Impact of CT-Defined Sarcopenia on Clinical Outcomes in Elderly Trauma Patients: A Retrospective Korean Cohort Study
Abstract
1. Introduction
2. Materials and Methods
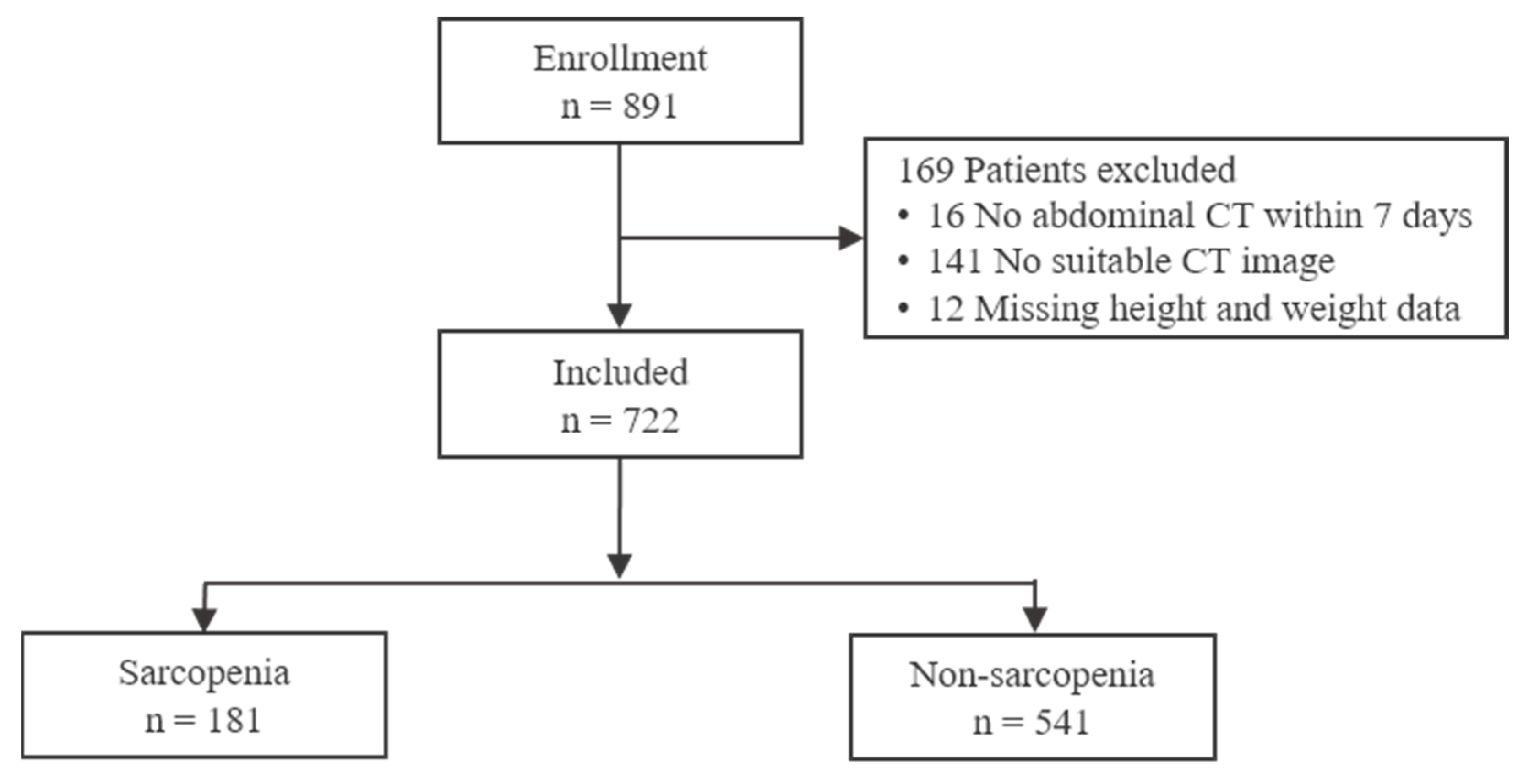
2.1. Patient Selection
2.2. Data Collection
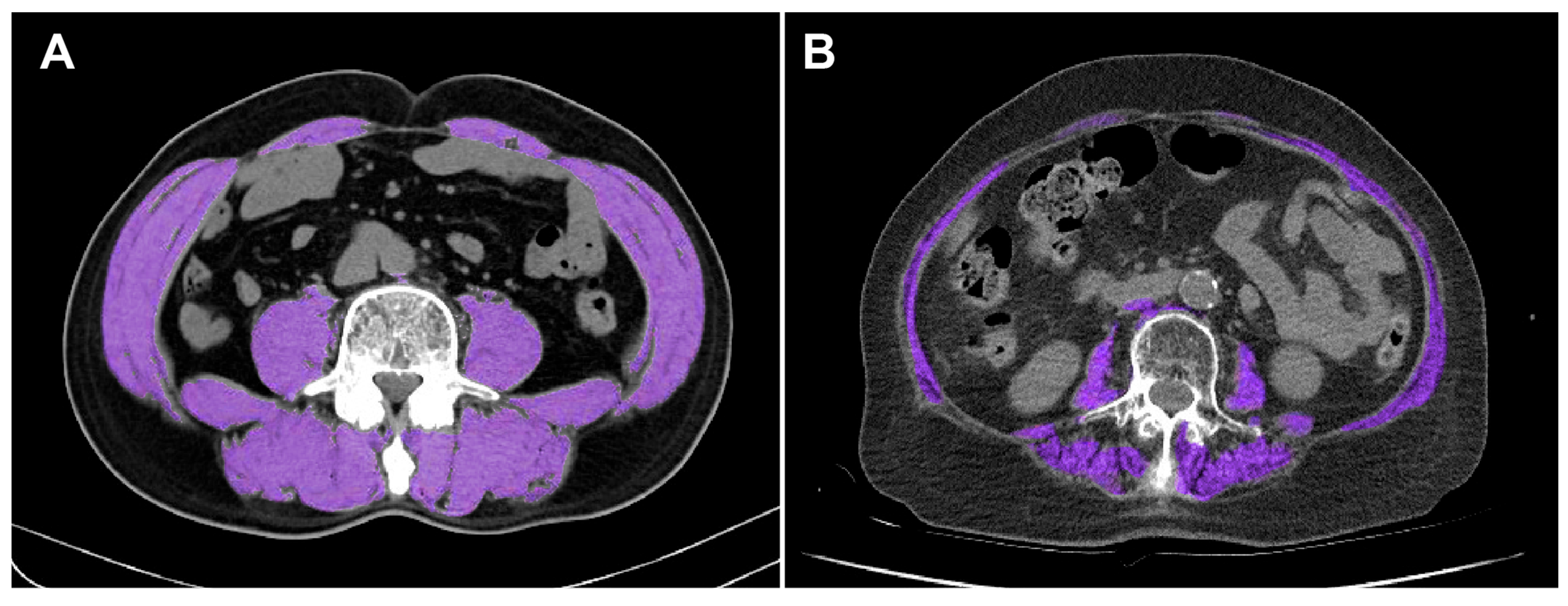
2.3. SMI Calculation
2.4. Definition of Sarcopenia in This Study
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics and Characteristics
3.2. Clinical Outcomes and Complications
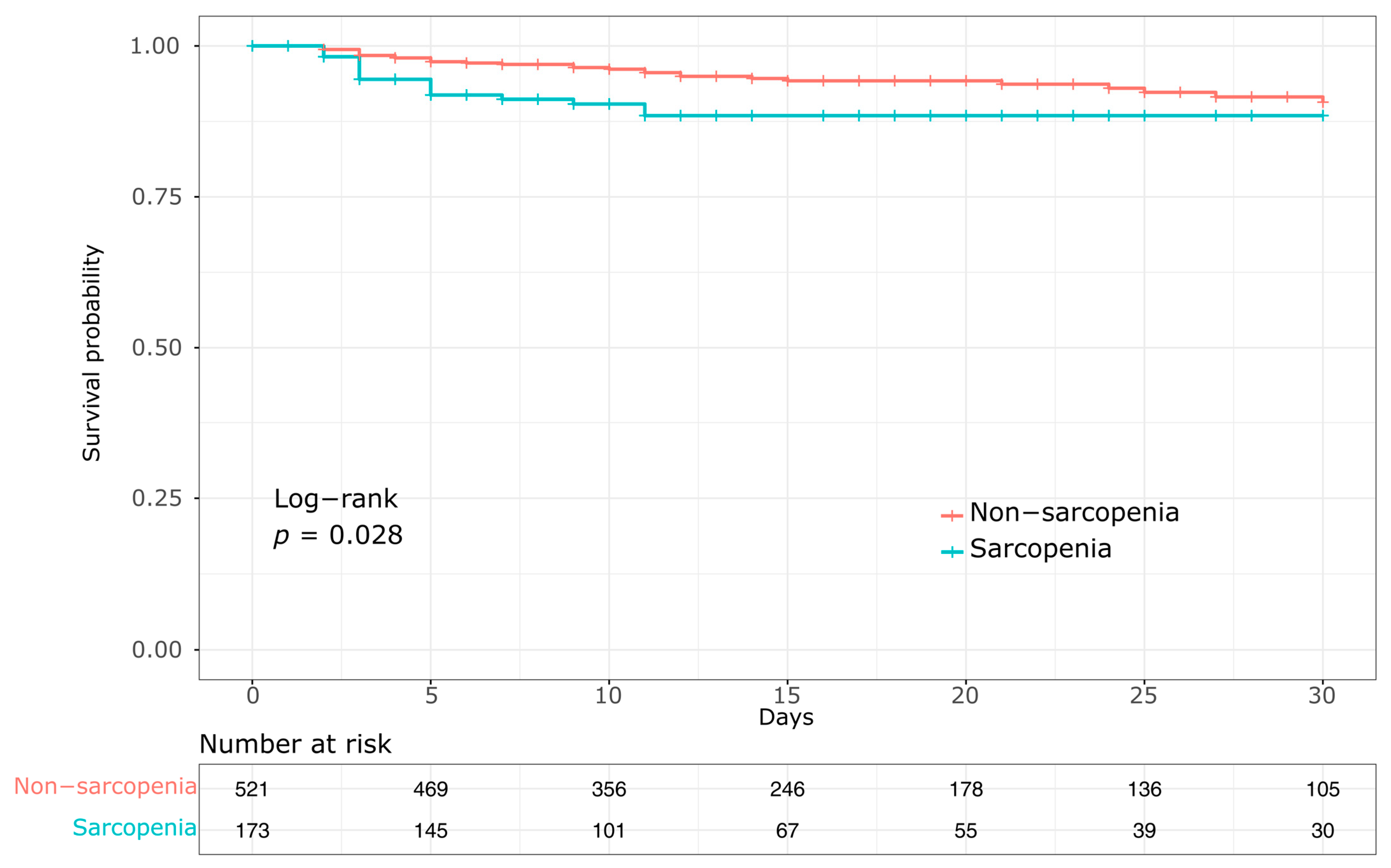
3.3. Mortality
3.4. Cox Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Guidelines for Trauma Quality Improvement Programmes; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Trunkey, D.D. Trauma. Sci. Am. 1983, 249, 28–35. [Google Scholar] [CrossRef]
- Hashmi, A.; Ibrahim-Zada, I.; Rhee, P.; Aziz, H.; Fain, M.J.; Friese, R.S.; Joseph, B. Predictors of mortality in geriatric trauma patients: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2014, 76, 894–901. [Google Scholar] [CrossRef]
- Stonko, D.P.; Etchill, E.W.; Giuliano, K.A.; DiBrito, S.R.; Eisenson, D.; Heinrichs, T.; Morrison, J.J.; Haut, E.R.; Kent, A.J. Failure to rescue in geriatric trauma: The impact of any complication increases with age and injury severity in elderly trauma patients. Am. Surg. 2021, 87, 1760–1765. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Marzetti, E. Sarcopenia and physical frailty: Two sides of the same coin. Front. Aging Neurosci. 2014, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from the ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Zumsteg, D.M.; Chu, C.E.; Midwinter, M.J. Radiographic assessment of sarcopenia in the trauma setting: A systematic review. Trauma Surg. Acute Care Open 2020, 5, e000414. [Google Scholar] [CrossRef]
