Integrating Multimorbidity Assessment into Rheumatology Care: Prognostic Role of the Charlson Comorbidity Index in Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Extraction
- Study design and location,
- Sample size and demographic characteristics,
- Method of comorbidity assessment (CCI or specific comorbidities),
- Follow-up duration,
- Reported effect sizes (ORs or HRs with 95% CIs),
- Variables adjusted for in multivariable models.
2.5. Risk of Bias Assessment
2.6. Data Synthesis and Statistical Analysis
3. Results
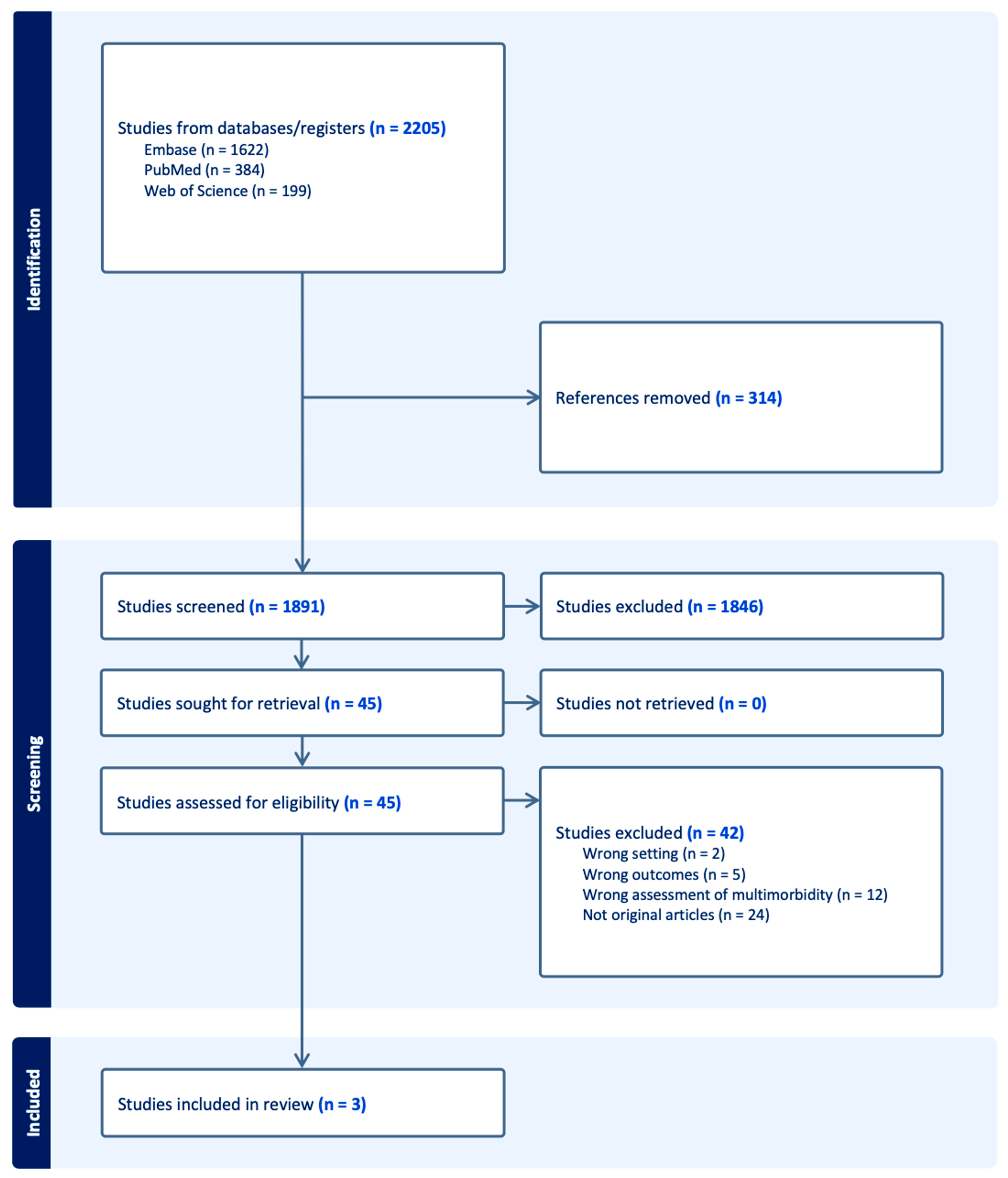
3.1. Study Selection: PRISMA Flow Diagram
3.2. Study Characteristics
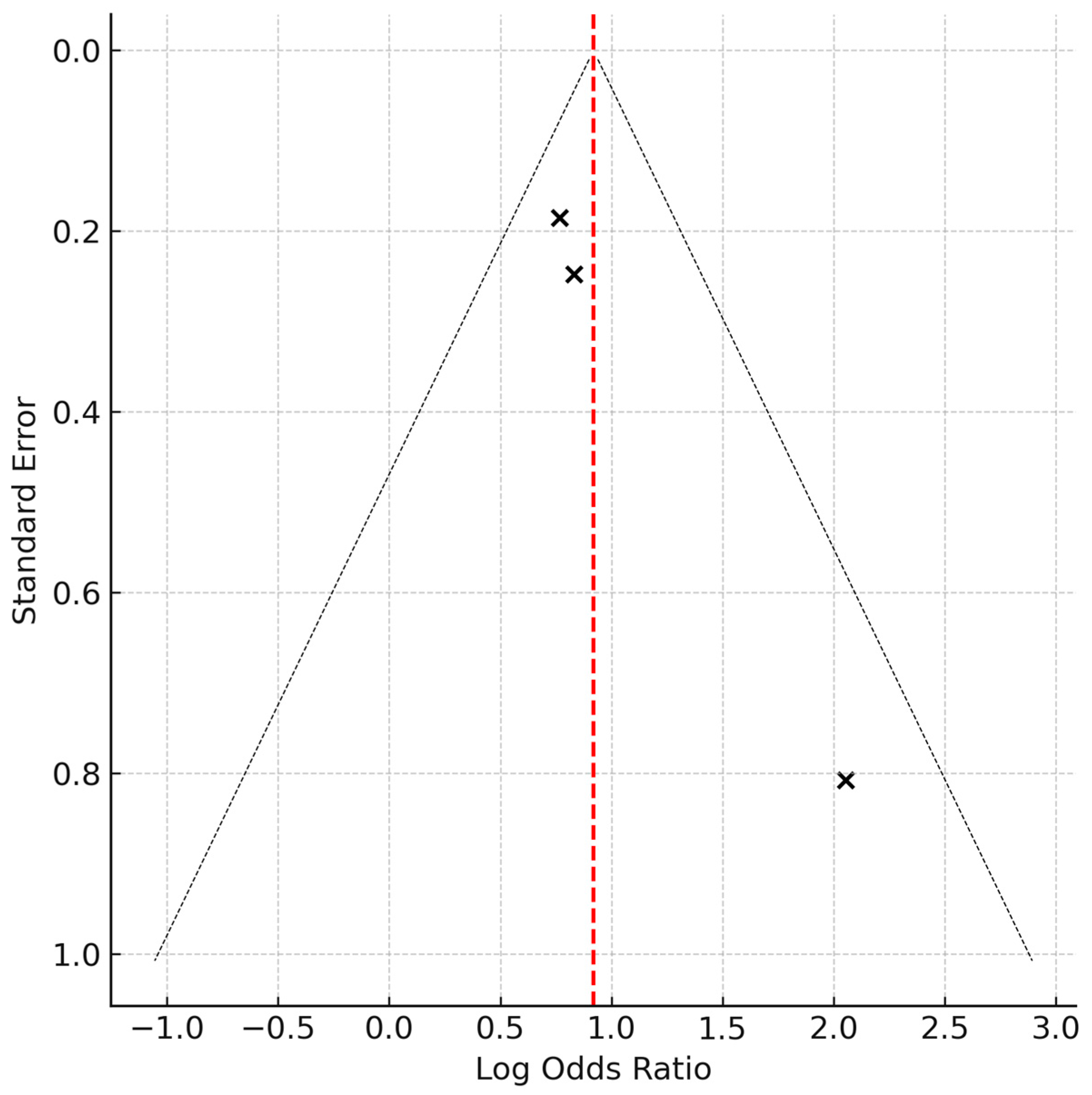
3.3. Risk of Bias Within Studies
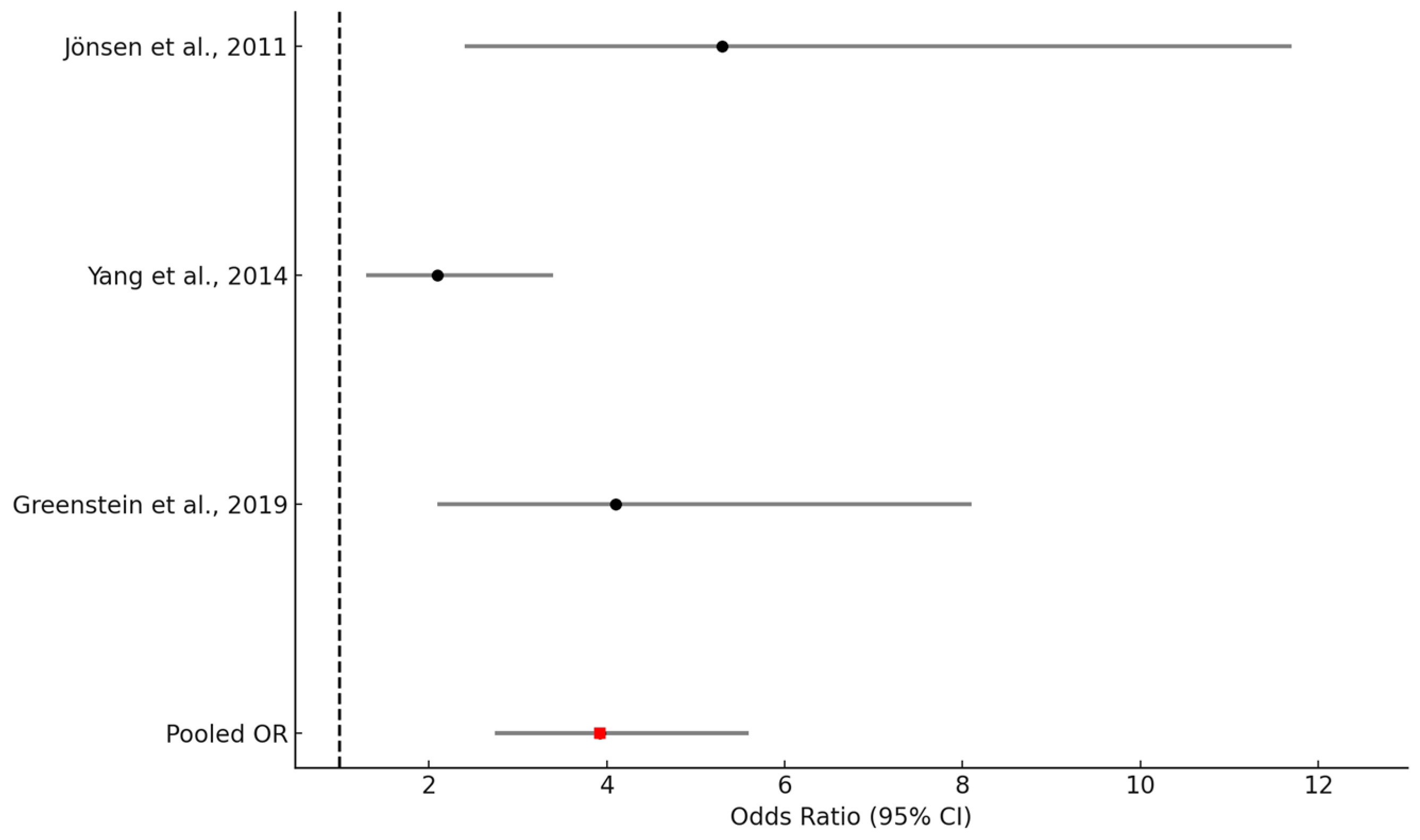
3.4. Results of Individual Studies and Synthesis
3.5. Legend
4. Discussion
4.1. Summary of Main Findings
4.2. Comparison with Previous Literature
4.3. Clinical Implications
4.4. Comparison of Comorbidity Indices in SLE
4.5. Limitations
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLE | Systemic Lupus Erythematosus |
| CCI | Charlson Comorbidity Index |
| OR | Odds Ratio |
| CI | Confidence Interval |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| ACR | American College of Rheumatology |
| SLICC | Systemic Lupus International Collaborating Clinics |
| NOS | Newcastle–Ottawa Scale |
| HR | Hazard Ratio |
| SD | Standard Deviation |
References
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Petri, M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, S4. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; A Khamashta, M.; Font, J.; Sebastiani, G.D.; Gil, A.; Lavilla, P.; Mejía, J.C.; Aydintug, A.O.; Chwalinska-Sadowska, H.; De Ramón, E.; et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1000 patients. Medicine 2003, 82, 299–308. [Google Scholar] [CrossRef]
