Associations Between Common Hip and Knee Osteoarthritis Treatments and All-Cause Mortality
Abstract
1. Introduction
Aims
2. Methods
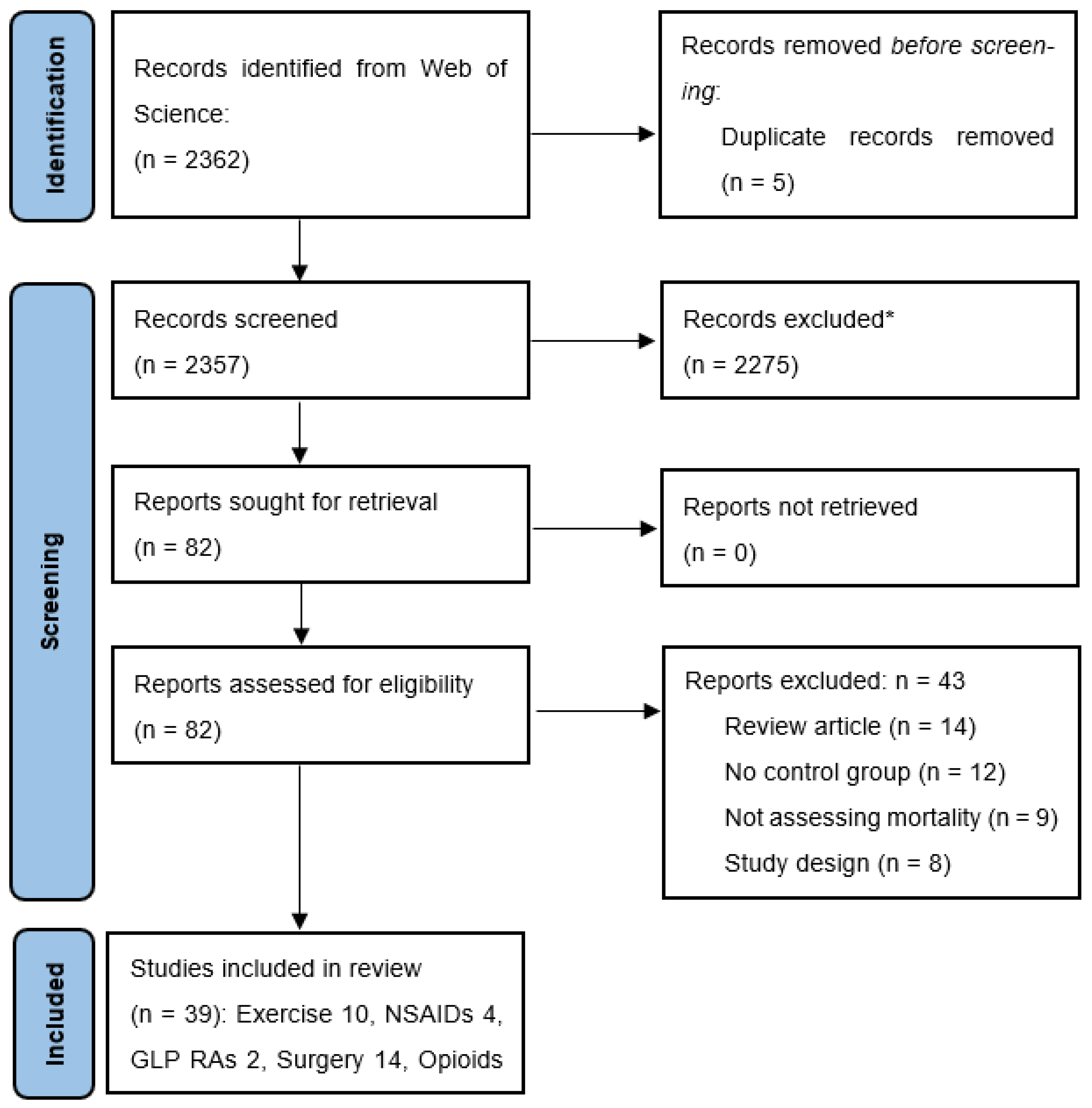
2.1. Literature Search
2.1.1. Inclusion and Exclusion Criteria
2.1.2. Data Extraction and Quality Assessment
3. Results
3.1. Quality
3.2. Exercise
3.3. Glucagon-like Peptide-1 Receptor Agonists
3.4. Non-Steroidal Anti-Inflammatory Drugs
3.5. Arthroplasty
3.5.1. All-Cause Mortality Following Hip Arthroplasty
| Authors | Cases | Follow Up Mean (Range) | Control | Results (Ratios and 95% CIs) |
|---|---|---|---|---|
| Cnudde et al., (2018) [40] | 131,808 | 5.9 years (1–14) | General population | At 1 year: R = 1.01 (1.01–1.0) At 5 years: R = 1.03 (1.03–1.03) At 10 years: R = 1.02 (1.02–1.03) At 12 years: R = 1.01 (0.99–1.02) |
| Cook et al., (2022) [39] | 103,563 | 90 days | OA population | 30 days: 1.05 (0.91, 1.23) 60 days: 0.82 (0.73, 0.92) 90 days: 0.68 (0.62, 0.76) |
| Gordon et al., (2016) [41] | 91,527 | 10 years (7–21) | General population | Overall 1.00 (0.99–1.01) 5–9 years 0.90 (0.88–0.92) 9–13 years 1.05 (1.03–1.08) 13–17 years 1.11 (1.07–1.14) 17–21 years 1.19 (1.13–1.26) Crossover occurred at 8.8 years (CI 8.3–9.3) |
| Kremers et al., (2016) [37] | 1645 | 11.9 years | General population | 0.82 (0.76–0.88) |
| Lie et al., (2000) [38] | 39,543 | 5.2 years (0–10.4) | General population | All THR patients 0.81 (0.79–0.83) OA patients 0.68 (0.66–0.70) First 60 days 1.39 (1.25–1.56) |
3.5.2. All-Cause Mortality Following Knee Arthroplasty
3.5.3. Opioids
3.5.4. Paracetamol
3.5.5. Duloxetine
4. Discussion
4.1. Exercise
4.2. GLP-1 RAs
4.3. NSAIDs
4.4. Arthroplasty
4.5. Opioids
4.6. Paracetamol
4.7. Strengths of This Review
4.8. Limitations of This Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, R.; Guo, Y.; Chen, Y.; Zhang, J. Osteoarthritis burden and inequality from 1990 to 2021: A systematic analysis for the global burden of disease Study 2021. Sci. Rep. 2025, 15, 8305. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Osteoarthritis; AIHW: Canberra, Australia, 2024. [Google Scholar]
- Bennell, K.L.; Bayram, C.; Harrison, C.; Brand, C.; Buchbinder, R.; Haas, R.; Hinman, R.S. Trends in management of hip and knee osteoarthritis in general practice in Australia over an 11-year window: A nationwide cross-sectional survey. Lancet Reg. Health West. Pac. 2021, 12, 100187. [Google Scholar] [CrossRef]
- Deveza, L.A.; Hunter, D.J.; Van Spil, W.E. Too much opioid, too much harm. Osteoarthr. Cartil. 2018, 26, 293–295. [Google Scholar] [CrossRef]
- Wood, G.; Neilson, J.; Cottrell, E.; Hoole, S.P. Osteoarthritis in people over 16: Diagnosis and management—Updated summary of NICE guidance. BMJ 2023, 380, 24. [Google Scholar] [CrossRef]
- Magni, A.; Agostoni, P.; Bonezzi, C.; Massazza, G.; Menè, P.; Savarino, V.; Fornasari, D. Management of Osteoarthritis: Expert Opinion on NSAIDs. Pain Ther. 2021, 10, 783–808. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bowden, J.L. Are you managing osteoarthritis appropriately? Nat. Rev. Rheumatol. 2017, 13, 703–704. [Google Scholar] [CrossRef]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the US bone and joint initiative. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2014; pp. 701–712. [Google Scholar]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Osteoarthritis of the Knee Clinical Care Standard; Australian Commission on Safety and Quality in Health Care: Sydney, Australia, 2024.
- Katz, J.N.; Smith, S.R.; Collins, J.E.; Solomon, D.H.; Jordan, J.M.; Hunter, D.J.; Suter, L.G.; Yelin, E.; Paltiel, A.D.; Losina, E. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthr. Cartil. 2016, 24, 409–418. [Google Scholar] [CrossRef]