- Kwon, I.; Kim, J.S.; Shin, C.H.; Park, Y.; Kim, J.H. Associations between skeletal muscle mass, grip strength, and physical and cognitive functions in elderly women: Effect of exercise with resistive theraband. J. Exerc. Nutr. Biochem. 2019, 23, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, Z.; Shen, Q.; Zhi, H.; Cai, W.; Wang, X.; Chen, X.; Shen, X.; Zhang, W. Body composition analysis using CT at three aspects of the lumbar third vertebra and its impact on the diagnosis of sarcopenia. World J. Surg. Oncol. 2025, 23, 64. [Google Scholar] [CrossRef]
- Moore, D.D.; Koehler, T.J.; Bradley, J.D.; O’Connor, J.V.; Scalea, T.M.; Hu, P. Sarcopenia as a predictor of mortality in elderly trauma patients. J. Trauma Acute Care Surg. 2015, 79, 413–418. [Google Scholar]
- Lee, C.; Kozar, R.A.; Moore, F.A.; Cox, C.S.; Szaflarski, N.; Moore, E.E. Computed tomographic skeletal muscle index is an independent predictor of mortality in severely injured trauma patients. J. Trauma Acute Care Surg. 2016, 80, 597–603. [Google Scholar]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar]
- Asan J-Morphometry. Available online: https://datasharing.aim-aicro.com/morphometry (accessed on 15 July 2025).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwatsuki, M.; Ishimoto, T.; Yoshida, N.; et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann. Surg. Oncol. 2015, 22, 2663–2668. [Google Scholar] [CrossRef]
- Voron, T.; Tselikas, L.; Pietrasz, D.; Pigneur, F.; Laurent, A.; Compagnon, P.; Salloum, C.; Luciani, A.; Azoulay, D. Sarcopenia impacts on short- and long-term results of hepatic resection for hepatocellular carcinoma. Ann. Surg. Oncol. 2015, 22, 4363–4369. [Google Scholar]
