrossRef]
- Shellikeri, S.; Keith, J.; Black, S.E.; Zinman, L.; Yunusova, Y. Neuropathology of speech network distinguishes bulbar from non-bulbar amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2020, 79, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Tomik, B.; Guiloff, R.J. Dysarthria in amyotrophic lateral sclerosis: A review. Amyotroph. Lateral Scler. 2010, 11, 4–15. [Google Scholar] [CrossRef] [PubMed]
- DePaul, R.; Brooks, B.R. Multiple orofacial indices in amyotrophic lateral sclerosis. J. Speech Hear. Res. 1993, 36, 1158–1167. [Google Scholar] [CrossRef]
- Kent, R.D.; Kent, J.F.; Weismer, G.; Sufit, R.L.; Rosenbek, J.C.; Martin, R.E.; Brooks, B.R. Impairment of speech intelligibility in men with amyotrophic lateral sclerosis. J. Speech Hear. Disord. 1990, 55, 721–728. [Google Scholar] [CrossRef]
- Rong, P.; Yunusova, Y.; Wang, J.; Green, J.R. Predicting Early Bulbar Decline in Amyotrophic Lateral Sclerosis: A Speech Subsystem Approach. Behav. Neurol. 2015, 2015, 183027. [Google Scholar] [CrossRef]
- Hecht, M.; Hillemacher, T.; Grasel, E.; Tigges, S.; Winterholler, M.; Heuss, D.; Hilz, M.J.; Neundorfer, B. Subjective experience and coping in ALS. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2002, 3, 225–231. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Motor Neurone Disease: Assessment and Management. NICE Guidel. 2019. Available online: https://www.nice.org.uk/guidance/ng42 (accessed on 8 December 2024).
- Van Damme, P.; Al-Chalabi, A.; Andersen, P.M. European Academy of Neurology (EAN) guideline on the management of amyotrophic lateral sclerosis in collaboration with European Reference Network for Neuromuscular Disease (ERN EURO-NMD). Eur. J. Neurol. 2024, 31, e16264. [Google Scholar] [CrossRef] [PubMed]
- Shoesmith, C.; Abrahao, A.; Benstead, T.; Chum, M.; Dupre, N.; Izenberg, A.; Johnston, W.; Kalra, S.; Leddin, D.; O’Connell, C.; et al. Canadian best practice recommendations for the management of amyotrophic lateral sclerosis. Can. Med. Assoc. J. 2020, 192, E1453–E1468. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.R. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 4th ed.; Elsevier: St. Louis, MO, USA, 2020. [Google Scholar]
- Sinaki, M.; Mulder, D.W. Rehabilitation techniques for patients with amyotrophic lateral sclerosis. Mayo Clin. Proc. 1978, 53, 173–178. [Google Scholar]
- Mahoney, D.J.; Rodriguez, C.; Devries, M.; Yasuda, N.; Tarnopolsky, M.A. Effects of high-intensity endurance exercise training in the G93A mouse model of amyotrophic lateral sclerosis. Muscle Nerve 2004, 29, 656–662. [Google Scholar] [CrossRef]
- Hanson, E.K.; Yorkston, K.M.; Britton, D. Dysarthria in amyotrophic lateral sclerosis: A systematic review of characteristics, speech treatment, and augmentative and alternative communication options. J. Med. Speech-Lang. Pathol. 2011, 19, 12–30. [Google Scholar]
- Dworkin, J.P.; Hartman, D.E. Progressive speech deterioration and dysphagia in amyotrophic lateral sclerosis: Case report. Arch. Phys. Med. Rehabil. 1979, 60, 423–425. [Google Scholar]
- Watts, C.R.; Vanryckeghem, M. Laryngeal dysfunction in Amyotrophic Lateral Sclerosis: A review and case report. BMC Ear Nose Throat Disord. 2001, 1, 1. [Google Scholar] [CrossRef]
- Meng, L.; Li, X.; Li, C.; Tsang, R.; Chen, Y.; Ge, Y.; Gao, Q. Effects of exercise in patients with amyotrophic lateral sclerosis: A systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 2020, 99, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Plowman, E.K. Is There a Role for Exercise in the Management of Bulbar Dysfunction in Amyotrophic Lateral Sclerosis? J Speech Lang Hear Res 2015, 58, 1151–1166. [Google Scholar] [CrossRef]