- Kariniemi, S.; Rantalaiho, V.; Virta, L.J.; Puolakka, K.; Sokka-Isler, T.; Elfving, P. Multimorbidity among incident Finnish systemic lupus erythematosus patients during 2000–2017. Lupus 2021, 30, 165–171. [Google Scholar] [CrossRef]
- Vacchi, C.; Sebastiani, M.; Cassone, G.; Cerri, S.; Della Casa, G.; Salvarani, C.; Manfredi, A. Therapeutic Options for the Treatment of Interstitial Lung Disease Related to Connective Tissue Diseases. A Narrative Review. J. Clin. Med. 2020, 9, 407. [Google Scholar] [CrossRef]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- Chang, C.M.; Yin, W.Y.; Wei, C.K.; Wu, C.C.; Su, Y.C.; Yu, C.H.; Lee, C.C.; Palaniyandi, S. Adjusted Age-Adjusted Charlson Comorbidity Index Score as a Risk Measure of Perioperative Mortality before Cancer Surgery. PLoS ONE 2016, 11, e0148076. [Google Scholar] [CrossRef]
- Jönsen, A.; Clarke, A.E.; Joseph, L.; Belisle, P.; Bernatsky, S.; Nived, O.; Bengtsson, A.A.; Sturfelt, G.; Pineau, C.A. Association of the Charlson comorbidity index with mortality in systemic lupus erythematosus. Arthritis Care Res. 2011, 63, 1233–1237. [Google Scholar] [CrossRef]
- Chuang, M.H.; Chuang, T.L.; Huang, K.Y.; Wang, Y.F. Age-adjusted Charlson Comorbidity Index scores predict major adverse cardiovascular events and all-cause mortality among systemic lupus erythematosus patients. Tzu Chi Med. J. 2017, 29, 154–158. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Straus, S.; Fortin, M.; Guthrie, B.; James, M.T.; Klarenbach, S.W.; Tam-Tham, H.; Lewanczuk, R.; Manns, B.J.; et al. Multimorbidity, dementia and health care in older people:a population-based cohort study. CMAJ Open 2017, 5, E623–E631. [Google Scholar] [CrossRef]