- Xia, W.; Barazanchi, A.W.H.; MacFater, W.S.; Hill, A.G. The impact of computed tomography-assessed sarcopenia on outcomes for trauma patients: A systematic review and meta-analysis. Injury 2019, 50, 1565–1576. [Google Scholar] [CrossRef]
- Varma, S.; Wilson, M.S.J.; Naik, M.; Sandhu, A.; Ota, H.C.U.; Aylwin, C.; Fertleman, M.; Peck, G. The associations of psoas and masseter muscles with sarcopenia and related adverse outcomes in older trauma patients: A retrospective study. Aging Clin. Exp. Res. 2022, 34, 1901–1908. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–organ crosstalk: The emerging roles of myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Sklar, M.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Rittayamai, N.; Harhay, M.O.; Reid, W.D.; Tomlinson, G.; et al. Association of low baseline diaphragm muscle mass with prolonged mechanical ventilation and mortality among critically ill adults. JAMA Netw. Open 2020, 3, e1921520. [Google Scholar] [CrossRef]
- Landi, F.; Camprubi-Robles, M.; Bear, D.E.; Cederholm, T.; Malafarina, V.; Welch, A.A.; Cruz-Jentoft, A.J. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association between malnutrition and clinical outcomes in the intensive care unit: A systematic review. JPEN J. Parenter. Enter. Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Age Ageing 2017, 46, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Aschkenasy, M.T.; Rothenhaus, T.C. Trauma and falls in the elderly. Emerg. Med. Clin. N. Am. 2006, 24, 413–432. [Google Scholar] [CrossRef] [PubMed]
- De Simone, B.; Chouillard, E.; Podda, M.; Pararas, N.; de Carvalho Duarte, G.; Fugazzola, P.; Birindelli, A.; Coccolini, F.; Polistena, A.; Sibilla, M.G.; et al. The 2023 WSES guidelines on the management of trauma in elderly and frail patients. World J. Emerg. Surg. 2024, 19, 18. [Google Scholar] [CrossRef]