- Brand, C.A.; Harrison, C.; Tropea, J.; Hinman, R.S.; Britt, H.; Bennell, K. Management of osteoarthritis in general practice in Australia. Arthritis Care Res. 2014, 66, 551–558. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Zomer, E.; Gilmartin-Thomas, J.F.; Liew, D. Forecasting the future burden of opioids for osteoarthritis. Osteoarthr. Cartil. 2018, 26, 350–355. [Google Scholar] [CrossRef]
- Roberts, E.; Delgado Nunes, V.; Buckner, S.; Latchem, S.; Constanti, M.; Miller, P.; Doherty, M.; Zhang, W.; Birrell, F.; Porcheret, M.; et al. Paracetamol: Not as safe as we thought? A systematic literature review of observational studies. Ann. Rheum. Dis. 2016, 75, 552–559. [Google Scholar] [CrossRef]
- Bliddal, H.; Bays, H.; Czernichow, S.; Uddén Hemmingsson, J.; Hjelmesæth, J.; Hoffmann Morville, T.; Koroleva, A.; Skov Neergaard, J.; Vélez Sánchez, P.; Wharton, S.; et al. Once-Weekly Semaglutide in Persons with Obesity and Knee Osteoarthritis. N. Engl. J. Med. 2024, 391, 1573–1583. [Google Scholar] [CrossRef]
- Lovald, S.T.; Ong, K.L.; Lau, E.C.; Schmier, J.K.; Bozic, K.J.; Kurtz, S.M. Mortality, cost, and health outcomes of total knee arthroplasty in Medicare patients. J. Arthroplast. 2013, 28, 449–454. [Google Scholar] [CrossRef]
- Therapeutic Guidelines Limited. Osteoarthritis. Therapeutic Guidelines. 2017. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://tgldcdp.tg.org.au/topicTeaser%3FguidelinePage%3DRheumatology%26etgAccess%3Dtrue (accessed on 26 September 2024).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- de Boer, W.I.J.; Mierau, J.O.; Koning, R.H. Do differences in sport participation contribute to socioeconomic health inequalities? Evidence from the Lifelines cohort study on all-cause mortality, diabetes and obesity. Prev. Med. Rep. 2023, 36, 102479. [Google Scholar] [CrossRef] [PubMed]
- Ekblom-Bak, E.; Ekblom, B.; Vikström, M.; de Faire, U.; Hellénius, M.L. The importance of non-exercise physical activity for cardiovascular health and longevity. Br. J. Sports Med. 2014, 48, 233–238. [Google Scholar] [CrossRef]
- Inoue, M.; Iso, H.; Yamamoto, S.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Tsugane, S.; Japan Public Hlth Centre-Based, p. Daily total physical activity level and premature death in men and women: Results from a large-scale population-based cohort study in Japan (JPHC study). Ann. Epidemiol. 2008, 18, 522–530. [Google Scholar] [CrossRef]
- Landi, F.; Russo, A.; Cesari, M.; Pahor, M.; Liperoti, R.; Danese, P.; Bernabei, R.; Onder, G. Walking one hour or more per day prevented mortality among older persons: Results from ilSIRENTE study. Prev. Med. 2008, 47, 422–426. [Google Scholar] [CrossRef]
- Leon-Munoz, L.M.; Martínez-Gómez, D.; Balboa-Castillo, T.; López-García, E.; Guallar-Castillón, P.; Rodríguez-Artalejo, F. Continued Sedentariness, Change in Sitting Time, and Mortality in Older Adults. Med. Sci. Sports Exerc. 2013, 45, 1501–1507. [Google Scholar] [CrossRef]
- Min, C.; Yoo, D.M.; Wee, J.H.; Lee, H.J.; Byun, S.H.; Choi, H.G. Mortality and cause of death in physical activity and insufficient physical activity participants: A longitudinal follow-up study using a national health screening cohort. Bmc Public Health 2020, 20, 1469. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Moshkovits, Y.; Chetrit, A.; Dankner, R. Self-reported physical activity properties and 20-year all-cause and cardiovascular mortality among community-dwelling older adults. Postgrad. Med. J. 2024, 101, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.Z.; Moon, Y.P.; Sherzai, A.; Cheung, Y.K.; Sacco, R.L.; Elkind, M.S.V. Leisure-time physical activity and mortality in a multiethnic prospective cohort study: The Northern Manhattan Study. Ann. Epidemiol. 2015, 25, 475–479. [Google Scholar] [CrossRef]