- Matthew, J.P.; Joanne, E.M.; Patrick, M.B.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Lerkvaleekul, B.; Chobchai, P.; Rattanasiri, S.; Vilaiyuk, S. Evaluating performance of the 2019 EULAR/ACR, 2012 SLICC, and 1997 ACR criteria for classifying adult-onset and childhood-onset systemic lupus erythematosus: A systematic review and meta-analysis. Front. Med. 2022, 9, 1093213. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In Proceedings of the 3rd Symposium on Systematic Review: Beyond the Basics, Oxford, UK, 3–5 July 2000. [Google Scholar]
- Greenstein, L.; Makan, K.; Tikly, M. Burden of comorbidities in South Africans with systemic lupus erythematosus. Clin. Rheumatol. 2019, 38, 2077–2082. [Google Scholar] [CrossRef]
- Chinn, S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat. Med. 2000, 19, 3127–3131. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2012, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Thumboo, J.; Earnest, A.; Yong, S.; Fong, K. The effect of comorbidity on hospital mortality in patients with SLE from an Asian tertiary hospital. Lupus 2014, 23, 714–720. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Palmer, K.; Marengoni, A.; Marzetti, E.; Lattanzio, F.; Roller-Wirnsberger, R.; Samaniego, L.L.; Rodríguez-Mañas, L.; Bernabei, R.; Onder, G. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. J. Gerontol. Ser. A 2019, 74, 659–666. [Google Scholar] [CrossRef]