- Brock, G.N.; Barnes, C.; Ramirez, J.A.; Myers, J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med. Res. Methodol. 2011, 11, 144. [Google Scholar]
- Kaplan, S.J.; Pham, T.N.; Arbabi, S.; Gross, J.A.; Damodarasamy, M.; Bentov, I.; Taitsman, L.A.; Mitchell, S.H.; Reed, M.J. Association of radiologic indicators of frailty with 1-year mortality in older trauma patients: Opportunistic screening for sarcopenia and osteopenia. JAMA Surg. 2017, 152, e164604. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Twisk, J.W.; Oudemans-van Straaten, H.M.; Weijs, P.J. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef] [PubMed]
- Tazerout, S.; Martinez, O.; Monsonis, B.; Millet, I.; Taourel, P.; Capdevila, X.; Charbit, J. Acute post-traumatic muscle atrophy on CT scan predicts prolonged mechanical ventilation and a worse outcome in severe trauma patients. Injury 2022, 53, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
| Variable | Sarcopenia (n = 181) | Non-Sarcopenia (n = 541) | p-Value |
|---|---|---|---|
| Age, years | 77 [72–82] | 72 [67–78] | <0.001 |
| Age ≥ 75 years, n (%) | 112 (61.9) | 195 (36.0) | <0.001 |
| Sex, n (%) | 0.976 | ||
| Male | 122 (67.4) | 364 (67.3) | |
| Female | 59 (32.6) | 177 (32.7) | |
| Weight, kg | 59.6 ± 11.4 | 64.3 ± 10.9 | <0.001 |
| BMI, kg/m2 | 22.0 ± 3.46 | 24.1 ± 3.06 | <0.001 |
| Blunt injury, n (%) | 178 (98.3) | 523 (96.7) | 0.247 |
| Mechanism of injury, n (%) | 0.037 | ||
| Fall | 97 (53.6) | 225 (41.6) | |
| Motor vehicle accident | 17 (9.4) | 84 (15.5) | |
| Motorcycle accident | 16 (8.8) | 49 (9.1) | |
| Bicycle accident | 5 (2.8) | 37 (6.8) | |
| Stabbing | 3 (1.7) | 15 (2.8) | |
| Pedestrian | 29 (16.0) | 97 (17.9) | |
| Struck by | 4 (2.2) | 15 (2.8) | |
| Other Mechanisms | 1 (0.6) | 6 (1.1) | |
