Comprehensive Assessment of Pain and Physiological Parameters of Sympathetic Blockade to Accurately Determine the Success of High Thoracic Erector Spinae Plane Block
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population and Sample
2.3. Data Collection and Assessments
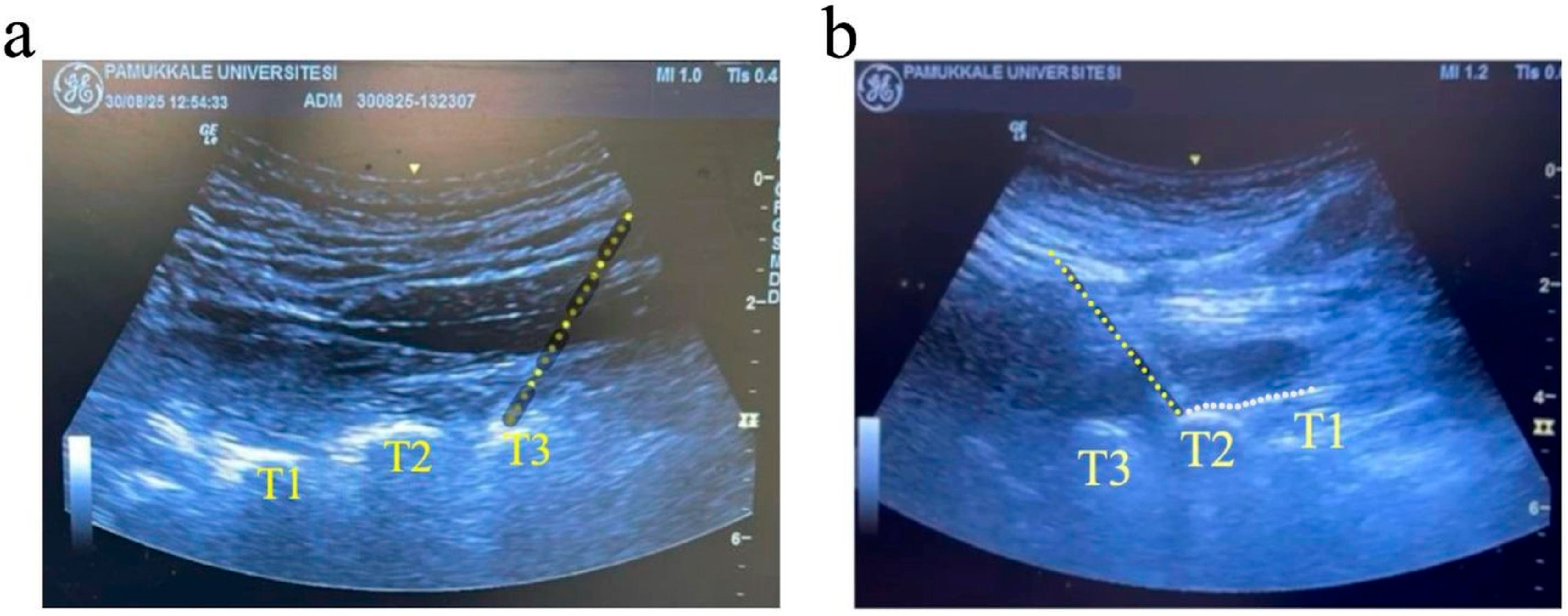
2.4. High Thoracic ESPB Procedure
2.5. Sample Size Calculations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| DBO | Diastolic Blood Pressure |
| ESPB | Erector Spinae Plane Block |
| GA | Graphical Abstract |
| ICMJE | International Committee of Medical Journal Editors |
| IQR | Interquartile Range |
| IRB | Institutional Review Board |
| ONSD | Optic Nerve Sheath Diameter |
| PI | Perfusion Index |
| SBP | Systolic Blood Pressure |
| SD | Standard Deviation |
| SpO2 | Peripheral Oxygen Saturation |
| US | Ultrasound |
| VAS | Visual Analog Scale |
References
- Bang, S.; Choi, J.; Kim, E.D. A High Thoracic Erector Spinae Plane Block Used for Sympathetic Block in Patients with Upper Extremity Complex Regional Pain Syndrome. J. Clin. Anesth. 2020, 60, 99–100. [Google Scholar] [CrossRef]
- Hernandez, N.; Guvernator, G.; Ansoanuur, G.; Ge, M.; Tabansi, P.; Le, T.T.; Obeidat, S.S.; de Haan, J. Relief of Secondary Headaches with High Thoracic Erector Spinae Plane Block. Local. Reg. Anesth. 2020, 13, 49–55. [Google Scholar] [CrossRef]
- Hong, J.H.; Park, J.H.; Park, K.B.; Lee, J.Y. Sympatholytic Effect of the High Thoracic Erector Spinae Plane Block. Pain Physician 2024, 27, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yoon, K.B.; Yoon, D.M.; Kim, D.H. Effect of Cervical Sympathetic Block on Optic Nerve Sheath Diameter Measured by Ultrasonography. Ultrasound Med. Biol. 2015, 41, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Polezi, M.; Oliveira, M.; Querioz, M.; Regatieri, F.; Schneider, D. Erector Spinae Plane Block as a New Treatment for Complex Regional Pain Syndrome in Upper Limbs: Two Case Reports. Ann. Clin. Med. Case Rep. 2023, 11, 1–3. [Google Scholar]
- Sethi, P.; Kaur, M.; Dang, D.; Bhatia, P. High Thoracic Erector Spinae Plane Block for Forequarter Amputation: A New Perspective. J. Anaesthesiol. Clin. Pharmacol. 2024, 40, 354–356. [Google Scholar] [CrossRef]
- Hong, J.H.; Park, K.B.; Lee, J.Y.; Park, J.H. Predictors of a Successful Outcome Following a Thoracic Erector Spinae Plane Block for Cervical Radiculopathy. Pain Physician 2024, 27, 235–242. [Google Scholar] [CrossRef]