- Yates, L.B.; Djouss, L.; Kurth, T.; Buring, J.E.; Gaziano, J.M. Exceptional longevity in men–Modifiable factors associated with survival and function to age 90 years. Arch. Intern. Med. 2008, 168, 284–290. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, W.; Wang, T.; Zhu, L.J.; Xu, Y.; Zhao, J.; Yu, L.L.; Bao, H.H.; Cheng, X.S. Independent and joint association of physical activity and sedentary behavior on all-cause mortality. Chin. Med. J. 2021, 134, 2857–2864. [Google Scholar] [CrossRef]
- Alenezi, B.T.; Elfezzani, N.; Uddin, R.; Patel, H.; Chester, S.; Abdelmaksoud, A.; Hussein, M.H.; Zaitone, S.A.; Fawzy, M.S.; Aiash, H.; et al. Beyond Glycemic Control: GLP-1 Receptor Agonists and Their Impact on Calcium Homeostasis in Real-World Patients. J. Clin. Med. 2024, 13, 4896. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Liao, W.L.; Huang, J.Y.; Lin, Y.J.; Yang, S.F.; Huang, C.C.; Wang, C.H.; Su, P.H. Long-term safety and efficacy of glucagon-like peptide-1 receptor agonists in individuals with obesity and without type 2 diabetes: A global retrospective cohort study. Diabetes Obes. Metab. 2024, 26, 5222–5232. [Google Scholar] [CrossRef]
- Bardia, A.; Ebbert, J.O.; Vierkant, R.A.; Limburg, P.J.; Anderson, K.; Wang, A.H.; Olson, J.E.; Vachon, C.M.; Cerhan, J.R. Association of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs with cancer incidence and mortality. Jnci-J. Natl. Cancer Inst. 2007, 99, 881–889. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Balkrishnan, R.; Hirth, R.A.; Hutton, D.W.; He, K.; Steffick, D.E.; Saran, R. Assessment of Prescription Analgesic Use in Older Adults with and Without Chronic Kidney Disease and Outcomes. Jama Netw. Open 2020, 3, e2016839. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, J.B.; Li, H.; Ke, Y.; Li, R.J.; Zhang, Y.Q.; Lin, J.H. Knee Symptomatic Osteoarthritis, Walking Disability, NSAIDs Use and All-cause Mortality: Population-based Wuchuan Osteoarthritis Study. Sci. Rep. 2017, 7, 3309. [Google Scholar] [CrossRef]
- Nash, D.M.; Markle-Reid, M.; Brimble, K.S.; McArthur, E.; Roshanov, P.S.; Fink, J.C.; Weir, M.A.; Garg, A.X. Nonsteroidal anti-inflammatory drug use and risk of acute kidney injury and hyperkalemia in older adults: A population-based study. Nephrol. Dial. Transplant. 2019, 34, 1145–1154. [Google Scholar] [CrossRef]
- Maradit Kremers, H.; Larson, D.R.; Noureldin, M.; Schleck, C.D.; Jiranek, W.A.; Berry, D.J. Long-Term Mortality Trends After Total Hip and Knee Arthroplasties: A Population-Based Study. J. Arthroplast. 2016, 31, 1163–1169. [Google Scholar] [CrossRef]
- Lie, S.A.; Engesaeter, L.B.; Havelin, L.I.; Gjessing, H.K.; Vollset, S.E. Mortality after total hip replacement: 0–10-year follow-up of 39,543 patients in the Norwegian Arthroplasty Register. Acta Orthop. Scand. 2000, 71, 19–27. [Google Scholar] [CrossRef]
- Cook, M.J.; Lunt, M.; Board, T.; O’Neill, T.W. The impact of frailty on short-term mortality following primary total hip and knee arthroplasty due to osteoarthritis. Age Ageing 2022, 51, afac118. [Google Scholar] [CrossRef]
- Cnudde, P.; Rolfson, O.; Timperley, A.J.; Garland, A.; Karrholm, J.; Garellick, G.; Nemes, S. Do Patients Live Longer After THA and Is the Relative Survival Diagnosis-specific? Clin. Orthop. Relat. Res. 2018, 476, 1166–1175. [Google Scholar] [CrossRef]