- Atkinson-Clement, C.; Sadat, J.; Pinto, S. Behavioural treatments for speech in Parkinson’s disease: Meta-analyses and review of the literature. Neurodegener. Dis. Manag. 2015, 5, 233–248. [Google Scholar] [CrossRef]
- Pu, T.; Huang, M.; Kong, X.T.; Wang, M.; Chen, X.; Feng, X.; Wei, C.; Weng, X.; Xu, F. Lee Silverman Voice Treatment to improve speech in Parkinson’s disease: A systematic review and met-analysis. Park. Dis. 2021, 27, 3366870. [Google Scholar] [CrossRef]
- Ramig, L.; Halpern, A.; Spielman, J.L.; Fox, C.; Freeman, K. Speech treatment in Parkinson’s disease: Randomised Controlled Trial (RCT). Mov. Disord. 2018, 33, 1777–1791. [Google Scholar] [CrossRef]
- Sapir, S.; Spielman, J.; Ramig, L.; Story, B.H.; Fox, C. Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on vowel articulation in dysarthric individuals with Parkinson’s disease: Acoustic and percpetual findings. J. Speech Lang. Hear. Res. 2007, 50, 899–912. [Google Scholar] [CrossRef]
- Dromey, C.; Ramig, L.; Johnson, A. Phonatory and articulatory changes associated with increased vocal intensity in Parkinson’s disease: A case study. J. Speech Hear. Res. 1995, 38, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Spielman, J.; Borod, J.; Ramig, L. Effects of intensive voice treatment (LSVT) on facial expressiveness in Parkinson’s disease: Preliminary data. Cogn. Behav. Neurol. 2003, 16, 177–188. [Google Scholar] [CrossRef]
- Dumer, A.I.; Oster, H.; McCabe, D.; Rabin, L.A.; Spielman, J.; Ramig, L.; Borod, J.C. Effects of the Lee Silverman Voice Treatment (LSVT LOUD) on hypomimia in Parkinson’s disease. J. Int. Neuropsychol. Soc. 2014, 20, 302–312. [Google Scholar] [CrossRef]
- El Sharkawi, A.; Ramig, L.; Logemann, J.; Pauloski, B.; Rademaker, A.; Smith, C.; Pawlas, A.; Baum, S.; Werner, C. Swallowing and voice effects of Lee Silverman Voice Treatment: A pilot study. J. Neurol. Neurosurg. Psychiatry 2002, 72, 31–36. [Google Scholar] [CrossRef]
- Miles, A.; Jardine, M.; Johnston, F.; de Lisle, M.; Friary, P.; Allen, J. Effect of Lee Silverman Voice Treatment (LSVT LOUD) on swallowing and cough in Parkinson’s disease: A pilot study. J. Neurol. Sci. 2017, 383, 180–187. [Google Scholar] [CrossRef]
- Vogel, A.P.; Stoll, L.H.; Oettinger, A.; Rommel, N.; Kraus, E.-M.; Timman, D.; Scott, D.; Atay, C.; Storey, E.; Schols, L.; et al. Speech treatment improves dysarthria in multisystemic dysarthria: A rater-blinded, controlled pilot-study in ARSACS. J. Neurol. 2019, 266, 1260–11266. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.P.; Graf, L.H.; Magee, M.; Schols, L.; Rommel, N.; Synofzik, M. Home-based biofeedback speech treatment improves dysarthria in repeat-expansion SCAs. Ann. Clin. Transl. Neurol. 2022, 9, 1310–1315. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Baldini Soares, C. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhon, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public. Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S.; et al. Improving translation of search strategies using the Polyglot search translator: A randomised controlled trial. J. Med. Libr. Assoc. 2020, 108, 195–207. [Google Scholar] [CrossRef]
- Clark, J.; Glasziou, P.; Del Mar, C.; Bannach-Brown, A.; Stehlik, P.; Scott, A.M. A full systematic review was completed in 2 weeks using automation tools: A case study. J. Clin. Epidemiol. 2020, 121, 81–90. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne Australia. Available online: www.covidence.org (accessed on 15 December 2022).