- Rahimzadeh, P.; Salehi, S.; Saadat, S.; Vaziri, M.; Azar, P.H.; Ganji, M.F.; Rahimzadeh, P.; Salehi, S.; Saadat, S.; Vaziri, M.; et al. Evaluating the Efficacy of Cervical Erector Spinae Plane Block Using Ultrasound Versus Fluoroscopic Guidance for Cervical Pain: A Case Series. Anesthesiol. Pain Med. 2025, 15, e160776. [Google Scholar] [CrossRef]
- Hong, J.H.; Park, J.; Lee, S.; Lee, J. Comparison of the Low Back Pain Relief and Spread Level After Upper and Lower Lumbar Erector Spinae Plane Block. Pain Physician 2023, 26, 549–556. [Google Scholar] [CrossRef]
- Patil, A.; Vyshnavi, S.; Raja, T.; Shastry, V.; Thammaiah, S.H.; Archana, K.N. A Randomized Clinical Trial Comparing the Efficacy of Ultrasound-Guided Erector Spinae Block and Paravertebral Block in Preventing Postherpetic Neuralgia in Patients with Zoster-Associated Pain. J. Anaesthesiol. Clin. Pharmacol. 2024, 40, 510–515. [Google Scholar] [CrossRef]
- Gu, Y.; Qin, Y.; Wu, J. The Evolving Role of Truncal Fascial Plane Blocks in Non-Surgical Pain Therapy: A Narrative Review. Front. Med. 2025, 12, 1612201. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.V.; Moriggl, B.; Hoermann, R.; Nielsen, T.D.; Bendtsen, T.F.; Børglum, J. Are Single-Injection Erector Spinae Plane Block and Multiple-Injection Costotransverse Block Equivalent to Thoracic Paravertebral Block? Acta Anaesthesiol. Scand. 2019, 63, 1231–1238. [Google Scholar] [CrossRef]
- Dautzenberg, K.H.W.; Zegers, M.J.; Bleeker, C.P.; Tan, E.C.T.H.; Vissers, K.C.P.; Van Geffen, G.J.; Van Der Wal, S.E.I. Unpredictable Injectate Spread of the Erector Spinae Plane Block in Human Cadavers. Anesth. Analg. 2019, 129, E163–E166. [Google Scholar] [CrossRef]
- Ciftci, B.; Ekinci, M.; Gölboyu, B.E.; Kapukaya, F.; Atalay, Y.O.; Kuyucu, E.; Demiraran, Y. The Efficacy of High Thoracic Erector Spinae Plane Block. Pain Med. 2021, 22, 3105–3106. [Google Scholar] [CrossRef]
- Dickey, Z.; Sharma, N. Lumbar Sympathetic Block Leading to Increased Arterial Diameter and Blood Flow: A Mechanism of Therapeutic Benefit. Cureus 2024, 16, e61755. [Google Scholar] [CrossRef]
- Raja, S.N.; Treede, R.D. Testing the Link between Sympathetic Efferent and Sensory Afferent Fibers in Neuropathic Pain. Anesthesiology 2012, 117, 173–177. [Google Scholar] [CrossRef]
- Vadhanan, P.; Tripaty, D.K.; Adinarayanan, S. Physiological and Pharmacologic Aspects of Peripheral Nerve Blocks. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 384–393. [Google Scholar] [CrossRef]
- Stevens, R.A.; Stotz, A.; Kao, T.C.; Powar, M.; Burgess, S.; Kleinman, B. The Relative Increase in Skin Temperature after Stellate Ganglion Block Is Predictive of a Complete Sympathectomy of the Hand. Reg. Anesth. Pain. Med. 1998, 23, 266–270. [Google Scholar] [CrossRef]