- Gordon, M.; Rysinska, A.; Garland, A.; Rolfson, O.; Aspberg, S.; Eisler, T.; Garellick, G.; Stark, A.; Hailer, N.P.; Skoldenberg, O. Increased Long-Term Cardiovascular Risk After Total Hip Arthroplasty: A Nationwide Cohort Study. Medicine 2016, 95, e2662. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Won, S.J.; Lee, N.K.; Chang, C.B. Life Expectancy of Patients Undergoing Total Knee Arthroplasty: Comparison with General Population. J. Korean Med. Sci. 2024, 39, e106. [Google Scholar] [CrossRef]
- Choi, H.G.; Kwon, B.C.; Kim, J.I.; Lee, J.K. Total knee arthroplasty reduces the risk of mortality in osteoarthritis patients up to 12 years: A Korean national cohort longitudinal follow-up study. J. Orthop. Surg. 2020, 28, 2309499020902589. [Google Scholar] [CrossRef] [PubMed]
- Lizaur-Utrilla, A.; Gonzalez-Parreno, S.; Miralles-Munoz, F.A.; Lopez-Prats, F.A. Ten-year mortality risk predictors after primary total knee arthroplasty for osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Robertsson, O.; Stefansdottir, A.; Lidgren, L.; Ranstam, J. Increased long-term mortality in patients less than 55 years old who have undergone knee replacement for osteoarthritis: Results from the Swedish Knee Arthroplasty Register. J. Bone Jt. Surg. Br. 2007, 89, 599–603. [Google Scholar] [CrossRef]
- Visuri, T.; Makela, K.; Pulkkinen, P.; Artama, M.; Pukkala, E. Long-term mortality and causes of death among patients with a total knee prosthesis in primary osteoarthritis. Knee 2016, 23, 162–166. [Google Scholar] [CrossRef]
- Yeh, H.W.; Chan, C.H.; Yang, S.F.; Chen, Y.C.; Yeh, Y.T.; Yeh, Y.T.; Huang, J.Y.; Yeh, C.B.; Chiu, C.H. Total knee replacement in osteoarthritis patients on reducing the risk of major adverse cardiac events: A 18-year retrospective cohort study. Osteoarthr. Cartil. 2022, 30, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Frampton, C.; Dowsey, M.; Choong, P.; Schilling, C.; Hirner, M. Assessing the Mortality Rate After Primary Total Knee Arthroplasty: An Observational Study to Inform Future Economic Analysis. J. Arthroplast. 2023, 38, 2328–2335.e2323. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, O.; Kurita, G.P.; Hjsted, J.; Juel, K.; Sjgren, P. Chronic pain, opioid prescriptions, and mortality in Denmark: A population-based cohort study. Pain 2014, 155, 2486–2490. [Google Scholar] [CrossRef]
- Khodneva, Y.; Richman, J.; Kertesz, S.; Safford, M.M. Gender differences in association of prescription opioid use and mortality: A propensity-matched analysis from the REasons for Geographic and Racial Differences in Stroke (REGARDS) prospective cohort. Subst. Abus. 2021, 42, 94–103. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Beasley, M.; Jones, G.T.; Stannard, C. The epidemiology of regular opioid use and its association with mortality: Prospective cohort study of 466 486 UK biobank participants. EClinicalMedicine 2020, 21, 100321. [Google Scholar] [CrossRef]
- Musich, S.; Wang, S.S.; Schaeffer, J.A.; Slindee, L.; Kraemer, S.; Yeh, C.S. Safety Events Associated with Tramadol Use Among Older Adults with Osteoarthritis. Popul. Health Manag. 2021, 24, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Jeon, Y.T.; Choi, J.W. Trends in chronic opioid use and association with five-year survival in South Korea: A population-based cohort study. Br. J. Anaesth. 2019, 123, 655–663. [Google Scholar] [CrossRef]
- Sjøgren, P.; Grønbæk, M.; Peuckmann, V.; Ekholm, O. A population-based cohort study on chronic pain: The role of opioids. Clin. J. Pain 2010, 26, 763–769. [Google Scholar] [CrossRef]