- Ukhanova, M.A.; Tillotson, C.J.; Marino, M.; Huguet, N.; Quiñones, A.R.; Hatch, B.A.; Schmidt, T.; DeVoe, J.E. Uptake of Preventive Services Among Patients With and Without Multimorbidity. Am. J. Prev. Med. 2020, 59, 621–629. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.; Ronksley, P.E.; Cook, L.L.; Patel, A.B.; Seidel, J.; Lethebe, B.C.; Green, L.A. The Impact of Primary Care Clinic and Family Physician Continuity on Patient Health Outcomes: A Retrospective Analysis From Alberta, Canada. Ann. Fam. Med. 2024, 22, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ohta, R.; Sano, C. Family Physicians as System-Specific Specialists in Japan’s Aging Society. Cureus 2022, 14, e30811. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Lee, C.T. Explore comorbidities associated with systemic lupus erythematosus: A total population-based case–control study. QJM 2022, 115, 17–23. [Google Scholar] [CrossRef]
- Musa, M.; Chukwuyem, E.; Ojo, O.M.; Topah, E.K.; Spadea, L.; Salati, C.; Gagliano, C.; Zeppieri, M. Unveiling Ocular Manifestations in Systemic Lupus Erythematosus. J. Clin. Med. 2024, 13, 1047. [Google Scholar] [CrossRef]
- Gamal, S.M.; Mokbel, A.; Niazy, M.H.; Elgengehy, F.T.; Elsaid, N.Y.; Fouad, N.A.; Sobhy, N.; Tantawy, M.; Mohamed, S.S.; Ghaleb, R.M.; et al. Comorbidities among Egyptian systemic lupus erythematosus: The COMOSLE-EGYPT study. Chronic Illn. 2023, 19, 791–803. [Google Scholar] [CrossRef]
- Sutton, E.J.; Davidson, J.E.; Bruce, I.N. The systemic lupus international collaborating clinics (SLICC) damage index: A systematic literature review. Semin. Arthritis Rheum. 2013, 43, 352–361. [Google Scholar] [CrossRef]
- England, B.R.; Sayles, H.; Mikuls, T.R.; Johnson, D.S.; Michaud, K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res. 2015, 67, 865–872. [Google Scholar] [CrossRef]