- National Health and Medical Research Council (NHMRC). NHMRC Additional Levels of Evidence and Grades for Recommendations for Developers of Guidelines. 2009. Available online: https://www.mja.com.au/sites/default/files/NHMRC.levels.of.evidence.2008-09.pdf (accessed on 8 December 2024).
- Hillier, S.; Grimmer-Somers, K.; Merlin, T.; Middleton, P.; Salisbury, J.; Tooher, R.; Weston, A. FORM: An Australian method for formulating and grading recommendations in evidence-based clinical guidelines. BMC Med. Res. Methodol. 2011, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Systematic reviews of etiology and risk. In JBI Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; JBI: North Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 15 December 2022). [CrossRef]
- Moher, D.; Liberati, A.; Tetziaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 6, 339. [Google Scholar] [CrossRef]
- Apreleva Kolomeytseva, A.T.; Brylev, L.; Eshghi, M.; Bottaeva, Z.; Zhang, J.; Fachner, J.C.; Street, A.J. Home-Based Music Therapy to Support Bulbar and Respiratory Functions of Persons with Early and Mid-Stage Amyotrophic Lateral Sclerosis-Protocol and Results from a Feasibility Study. Brain Sci. 2022, 12, 494. [Google Scholar] [CrossRef]
- Rohers, D.E.; Mundt, A.A.; Rodrigues Portalete, C.; Cantele Malavolta, V.; Goulart Moreira, H.; Keske-Soares, M.; Skarzynski, P.H.; Dominici Sanfins, M.; Vargas Garcia, M. Quality of life questionnaire and frequency following response in two dysarthric subjects with neurodegenerative disease: A case study. J. Hear. Sci. 2022, 12, 57–63. [Google Scholar] [CrossRef]
- Rooney, J.; Burke, T.; Vajda, A.; Heverin, M.; Hardiman, O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Chernenkaya, V.Y.; Gorbachev, K.V.; Gorbachev, D.V.; Ataulina, A.I.; Fominykh, V.V.; Brylev, L.V.; Guekht, A.B. The Edinburgh Cognitive and Behavioral ALS Screen (ECAS): A Russian version. Korsakov J. Neurol. Psychiatry 2018, 118, 36–39. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Bidelman, G.M.; Sisson, A.; Rizzi, R.; MacLean, J.; Baer, K. Myogenic artifacts masquerade as neuroplasticity in the auditory frequency-following response. Fontiers Neurosci. 2024, 18, 1422903. [Google Scholar] [CrossRef]
- Marques, M.C.S.; Griz, S.; Lira de Andrade, K.C.; Menezes, P.L.; Menezes, D.C. Frequency following response in childhood apraxia of speech. Int. J. Peditaric Otorhinolaryngol. 2021, 145, 110742. [Google Scholar] [CrossRef]
- Bidelman, G.M. Sonification of scalp-recorded frequency-following responses (FRRs) offers improved response detection over conventional statistical metrics. J. Neurosci. Methods 2018, 293, 59–66. [Google Scholar] [CrossRef]
- Puhl, A.E.; Diaferia, G.; Padovni, M.M.; Behlau, M.S. Living with dysarthria self reported questionnaire in Parkinson’s disease. In Proceedings of the 28th IALP Conference, Athens, Greece, 22–26 August 2010. [Google Scholar]
- Ramig, L.; Countryman, S.; O’Brien, C.; Hoehn, M.; Thompson, L. Intensive speech treatment for patients with Parkinson disease: Short and long-term comparison of two techniques. Neurology 1996, 47, 1496–1504. [Google Scholar] [CrossRef]
- Verdolini-Marston, K.; Burke, M.D.; Lessac, A.; Glaze, L.E.; Caldwell, E. A preliminary study on two methods of treatment for laryngeal nodules. J. Voice 1995, 9, 74–85. [Google Scholar] [CrossRef]