- Song, I.A.; Choi, H.R.; Oh, T.K. Long-term opioid use and mortality in patients with chronic non-cancer pain: Ten-year follow-up study in South Korea from 2010 through 2019. EClinicalMedicine 2022, 51, 101558. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Sourdet, S.; Cantet, C.; de Souto Barreto, P.; Rolland, Y. Acetaminophen Safety: Risk of Mortality and Cardiovascular Events in Nursing Home Residents, a Prospective Study. J. Am. Geriatr. Soc. 2019, 67, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Friis, S.; Mellemkjaer, L.; Signorello, L.B.; Johnsen, S.P.; Nielsen, G.L.; McLaughlin, J.K.; Blot, W.J.; Olsen, J.H. A population-based cohort study of mortality among adults prescribed paracetamol in Denmark. J. Clin. Epidemiol. 2003, 56, 796–801. [Google Scholar] [CrossRef]
- Schnohr, P.; O’Keefe, J.H.; Marott, J.L.; Lange, P.; Jensen, G.B. Dose of jogging and long-term mortality: The Copenhagen City Heart Study. J. Am. Coll. Cardiol. 2015, 65, 411–419. [Google Scholar] [CrossRef]
- Watts, E.L.; Matthews, C.E.; Freeman, J.R.; Gorzelitz, J.S.; Hong, H.G.; Liao, L.M.; McClain, K.M.; Saint-Maurice, P.F.; Shiroma, E.J.; Moore, S.C. Association of leisure time physical activity types and risks of all-cause, cardiovascular, and cancer mortality among older adults. JAMA Netw. Open 2022, 5, e2228510. [Google Scholar] [CrossRef]
- Andersen, K.; Farahmand, B.; Ahlbom, A.; Held, C.; Ljunghall, S.; Michaelsson, K.; Sundström, J. Risk of arrhythmias in 52 755 long-distance cross-country skiers: A cohort study. Eur. Heart J. 2013, 34, 3624–3631. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Patil, H.R.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Potential adverse cardiovascular effects from excessive endurance exercise. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 587–595. [Google Scholar]
- Ector, J.; Ganame, J.; van der Merwe, N.; Adriaenssens, B.; Pison, L.; Willems, R.; Gewillig, M.; Heidbüchel, H. Reduced right ventricular ejection fraction in endurance athletes presenting with ventricular arrhythmias: A quantitative angiographic assessment. Eur. Heart J. 2007, 28, 345–353. [Google Scholar] [CrossRef]
- Parry-Williams, G.; Sharma, S. The effects of endurance exercise on the heart: Panacea or poison? Nat. Rev. Cardiol. 2020, 17, 402–412. [Google Scholar] [CrossRef]
- Newton, R.U.; Galvao, D.A. Exercise in prevention and management of cancer. Curr. Treat. Options Oncol. 2008, 9, 135–146. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018, 5, 408204. [Google Scholar] [CrossRef]
- Silverman, M.N.; Deuster, P.A. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus 2014, 4, 20140040. [Google Scholar] [CrossRef]
- Idorn, M.; thor Straten, P. Exercise and cancer: From “healthy” to “therapeutic”? Cancer Immunol. Immunother. 2017, 66, 667–671. [Google Scholar] [CrossRef]
- Timmons, B.W.; Cieslak, T. Human natural killer cell subsets and acute exercise: A brief review. Exerc. Immunol. Rev. 2008, 14, 8–23. [Google Scholar]
- Safdar, A.; Hamadeh, M.J.; Kaczor, J.J.; Raha, S.; Debeer, J.; Tarnopolsky, M.A. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS ONE 2010, 5, e10778. [Google Scholar] [CrossRef]
- Mariam, Z.; Niazi, S.K. Glucagon–like peptide agonists: A prospective review. Endocrinol. Diabetes Metab. 2024, 7, e462. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Marsico, F.; Paolillo, S.; Filardi, P.P. NSAIDs and cardiovascular risk. J. Cardiovasc. Med. 2017, 18, e40–e43. [Google Scholar] [CrossRef]
- Kearney, P.M.; Baigent, C.; Godwin, J.; Halls, H.; Emberson, J.R.; Patrono, C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006, 332, 1302–1308. [Google Scholar] [CrossRef]