- Chung, K.; Kim, K.H.; Kim, E.D. Perfusion Index as a Reliable Parameter of Vasomotor Disturbance in Complex Regional Pain Syndrome. Br. J. Anaesth. 2018, 121, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Yi, M.S.; Park, P.G.; Kang, H.; Lee, J.S.; Shin, H.Y. Effect of Stellate Ganglion Block on the Regional Hemodynamics of the Upper Extremity: A Randomized Controlled Trial. Anesth. Analg. 2018, 126, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B.; Graf, R. Different Compartments of Intracranial Pressure and Its Relationship to Cerebral Blood Flow. J. Trauma—Inj. Infect. Crit. Care 2005, 59, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Yayik, A.M.; Ahiskalioglu, E.O.; Celik, E.C.; Yalin, M.S.O.; Firinci, B.; Ates, I.; Aydin, M.E.; Ahiskalioglu, A. Evaluating the Effect of Caudal Epidural Block on Optic Nerve Sheath Diameter in Pediatric Patients: Randomized Controlled Study. BMC Anesth. 2025, 25, 138. [Google Scholar] [CrossRef]
- Lee, B.; Koo, B.N.; Choi, Y.S.; Kil, H.K.; Kim, M.S.; Lee, J.H. Effect of Caudal Block Using Different Volumes of Local Anaesthetic on Optic Nerve Sheath Diameter in Children: A Prospective, Randomized Trial. Br. J. Anaesth. 2017, 118, 781–787. [Google Scholar] [CrossRef]
- Kim, H.; Hyeon, J.; Hong, S.J.; Shin, K.M.; Park, J.; Kang, S.S. Cervical Interlaminar Epidural Injections at High Doses Do Not Increase Optic Nerve Sheath Diameter on a Long-Term Basis. Pain Physician 2016, 19, E1173–E1179. [Google Scholar] [CrossRef]
- Gundogdu, O.; Avci, O. Evaluation of the Effect of Interscalene Brachial Plexus Block on Intracranial Pressure Using Optic Nerve Sheath Diameter and Internal Jugular Vein Collapsibility Index. J. Coll. Physicians Surg. Pak. 2022, 32, 1249–1254. [Google Scholar] [CrossRef]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A Review of ASA Physical Status—Historical Perspectives and Modern Developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Forero, M.; Peng, P.; Chan, P. Horner Syndrome Following High Thoracic Erector Spinae Plane Block. Can. J. Anesth. 2022, 69, 400–401. [Google Scholar] [CrossRef]
- Kim, E.; Lim, J.A.; Choi, C.H.; Lee, S.Y.; Kwak, S.; Kim, J. Assessment of the Changes in Cardiac Sympathetic Nervous Activity Using the Pupil Size Changes Measured in Seated Patients Whose Stellate Ganglion Is Blocked by Interscalene Brachial Plexus Block. Korean J. Anesth. 2023, 76, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Cetgen, N.; Ener, D.; Dogukan, M.; Duran, M.; Uludag, O. Perfusion Index Value in Determining the Success of Axillary Block. J. Coll. Physicians Surg. Pak. 2022, 32, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasser, A.; Abdelhamid, B.; Elsonbaty, A.; Hasanin, A.; Rady, A. Predicting Successful Supraclavicular Brachial Plexus Block Using Pulse Oximeter Perfusion Index. Br. J. Anaesth. 