- Huang, Y.J.; Chen, J.S.; Luo, S.F.; Kuo, C.F. Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 5460. [Google Scholar] [CrossRef]
- Mysler, E.; Monticielo, O.A.; Al-Homood, I.A.; Lau, C.S.; Hussein, H.; Chen, Y.-H. Opportunities and challenges of lupus care in Latin America, the Middle East, and Asia-Pacific: A call to action. Mod. Rheumatol. 2024, 34, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Gilcrease, W.; Manfredi, L.; Sciascia, S.; Ricceri, F. From Multimorbidity to Network Medicine in Patients with Rheumatic Diseases. Rheumatol. Ther. 2025, 12, 1–24. [Google Scholar] [CrossRef]
- Ohta, R.; Yakabe, T.; Sano, C. Decision-Making in the Admission of Older Patients: A Thematic Analysis From Multiple-Stakeholder Perspectives. Cureus 2024, 16, e51966. [Google Scholar] [CrossRef] [PubMed]
- Kotb, H.A.; Khalad, S.; Moghazy, A. Hospitalization and outcome of systemic lupus erythematosus patients admitted to the Rheumatology ward of Kasr Al-Ainy University Hospital. Egypt. Rheumatol. 2023, 45, 235–239. [Google Scholar] [CrossRef]
- Cruciani, C.; Zen, M.; Gatto, M.; Morand, E.; Doria, A. Assessment of disease activity and damage in SLE: Are we there yet? Best Pract. Res. Clin. Rheumatol. 2023, 37, 101896. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Ohta, R.; Sano, C. Relationship between Anemia and Readmission among Older Patients in Rural Community Hospitals: A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 539. [Google Scholar] [CrossRef]
- Ohta, R.; Sano, C. The Effectiveness of Family Medicine-Driven Interprofessional Collaboration on the Readmission Rate of Older Patients. Healthcare 2023, 11, 269. [Google Scholar] [CrossRef]