- Fosbol, E.L.; Folke, F.; Jacobsen, S.; Rasmussen, J.N.; Sorensen, R.; Schramm, T.K.; Andersen, S.S.; Rasmussen, S.; Poulsen, H.E.; Kober, L.; et al. Cause-Specific Cardiovascular Risk Associated with Nonsteroidal Antiinflammatory Drugs Among Healthy Individuals. Circ.–Cardiovasc. Qual. Outcomes 2010, 3, 395–405. [Google Scholar] [CrossRef]
- Olsen, A.M.S.; Fosbol, E.L.; Lindhardsen, J.; Andersson, C.; Folke, F.; Nielsen, M.B.; Kober, L.; Hansen, P.R.; Torp-Pedersen, C.; Gislason, G.H. Cause-Specific Cardiovascular Risk Associated with Nonsteroidal Anti-Inflammatory Drugs among Myocardial Infarction Patients–A Nationwide Study. PLoS ONE 2013, 8, e54309. [Google Scholar] [CrossRef]
- Trelle, S.; Reichenbach, S.; Wandel, S.; Hildebrand, P.; Tschannen, B.; Villiger, P.M.; Egger, M.; Jüni, P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ 2011, 342, c7086. [Google Scholar] [CrossRef]
- Tomić, T.; Domínguez-López, S.; Barrios-Rodríguez, R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: A systematic review and meta-analysis. Cancer Epidemiol. 2019, 58, 52–62. [Google Scholar] [CrossRef]
- Fang, F.; Valdimarsdóttir, U.; Mucci, L.; Sparén, P.; Ye, W.; Fall, K. Hospitalization for osteoarthritis and prostate cancer specific mortality among Swedish men with prostate cancer. Cancer Epidemiol. 2010, 34, 644–647. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Zeng, C.; Doherty, M.; Persson, M.S.M.; Yang, Z.; Sarmanova, A.; Zhang, Y.; Wei, J.; Kaur, J.; Li, X.; Lei, G.; et al. Comparative efficacy and safety of acetaminophen, topical and oral non-steroidal anti-inflammatory drugs for knee osteoarthritis: Evidence from a network meta-analysis of randomized controlled trials and real-world data. Osteoarthr. Cartil. 2021, 29, 1242–1251. [Google Scholar] [CrossRef]
- Michet, C.J., 3rd; Schleck, C.D.; Larson, D.R.; Maradit Kremers, H.; Berry, D.J.; Lewallen, D.G. Cause-Specific Mortality Trends Following Total Hip and Knee Arthroplasty. J. Arthroplast. 2017, 32, 1292–1297. [Google Scholar] [CrossRef][Green Version]
- Tolle, T.; Fitzcharles, M.A.; Hauser, W. Is opioid therapy for chronic non-cancer pain associated with a greater risk of all-cause mortality compared to non-opioid analgesics? A systematic review of propensity score matched observational studies. Eur. J. Pain 2021, 25, 1195–1208. [Google Scholar] [CrossRef]
- Larney, S.; Peacock, A.; Tran, L.T.; Stockings, E.; Santomauro, D.; Santo, T.; Degenhardt, L. All-Cause and Overdose Mortality Risk Among People Prescribed Opioids: A Systematic Review and Meta-analysis. Pain Med. 2020, 21, 3700–3711. [Google Scholar] [CrossRef]
- Hoppe, D.J.; Schemitsch, E.H.; Morshed, S.; Tornetta, P., 3rd; Bhandari, M. Hierarchy of evidence: Where observational studies fit in and why we need them. J. Bone Jt. Surg. Am. 2009, 91 (Suppl. S3), 2–9. [Google Scholar] [CrossRef]
- Hurtado, I.; Robles, C.; Garcia-Sempere, A.; Llopis-Cardona, F.; Sanchez-Saez, F.; Rodriguez-Bernal, C.; Peiro, S.; Sanfelix-Gimeno, G. Long-term use of prescription opioids for non-cancer pain and mortality: A population-based, propensity-weighted cohort study. Public Health 2024, 232, 4–13. [Google Scholar] [CrossRef]
- MacMahon, S.; Collins, R. Reliable assessment of the effects of treatment on mortality and major morbidity, II: Observational studies. Lancet 2001, 357, 455–462. [Google Scholar] [CrossRef]
- Bosdriesz, J.R.; Stel, V.S.; van Diepen, M.; Meuleman, Y.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Evidence-based medicine-When observational studies are better than randomized controlled trials. Nephrology 2020, 25, 737–743. [Google Scholar] [CrossRef]