| Unknown | 9 (5.0) | 13 (2.4) | |
| SBP, mmHg | 143 [121–158] | 143 [124–161] | 0.997 |
| SBP ≤ 90 mmHg, n (%) | 9 (5.0) | 22 (4.1) | 0.603 |
| GCS | 15 [14,15] | 15 [14,15] | 0.953 |
| ISS | 18 [10–26] | 19 [14–29] | 0.013 |
| ISS ≥ 16, n (%) | 104 (57.5) | 358 (66.2) | 0.034 |
| AIS Head ≥ 3, n (%) | 21 (11.6) | 88 (16.3) | 0.129 |
| AIS Thorax ≥ 3, n (%) | 94 (51.9) | 367 (67.8) | <0.001 |
| AIS Abdomen ≥ 3, n (%) | 7 (3.9) | 60 (11.1) | 0.004 |
| Albumin, g/dL | 3.8 [3.4–4.1] | 3.9 [3.6–4.2] | 0.010 |
| Hemoglobin, g/dL | 11.7 [10.1–12.9] | 12.4 [10.9–13.4] | <0.001 |
| SMA, cm2 | 83.8 [65.9–97.0] | 114.0 [89.5–127.1] | <0.001 |
| SMI, cm2/m2 | 29.47 ± 5.05 | 41.20 ± 7.33 | <0.001 |
| Outcome | Sarcopenia (n = 181) | Non-Sarcopenia (n = 541) | p-Value |
|---|---|---|---|
| 24 h mortality, n (%) | 8 (4.4) | 20 (3.7) | 0.663 |
| In-hospital mortality, n (%) | 28 (15.5) | 53 (9.8) | 0.036 |
| Hospital LOS | 12 [6–22] | 14 [8–26] | 0.026 |
| ICU LOS | 6.5 [3–20] | 6.5 [2–17] | 0.654 |
| Mechanical ventilation, n (%) | 58 (32.0) | 192 (35.5) | 0.399 |
| Ventilator days | 5 [4–8] | 3 [2–7] | 0.695 |
| RBC transfusion, n (%) | 11 (6.1) | 44 (8.1%) | 0.367 |
| RBC transfusion within 24 h, unit | 13.5 [8–15] | 12 [7–16] | 1.000 |
| Massive transfusion * n (%) | 11 (6.1) | 44 (8.1) | 0.367 |
| Hospital cost, USD ** × 104 $ | 2.87 [2.29–4.38] | 3.62 [1.50–5.87] | 0.053 |
| Complication (n, %) | Sarcopenia (n = 181) | Non-Sarcopenia (n = 541) | p-Value |
|---|---|---|---|
| Sepsis | 3 (1.7) | 16 (3.0) | 0.431 |
| CAUTI | 3 (1.7) | 13 (2.4) | 0.772 |
| Deep or Organ SSI | 0 | 3 (0.6) | 0.577 |
| CLABSI | 0 | 3 (0.6) | 0.577 |
| Acute kidney injury | 5 (2.8) | 29 (5.4) | 0.153 |
| VAP | 8 (4.4) | 33 (6.1) | 0.398 |
| ARDS | 4 (2.2) | 8 (1.5) | 0.508 |
| Cardiac arrest | 6 (3.3) | 14 (2.6) | 0.606 |