2017, 119, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.K.S.D.; Bhargav Ram, A.; Prasad, C.R.K.; Garre, S.; Waghray, A. Effect on the Size of Optic Nerve Sheath Diameter in Patients Undergoing Surgeries under Spinal Anaesthesia versus Peripheral Nerve Blocks—A Randomised Controlled Study. Indian J. Anaesth. 2025, 69, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.B.; Bland, J.D.P.; Bhat, M.A.; Bennett, D.L.H. The Relationship of Nerve Fibre Pathology to Sensory Function in Entrapment Neuropathy. Brain 2014, 137, 3186–3199. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kim, H.J.; Back, S.K.; Na, H.S. Common and Discrete Mechanisms Underlying Chronic Pain and Itch: Peripheral and Central Sensitization. Pflug. Arch. 2021, 473, 1603–1615. [Google Scholar] [CrossRef]
| Variables | Overall (n = 35) | Responders (VAS > 50%) (n = 29) | Non-Responders (VAS ≤ 50%) (n = 6) | p |
|---|---|---|---|---|
| Age (years) | 53.0 [27.0–79.0] | 49.0 [27.0–79.0] | 55.0 [44.0–67.0] | 0.380 * |
| Sex | ||||
| Female | 19 (54.3) | 16 (55.2) | 3 (50.0) | 0.999 ** |
| Male | 16 (45.7) | 13 (44.8) | 3 (50.0) | |
| Body mass index (kg/m2) | 26.2 [20.8–33.3] | 26.2 [20.8–33.3] | 29.1 [21.3–31.6] | 0.418 * |
| Pain duration (months) | 1.0 [1.0–2.0] | 1.0 [1.0–2.0] | 1.5 [1.0–2.0] | 0.839 * |
| Side | ||||
| Right | 18 (51.4) | 15 (51.7) | 3 (50.0) | 0.999 ** |
| Left | 17 (48.6) | 14 (48.3) | 3 (50.0) |
| Variables | Responders (VAS > 50%) (n = 29) | Non-Responders (VAS ≤ 50%) (n = 6) | p |
|---|---|---|---|
| Heart rate (beats/min) | |||
| Pre-procedure | 79.0 [65.0–100.0] | 84.5 [70.0–98.0] | 0.381 * |
| Post-procedure 30 min | 79.0 [66.0–93.0] | 78.5 [73.0–94.0] | 0.456 * |
| p ** | 0.131 | 0.999 | |
| SpO2 (%) | |||
| Pre-procedure | 98.0 [95.0–100.0] | 96.5 [95.0–99.0] | 0.168 * |
| Post-procedure 30 min | 99.0 [95.0–100.0] | 96.5 [95.0–99.0] | 0.136 * |
| p ** | 0.157 | 0.999 | |
| SBP (mmHg) | |||
| Pre-procedure | 124.0 [93.0–166.0] | 137.5 [127.0–163.0] | 0.042 * |
| Post-procedure 30 min | 130.0 [90.0–165.0] | 142.0 [128.0–159.0] | 0.110 * |
| p ** | 0.075 | 0.245 | |
| DBP (mmHg) | |||
| Pre-procedure | 63.0 [50.0–94.0] | 77.5 [52.0–97.0] | 0.368 * |
| Post-procedure 30 min | 67.0 [50.0–91.0] | 66.0 [54.0–95.0] | 0.792 * |
| p ** | 0.086 | 0.917 | |
| Ipsilateral skin temperature (°C) | |||
| Pre-procedure | 34.7 [33.4–35.8] | 34.4 [33.9–35.5] | 0.693 * |