- Elias, V.S.; Cielo, C.A.; Jotz, G.P.; Christmann, M.K. Effect of vocal fry on voice and velopharyngeal sphincter. Int. Arch. Otorhinolaryngol. 2016, 20, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Netsell, R. The acquisition of speech motor control: A perspective with directions for research. In Language Behaviour in Infancy and Early Childhood; Stark, R., Ed.; Elsevier: New York, NY, USA, 1981; pp. 127–156. [Google Scholar]
- World Health Organisation. International Classification of Functioning, Disability and Health (ICF); World Health Organisation: Geneva, Switzerland, 2001. [Google Scholar]
- Wenke, R.J.; Theodoros, D.G.; Cornwell, P. The short-and long-term effectiveness of LSVT for dysarthria following TBI and stroke. Brain Inj. 2008, 22, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.; Sufit, R.L.; Rosenbek, J.C.; Kent, J.F.; Weismer, G.; Martin, R.E.; Brooks, B.R. Speech deterioration in amyotrophic lateral sclerosis: A case study. J. Speech Hear. Res. 1991, 34, 1269–1275. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Jones, H.N. Principles of Treatment for Sensorimotor Speech Disorders. In Clinical Management of Sensorimotor Speech Disorders; McNeil, M.R., Ed.; Thieme Medical Publishers: New York, NY, USA, 2009; pp. 269–286. [Google Scholar]
- Hayden, D.; Square, P. Motor speech treatment hierarchy: A systems approach. Clin. Commun. Disord. 1994, 4, 162–174. [Google Scholar]
- Mackenzie, C.; Lowitt, A. Behavioural intervention effects in dysarthria following stroke: Communication effectiveness, intelligibility and dysarthria impact. Int. J. Lang. Commun. Disord. 2007, 42, 131–153. [Google Scholar] [CrossRef]
- Lowit, A.; Egan, A.; Hadjivassiliou, M. Feasibility and acceptability of Lee Silverman Voice Treatment in progressive ataxias. Cerebellum 2020, 19, 701–714. [Google Scholar] [CrossRef]
- White-Schwoch, T.; Woodruff Carr, K.; Thompson, E.C.; Anderson, S.; Nicol, T.; Bradlow, A.R.; Zecker, S.G.; Kraus, N. Auditory processing in noise: A preschool biomarker for literacy. PLOS Biol. 2015, 13, e1002196. [Google Scholar] [CrossRef]
- Smith, R.A.; Macklin, E.A.; Myers, K.J.; Pattee, G.L.; Goslin, K.L.; Meekins, G.D.; Green, J.R.; Shefner, J.M.; Pioro, E.P. Assessment of bulbar function in amyotrophic lateral sclerosis: Validation of a self-report scale (Centre for Neurologic Study Bulbar Function Scale. Eur. J. Neurol. 2018, 25, 907-e66. [Google Scholar] [CrossRef] [PubMed]
- Yorkston, K.M.; Beukelman, D.R.; Strand, E.; Bell, K.R. Management of Motor Speech Disorders in Children and Adults, 2nd ed.; Pro-Ed: Austin, TX, USA, 1999. [Google Scholar]
- Fox, C.; Ramig, L.; Ciucci, M.R.; Sapir, S.; McFarland, D.H.; Farley, B.G. The science and practice of LSVT/LOUD: Neural plasticity-principled approach to treating individuals with Parkinson Disease and other neurological disorders. Semin. Speech Lang. 2006, 27, 283–299. [Google Scholar] [CrossRef]
- Dworkin, J. Motor Speech Disorders: A Treatment Guide; Mosby: St. Louis, MO, USA, 1991. [Google Scholar]
- Colton, R.H.; Casper, J.K. Understanding Voice Problems: A Physiological Perspective for Diagnosis and Treatment; Williams and Wilkins: Baltimore, MD, USA, 1990. [Google Scholar]