- RACGP. Guideline for the Management of Knee and Hip Osteoarthritis; The Royal Australian College of General Practitioners: East Melbourne, VIC, Australia, 2018. [Google Scholar]
| #1 | (All cause mortality OR cardiovascular mortality OR life expectancy OR mortality) |
| #2 | (Cohort OR cross-sectional OR case–control) |
| #3 | #1 AND #2 |
| #4 | (GLP-1 agonists OR Glucagon-like peptide-1 receptor agonists) |
| #5 | (Exercise OR Physiotherapy OR Exercise Physiology) |
| #6 | (Opioid OR Opiate) |
| #7 | (Arthroplasty OR Hip Replacement OR Knee Replacement) |
| #8 | #4 OR #5 OR #6 OR #7 |
| #9 | (Obesity OR Osteoarthritis OR Chronic non-cancer pain) |
| #10 | #8 AND #9 |
| #11 | (NSAID OR NSAIDs OR Paracetamol OR Acetaminophen OR Duloxetine OR SNRI) |
| #12 | #10 OR #11 |
| #13 | #3 AND #12 |
| Authors | Cases | Duration of Follow-Up (Average) | Population and Exposure | Results (Ratios and 95% CIs) |
|---|---|---|---|---|
| de Boer et al. (2023) [20] | 84,230 | 7.8 years | Age between 25 and 75 years, without T2DM, CVD or cancer at baseline. Moderate-to-vigorous physical activity (MVPA) minutes/week score quintiles | No MVPA: 1.00 (reference) Practicing MVPA: 0.77 (0.64–0.93) By MVPA quintiles: Q1: 0.82 (0.65–1.04) Q2: 0.77 (0.60–0.99) Q3: 0.71 (0.55–0.91) Q4: 0.73 (0.57–0.92) Q5: 0.82 (0.66–1.02) |
| Ekblom-Bak et al. (2014) [21] | 4232 | 12.5 years | Intake 60 year olds. Self-reported frequency on questionnaire of non-exercise physical activity (NEPA) score tertiles | Low NEPA: 1.00 (reference) Moderate NEPA: 0.85 (0.67–1.08) High NEPA: 0.7 (0.53–0.98) |
| Inoue et al. (2008) [22] | 83,034 (Men n = 39,183; Women n = 43,851) | 5 years | 45–74, daily total physical activity (PA) level by metabolic equivalent of task (METs)/day score quartiles | Men Lowest: 1.00 (reference) Second: 0.76 (0.68–0.83) Third: 0.79 (0.71–0.87) Highest: 0.67 (0.61–0.74) Women Lowest: 1.00 (reference) Second: 0.70 (0.62–1.18) Third: 0.61 (0.53–0.70) Highest: 0.54 (0.52–0.82) |
| Landi et al. (2008) [23] | 364 | 2 years | Age > 80. Sedentary defined as subjects walking less than 1 h/day, active walking ≥1 h/day | Sedentary: 1.00 (reference) Active: 0.36 (0.12–0.98) |
| Leon-Munoz et al. (2013) [24] | 2635 | 2 years | Age > 60. Sedentary behaviours defined by comparing subjects self-reported sitting time (ST) behaviour at 2 points in time (2001 and 2003). Consistently sedentary (>Median ST in 2001 and 2003) Newly sedentary (≤Median ST in 2001 and >Median in 2003) Formerly sedentary (>Median in 2001 and ≤Median in 2003) Consistently non-sedentary (≤Median in 2001 and 2003) | Consistently sedentary: 1.00 (reference) Newly sedentary: 0.91 (0.76–1.10) Formerly sedentary: 0.86 (0.70–1.05) Consistently non-sedentary: 0.75 (0.62–0.90) |
| Min et al. (2020) [25] | 167,413 | 5.4 years | Age 40–79. PA ‘sufficiency’ according to the International Physical Activity Questionnaire (IPAQ) [26] | Insufficient PA: 1.00 (reference) Sufficient PA: 0.85 (0.82–0.88) |
| Moshkovits et al. (2024) [27] | 1210 | 13 years | Age 65+. Baseline self-reported physical activity intensity. Light-moderate (household activities, walking, yoga, dancing, Feldenkrais, aerobics, and physiotherapy-associated activity) and vigorous (running, cycling, swimming, ball games, and gym) | Never/past: 1.00 (reference) Light-moderate: 0.72 (0.57–0.89) Vigorous: 0.74 (0.54–1.0) |
| Willey et al. (2015) [28] | 3298 | 11.8 years | Age > 39. Leisure time PA (LTPA) self-reported questionnaire based on prior 2 weeks | No LTPA: 1.00 (reference) Any LTPA: 0.84 (0.75–0.94) |