| Post-procedure 30 min | 36.0 [34.5–36.5] | 36.5 [35.3–36.6] | 0.186 * |
| p ** | <0.001 | 0.028 | |
| Contralateral skin temperature (°C) | |||
| Pre-procedure | 34.6 [33.4–36.0] | 34.2 [33.9–35.7] | 0.660 * |
| Post-procedure 30 min | 34.8 [33.5–36.2] | 34.2 [34.0–35.7] | 0.510 * |
| p ** | <0.001 | 0.498 | |
| VAS | |||
| Pre-procedure | 8.0 [6.0–10.0] | 6.0 [6.0–9.0] | 0.054 * |
| Post-procedure 30 min | 2.0 [1.0–3.0] | 3.0 [3.0–8.0] | NA |
| p ** | <0.001 | 0.024 |
| Variables | Overall (n = 35) | Responders (VAS > 50%) (n = 29) | Non-Responders (VAS ≤ 50%) (n = 6) | p |
|---|---|---|---|---|
| Ipsilateral optic nerve (mm, horizontal) | ||||
| Pre-block | 0.47 [0.36–0.65] | 0.48 [0.38–0.65] | 0.41 [0.36–0.61] | 0.254 * |
| Post-block | 0.51 [0.43–0.69] | 0.53 [0.43–0.69] | 0.48 [0.45–0.65] | 0.262 * |
| p ** | <0.001 | <0.001 | 0.027 | |
| Ipsilateral optic nerve (mm, vertical) | ||||
| Pre-block | 0.46 [0.36–0.65] | 0.46 [0.38–0.65] | 0.40 [0.36–0.61] | 0.227 * |
| Post-block | 0.52 [0.43–0.68] | 0.52 [0.43–0.68] | 0.51 [0.45–0.64] | 0.999 * |
| p ** | <0.001 | <0.001 | 0.028 | |
| Average ipsilateral optic nerve (mm) | ||||
| Pre-block | 0.47 [0.36–0.65] | 0.47 [0.38–0.65] | 0.40 [0.36–0.61] | 0.228 * |
| Post-block | 0.51 [0.44–0.69] | 0.52 [0.44–0.69] | 0.50 [0.45–0.64] | 0.584 * |
| p ** | <0.001 | <0.001 | 0.031 | |
| Contralateral optic nerve (mm, horizontal) | ||||
| Pre-block | 0.47 [0.39–0.65] | 0.47 [0.39–0.65] | 0.50 [0.39–0.63] | 0.614 * |
| Post-block | 0.48 [0.40–0.65] | 0.48 [0.40–0.65] | 0.50 [0.40–0.64] | 0.692 * |
| p ** | <0.001 | 0.001 | 0.102 | |
| Contralateral optic nerve (mm, vertical) | ||||
| Pre-block | 0.49 [0.40–0.62] | 0.49 [0.40–0.61] | 0.49 [0.40–0.62] | 0.861 * |
| Post-block | 0.49 [0.40–0.62] | 0.49 [0.40–0.62] | 0.49 [0.40–0.62] | 0.742 * |
| p ** | 0.285 | 0.655 | 0.317 | |
| Average contralateral optic nerve (mm) | ||||
| Pre-block | 0.48 [0.40–0.63] | 0.48 [0.40–0.63] | 0.50 [0.40–0.62] | 0.693 * |
| Post-block | 0.49 [0.40–0.63] | 0.49 [0.40–0.62] | 0.50 [0.40–0.63] | 0.742 * |
| p ** | <0.001 | 0.002 | 0.174 |
| Variables | Overall (n = 35) | Responders (VAS > 50%) (n = 29) | Non-Responders (VAS ≤ 50%) (n = 6) | p |
|---|---|---|---|---|
| Ipsilateral perfusion index | ||||
| Pre-procedure | 2.6 [0.4–7.3] | 2.6 [0.4–7.3] | 2.3 [1.4–4.5] | 0.540 * |
| Post-procedure 30 min | 3.0 [0.7–7.1] | 3.0 [0.7–7.1] | 3.0 [2.5–4.6] | 0.999 * |
| p ** | 0.002 | 0.022 | 0.036 | |
| %Δ PI-0/30 min (ipsilateral) | 11.1 [−23.1–100.0] | 11.1 [−23.1–100.0] | 29.9 [2.2–100.0] | 0.347 * |
| Contralateral perfusion index | ||||
| Pre-procedure | 3.3 [0.5–8.2] | 3.1 [0.5–8.2] | 4.4 [2.0–8.0] | 0.539 * |