- Allison, K.M.; Yunusova, Y.; Green, J.R. Shorter sentence length maximises intelligibility and speech motor performance in persons with dysarthria due to amyotrophic lateral sclerosis. Am. J. Speech-Lang. Pathol. 2019, 28, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, K.; Lam, J.; Wilding, G. Vowel acoustics in Parkinson’s disease and multiple sclerosis: Comparison of clear, loud and slow speaking conditions. J. Speech Lang. Hear. Res. 2013, 56, 1485–1502. [Google Scholar] [CrossRef]
- Murphy, J. Communication strategies of people with ALS and their partners. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5, 121–126. [Google Scholar] [CrossRef]
- Yorkston, K.M. Management of dysarthria in amyotrophic lateral sclerosis. Geriatr. Aging 2002, 5, 38–41. [Google Scholar]
- Bunton, K. Speech versus nonspeech: Differetn tasks, different neural organisation. Semin. Speech Lang. 2009, 29, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wightman, D.; Lintern, G. Part-task training for tracking and manual control. Hum. Factors 1985, 27, 267–283. [Google Scholar] [CrossRef]
- Tamura, T.; Tanaka, Y.; Watabnabe, Y.; Sato, K. Realationships between maximum tongue pressure and second formant transition in speakers with different types of dysarthria. PLoS ONE 2022, 17, e0264995. [Google Scholar] [CrossRef]
- Kuruvilla-Dugdale, M.; Chuqulin-Arista, M. An investigation of clear speech effects on articulatory kinematics in talkers with ALS. Clin. Linguist. Phon. 2017, 31, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Perry, A.; Bilney, B.; Curran, A.; Dodd, K.; Wittwer, J.E.; Dalton, G.W. Outcomes of physical therapy, speech pathology, and occupational therapy for people with motor neuron disease: A systematic review. Neurorehabil Neural Repair. 2006, 20, 424–434. [Google Scholar] [CrossRef]
- Stipancic, K.L.; Yunusova, Y.; Campbell, T.F.; Wang, J.; Berry, J.D.; Green, J.R. Two Distinct Clinical Phenotypes of Bulbar Motor Impairment in Amyotrophic Lateral Sclerosis. Front. Neurol. 2021, 12, 664713. [Google Scholar] [CrossRef]
- Chen, X.; Wei, Q.-Q.; Chen, Y.; Cao, B.; Ou, R.; Hou, Y.; Yuan, X.; Zhang, L.; Liu, H.X.; Shang, H. Clinical staging of amyotrophic lateral sclerosis in Chinese patients. Front. Neurol. 2018, 9, 442. [Google Scholar] [CrossRef]
- Yunusova, Y.; Green, J.R.; Greenwood, L.; Wang, J.; Pattee, G.L.; Zinman, L. Tongue movements and their acoustic consequences in amyotrophic lateral sclerosis. Folia Phoniatr. Logop. 2012, 64, 94–102. [Google Scholar] [CrossRef]
- Ball, L.J.; Beukelman, D.; Pattee, G.L. Timing of speech deterioration in people with amyotrophic lateral sclerosis. J. Medicl Speech-Lang. Pathol. 2002, 10, 231–235. [Google Scholar]
- Fried-Oken, M.; Fox, L.; Rau, M.T.; Tullman, J.; Baker, G.; Hindal, M.; Wilse, N.; Lou, J.-S. Purposes of AAc device for persons with ALS as reported by caregivers. Augment. Altern. Commun. 2006, 22, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Green, J.R.; Yunusova, Y.; Kuruvilla, M.S.; Wang, J.; Pattee, G.L.; Synhorst, L.; Zinman, L.; Berry, J.D. Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Barnwell, J.; Al-Chalabi, A.; Eisen, A. Young-onset amyotrophic lateral sclerosis: Historical and other observations. Brain 2012, 135, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Rogus-Pulia, N.M.; Plowman, E.K. Shifting Tides Toward a Proactive Patient-Centered Approach in Dysphagia Management of Neurodegenerative Disease. Am. J. Speech-Lang. Pathol. 2020, 29, 1093–1108. [Google Scholar] [CrossRef]