| Pulmonary embolism | 0 | 4 (0.7) | 0.577 |
| Stroke | 2 (1.1) | 7 (1.3) | 1.000 |
| Myocardial infarction | 0 | 2 (0.4) | 1.000 |
| Variable | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.03 (0.99–1.07) | 0.157 | 1.00 (0.95–1.06) | 0.927 |
| Male | 1.06 (0.57–2.00) | 0.847 | 0.68 (0.32–1.42) | 0.301 |
| Sarcopenia | 1.93 (1.06–3.51) | 0.032 | 2.36 (1.07–5.23) | 0.034 |
| SBP ≤ 90 | 1.01 (1.00–1.02) | 0.022 | 1.37 (0.34–5.57) | 0.663 |
| ISS | 1.05 (1.03–1.07) | <0.001 | 1.06 (1.02–1.09) | 0.002 |
| GCS | 0.73 (0.68–0.78) | <0.001 | 0.74 (0.68–0.80) | <0.001 |
| Albumin | 0.62 (0.39–0.97) | 0.038 | 1.02 (0.52–1.99) | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Oh, Y.; Kwon, S.; Lee, J.; Kim, M.; Choi, D.; Kwon, J. Impact of CT-Defined Sarcopenia on Clinical Outcomes in Elderly Trauma Patients: A Retrospective Korean Cohort Study. Healthcare 2025, 13, 2321. https://doi.org/10.3390/healthcare13182321
Park J, Oh Y, Kwon S, Lee J, Kim M, Choi D, Kwon J. Impact of CT-Defined Sarcopenia on Clinical Outcomes in Elderly Trauma Patients: A Retrospective Korean Cohort Study. Healthcare. 2025; 13(18):2321. https://doi.org/10.3390/healthcare13182321
Chicago/Turabian StylePark, Juhong, Yesung Oh, Songhee Kwon, Jihyun Lee, Mihyang Kim, Donghwan Choi, and Junsik Kwon. 2025. "Impact of CT-Defined Sarcopenia on Clinical Outcomes in Elderly Trauma Patients: A Retrospective Korean Cohort Study" Healthcare 13, no. 18: 2321. https://doi.org/10.3390/healthcare13182321
APA StylePark, J., Oh, Y., Kwon, S., Lee, J., Kim, M., Choi, D., & Kwon, J. (2025). Impact of CT-Defined Sarcopenia on Clinical Outcomes in Elderly Trauma Patients: A Retrospective Korean Cohort Study. Healthcare, 13(18), 2321. https://doi.org/10.3390/healthcare13182321