| Post-procedure 30 min | 3.5 [0.4–7.9] | 3.4 [0.4–7.9] | 4.4 [2.0–7.7] | 0.483 * |
| p ** | 0.111 | 0.073 | 0.999 | |
| %Δ PI-0/30 min (contralateral) | −2.0 [−23.1–46.7] | −2.0 [−23.1–46.7] | −1.0 [−3.8–12.0] | 0.479 * |
| VAS Pain Score | ||||
|---|---|---|---|---|
| r | p | |||
| Pre-intervention | Ipsilateral | Skin temperature | 0.051 | 0.770 |
| PI | 0.090 | 0.609 | ||
| ONSD | 0.295 | 0.085 | ||
| Contralateral | Skin temperature | 0.075 | 0.668 | |
| PI | −0.103 | 0.554 | ||
| ONSD | 0.259 | 0.132 | ||
| Post-intervention | Ipsilateral | Skin temperature | −0.023 | 0.896 |
| PI | −0.074 | 0.671 | ||
| ONSD | −0.253 | 0.143 | ||
| Contralateral | Skin temperature | −0.098 | 0.574 | |
| PI | 0.003 | 0.987 | ||
| ONSD | −0.040 | 0.819 | ||
| Variables | Overall (n = 35) | Responders (VAS > 50%) (n = 29) | Non-Responders (VAS ≤ 50%) (n = 6) | p * |
|---|---|---|---|---|
| Adverse effects, present | 17 (48.6) | 13 (44.8) | 4 (66.7) | 0.402 |
| Facial flushing, present | 12 (34.3) | 9 (31.0) | 3 (50.0) | 0.391 |
| Nasal congestion, present | 7 (20.0) | 6 (20.7) | 1 (16.7) | 0.999 |
| Horner syndrome, present | 2 (5.7) | 2 (6.9) | 0 (0.0) | 0.999 |
| Hoarseness, present | 1 (2.9) | 1 (3.4) | 0 (0.0) | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İlhan, S.; Akbudak, İ.H.; Evran, T.; Çopur, İ.; Erdoğan, Ç.; Gönüllü, E. Comprehensive Assessment of Pain and Physiological Parameters of Sympathetic Blockade to Accurately Determine the Success of High Thoracic Erector Spinae Plane Block. Healthcare 2025, 13, 2322. https://doi.org/10.3390/healthcare13182322
İlhan S, Akbudak İH, Evran T, Çopur İ, Erdoğan Ç, Gönüllü E. Comprehensive Assessment of Pain and Physiological Parameters of Sympathetic Blockade to Accurately Determine the Success of High Thoracic Erector Spinae Plane Block. Healthcare. 2025; 13(18):2322. https://doi.org/10.3390/healthcare13182322
Chicago/Turabian Styleİlhan, Seher, İlknur Hatice Akbudak, Turan Evran, İsmet Çopur, Çağla Erdoğan, and Edip Gönüllü. 2025. "Comprehensive Assessment of Pain and Physiological Parameters of Sympathetic Blockade to Accurately Determine the Success of High Thoracic Erector Spinae Plane Block" Healthcare 13, no. 18: 2322. https://doi.org/10.3390/healthcare13182322
APA Styleİlhan, S., Akbudak, İ. H., Evran, T., Çopur, İ., Erdoğan, Ç., & Gönüllü, E. (2025). Comprehensive Assessment of Pain and Physiological Parameters of Sympathetic Blockade to Accurately Determine the Success of High Thoracic Erector Spinae Plane Block. Healthcare, 13(18), 2322. https://doi.org/10.3390/healthcare13182322