- Donohue, C.; Carnaby, G.; Reilly, M.C.; Colquhon, R.J.; Lacomis, D.; Garand, K.L. A meta-analysis of post-exercise outcomes in people with amyotrophic lateral sclerosis. Neurol. Sci. 2023, 31, 100452. [Google Scholar] [CrossRef]
| Author, Publication Date/ Country of Origin | Study Aims | Participants/ Sample Size | Study Design | Dysarthria Treatment | Speech Outcome Measure(s) | Measurement Timepoints | Key Findings | Level of Evidence (NHMRC) | JBI Critical Appraisal Criteria Met |
|---|---|---|---|---|---|---|---|---|---|
| Apreleva et al., 2022 (Russia) [45] | To test the feasibility and efficacy of a music therapy protocol designed to support bulbar and respiratory functions of individuals with early- and mid-stage ALS. | N = 7 (6 female, 2 male participants with ALS, mean age 58.1 yrs) | ABA mixed-methods case series with repeated measures | Individualised music therapy, 2x/week for 1 h, in-home, over 6 consecutive weeks. ALS-specific vocal health guidelines to promote healthy voice use/habits in daily life. | CNS-BFS (speech subscore), MPT (sec), AMR (syllables uttered), SMR (syllables uttered), Jitter (%), Shimmer (%), HNR (dB), VSA (Hz2), F0 (Hz), Speaking rate (words/min), Speech-pause ratio (oral reading; secs/min), Pause frequency (oral reading; pauses/min), Hypernasality level (perceptual rating). Thematic analysis of semi-structured interviews with participants and carers regarding treatment experience. | Baseline (week 1), pre-treatment (week 6), post-treatment (week 12), and after wash-out period (week 16). | Most bulbar & respiratory functions maintained or improved during treatment phase compared with control period (weeks 1 to 6) | III-3 | 70% |
| Dworkin & Hartman, 1979 (USA) [19] | To describe the effects of progressive lingual and velopharyngeal involvement on speech and swallowing over a 6-month period post-diagnosis, and outline the medical, speech, and prosthetic measures taken to lessen the effects of ALS. | N = 1 (Male with ALS, 49 yrs, mixed spastic-flaccid dysarthria) | Case report | Tongue strengthening program (resistance exercises) with regular articulation training; discontinued after one month due to fatigue/breathing difficulties. | Tongue strength; Lingual AMR; Perceptual ratings of articulatory precision, hypernasality, voice quality, intelligibility. | Baseline, 6 months post-treatment | Reductions in tongue strength and lingual AMR; Moderately imprecise articulation, hypernasality and nasal snorting progressed to severely affected; Mildly strain-strangled voice progressed to severely strain-strangled; Moderately affected intelligibility became severely affected. | IV | 75% |