| First Author (Year) | Country | Study Design | Sample Size (n) | Mean Age (Years) | Female (%) | Comorbidity Assessment | Follow-Up Duration | Primary Outcome |
|---|---|---|---|---|---|---|---|---|
| Jönsen et al. (2011) [10] | Sweden | Retrospective cohort | 673 | 42 | 90% | CCI (cutoff ≥ 2) | 10 years | Mortality |
| Yang et al. (2014) [19] | Singapore | Retrospective cohort | 262 | 39 | 88% | CCI (cutoff ≥ 2) | In-hospital | Mortality |
| Greenstein et al. (2019) [16] | South Africa | Nested case-control | 240 | 32.1 | 88% | CCI | Median: 5 years | Mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, R.; Ryu, Y.; Sano, C.; Ichinose, K. Integrating Multimorbidity Assessment into Rheumatology Care: Prognostic Role of the Charlson Comorbidity Index in Systemic Lupus Erythematosus. Healthcare 2025, 13, 2285. https://doi.org/10.3390/healthcare13182285
Ohta R, Ryu Y, Sano C, Ichinose K. Integrating Multimorbidity Assessment into Rheumatology Care: Prognostic Role of the Charlson Comorbidity Index in Systemic Lupus Erythematosus. Healthcare. 2025; 13(18):2285. https://doi.org/10.3390/healthcare13182285
Chicago/Turabian StyleOhta, Ryuichi, Yoshinori Ryu, Chiaki Sano, and Kunihiro Ichinose. 2025. "Integrating Multimorbidity Assessment into Rheumatology Care: Prognostic Role of the Charlson Comorbidity Index in Systemic Lupus Erythematosus" Healthcare 13, no. 18: 2285. https://doi.org/10.3390/healthcare13182285
APA StyleOhta, R., Ryu, Y., Sano, C., & Ichinose, K. (2025). Integrating Multimorbidity Assessment into Rheumatology Care: Prognostic Role of the Charlson Comorbidity Index in Systemic Lupus Erythematosus. Healthcare, 13(18), 2285. https://doi.org/10.3390/healthcare13182285