| Rohers et al., 2022 (Brazil) [46] | To assess the effectiveness of the frequency-following response (FFR) to monitor progress of speech therapy for dysarthria in neurodegenerative disease. | N = 2 (1 male with PD, 71 yrs, hypokinetic dysarthria; 1 male with ALS, 58 yrs, mixed spastic-flaccid dysarthria) | Descriptive, longitudinal, and qualitative pilot study | 25 motor speech treatment sessions, 45 min each, over 6 months: 5 sessions targeted respiration, 5 targeted resonance, 5 targeted respiration & resonance together, 5 targeted prosody, and 5 targeted phonation. | Self-assessment questionnaire (Living with dysarthria); Electrophysiological assessment (FFR). | Pre- and post-treatment | Significant improvements in Living with Dysarthria scores for both participants across all domains. FFR –decreased latencies & increased amplitudes for some waves (both participants); responses more noticeable for amplitude measure. | IV | 38% |
| Watts & Vanryckeghem, 2001 (USA) [20] | To present a case report of a female with bulbar ALS, and describe the effects of voice/speech and swallowing therapy. | N = 1 (Female with ALS, 72 yrs, primary involvement of bulbar nerves, flaccid dysarthria) | Case report | Combination of Lee Silverman Voice Treatment (LSVT) and voice focus/resonant therapy to reduce laryngeal tension during initial stage of LSVT; 4x/week for 1 h, discontinued after 2 weeks. Voice focus therapy transitioning to articulation focus therapy over time; 1x/week for 1.5 hrs, over 4.5 months. Daily articulation therapy (motor motility & strengthening exercises). | Perceptual ratings of voice quality; Acoustic variables (F0 (Hz), Jitter (%), Shimmer (%), NHR (dB), vF0 (Hz), vAm (dB)); Sentence intelligibility ratings. | Pre-, during (monthly), and post-treatment | LSVT resulted in decline in voice quality (increased ventricular compression). No measurable/perceptible improvement in voice quality following voice focus/resonant therapy and resumption of LSVT. F0 increased during voice/articulation therapy while all other acoustic variables decreased over first 3 months/increased in last month of therapy. Intelligibility declined rapidly over course of therapy. | IV | 88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whelan, B.-M.; Aldridge, D.; Ruhle, J.; Whitelock, P.; Taubert, S.; Collins, A.; Kearney, E.; Charania, S.; Henderson, R.D.; Wallace, S.J.; et al. To Treat or Not to Treat: A Scoping Review of Speech Treatment for Dysarthria in Amyotrophic Lateral Sclerosis (ALS). Healthcare 2025, 13, 2434. https://doi.org/10.3390/healthcare13192434
Whelan B-M, Aldridge D, Ruhle J, Whitelock P, Taubert S, Collins A, Kearney E, Charania S, Henderson RD, Wallace SJ, et al. To Treat or Not to Treat: A Scoping Review of Speech Treatment for Dysarthria in Amyotrophic Lateral Sclerosis (ALS). Healthcare. 2025; 13(19):2434. https://doi.org/10.3390/healthcare13192434
Chicago/Turabian StyleWhelan, Brooke-Mai, Danielle Aldridge, Jessica Ruhle, Persephone Whitelock, Shana Taubert, Annette Collins, Elaine Kearney, Salma Charania, Robert D. Henderson, Sarah J. Wallace, and et al. 2025. "To Treat or Not to Treat: A Scoping Review of Speech Treatment for Dysarthria in Amyotrophic Lateral Sclerosis (ALS)" Healthcare 13, no. 19: 2434. https://doi.org/10.3390/healthcare13192434
APA StyleWhelan, B.-M., Aldridge, D., Ruhle, J., Whitelock, P., Taubert, S., Collins, A., Kearney, E., Charania, S., Henderson, R. D., Wallace, S. J., Mitchell, C., Stipancic, K. L., Kuruvilla-Dugdale, M., & Vogel, A. P. (2025). To Treat or Not to Treat: A Scoping Review of Speech Treatment for Dysarthria in Amyotrophic Lateral Sclerosis (ALS). Healthcare, 13(19), 2434. https://doi.org/10.3390/healthcare13192434