| Yates et al. (2008) [29] | 970 | 16 years | Initial mean age 72, followed up until 90. Self-reported frequency of vigorous exercise sufficient to cause sweat. | Rarely/never: 1.00 (reference) 1–4 time/month: 0.78 (0.67–0.91) 2–4 times/week: 0.72 (0.62–0.83) ≥5 times/week: 0.81 (0.69–0.96) |
| Zhou et al. (2021) [30] | 11,744 | 5.4 years | Age ≥ 35, self-reported PA according to IPAQ | Low: 1.00 (reference) Moderate: 0.75 (0.62–0.91) High: 0.48 (0.40–0.57) |
| Authors | Cases | Duration of Follow-Up (Average) | Exposure (No-Use Versus:) | Results (Ratios and 95% CIs) |
|---|---|---|---|---|
| Alenezi et al. (2024) [31] | 15,655 | 6 months | Age > 18, GLP-1 RA prescription vs. no GLP-1RA | 0.27 (0.21–0.36) |
| Huang et al. (2024) [32] | 12,123 | 5 years | Obese individuals without T2DM on GLP-1 RA vs. no GLP-1RA | 0.23 (0.15–0.34) |
| Authors | Cases | Duration of Follow-Up (Average) | Exposure | Results (Ratios and 95% CIs) |
|---|---|---|---|---|
| Bardia et al. (2007) [33] | 22,507 | 10 years | Post menopausal women age 55–69, NSAID vs. no NSAID | 0.97 (0.90–1.04) |
| Han et al. (2020) [34] | 649,339 | 10 years | Age > 65, ever used prescription NSAID vs. no NSAID | 0.84 (0.83–0.85) |
| Liu et al. (2017) [35] | 1025 | 8 years | Age > 50, Patient with symptomatic knee OA—NSAID use vs. no NSAID use | 1.45 (0.93–21.26) |
| Nash et al. (2019) [36] | 46,107 | 30 days | Age > 65, NSAID prescribed vs. no NSAID | 0.83 (0.60–1.16) |
| Study Authors | Cases | Follow Up Mean (Range) | Control | Results (Ratios and 95% CIs) |
|---|---|---|---|---|
| Choi, et al. [43] | 5072 | 58.1 months (1–12 years) | General population | 0.61 (0.54–0.70) |
| Cook et al. [39] | 125,367 | 90 days | OA population | 30 days: 1.14 (0.97, 1.34) 60 days, 0.83 (0.74, 0.95) 90 days, 0.70 (0.63, 0.78) |
| Kim et al. [42] | 601 | 13 years (10–16.5) | General population | Over 70 years: 0.63 (0.49–0.8) Aged 70–79: 0.67 (0.45–0.96) Aged > 80: 0.69 (0.58–0.82) |
| Maradit Kremers, Larson, Noureldin, Schleck, Jiranek and Berry [37] | 1980 | 10.8 years | General population | 0.80 (0.75–0.86) |
| Lizaur-Utrilla, et al. [44] | 11,569 | 8.1 years (5–13) | General population | 86.8 (79.9–91.2) |
| Lovald, Ong, Lau, Schmier, Bozic and Kurtz [16] | 134,458 | (1–7 years) | OA population | 1 year 0.49 3 years 0.48 5 years 0.50 7 years 0.54 |
| Robertsson, et al. [45] | 65,515 | (0–28 years) | General population | 0.77 (0.76–0.78) |
| Visuri et al. [46] | 9443 | 14 years (1–33) | General population | Overall 1.00 (0.98–1.02) 0–1 year: 0.50 (0.45–0.55) 2–9 years: 0.78 (0.75–0.81) 10–19 years: 1.23 (1.19–1.26) >20 years: 1.95 (1.79–2.11) |
| Yeh et al. [47] | 103,114 | 144 months (0–17 years) | OA population | 0.791 (0.746–0.840) |
| Zhou et al. [48] | 22,938 | 11 years (0–23) | General population | Overall: 1.08 (1.06–1.09) 0–5 yr: 0.59 (0.57–0.60) >11 yr: 3.13 (2.95–3.31) |
| Authors | Cases | Follow Up Mean (Range) | Exposure | Results (Ratios and 95% CIs) |
|---|---|---|---|---|
| Ekholm et al. [49] | 13,127 | 11 years | Long-term and short-term opioid use for chronic pain | Long term: 1.72 (1.23–2.41) Short term: 1.22 (0.93–1.59) Chronic pain and no opioid: 1.28 (1.10–1.49) |
| Khodneva et al. [50] | 1906 | 6 years | Prescription opioids | 1.15 (1.04–1.28) |
| Macfarlane et al. [51] | 25,864 | 10 years | Weak opioid and strong opioid | Weak: 1.18 (1.06–1.33) Strong: 1.20 (1.01–1.43) |
| Musich et al. [52] | 651,556 | 33 months | New tramadol, new other opioid, continued tramadol, continued other opioid | New tramadol 1.21 (1.12–1.31) New other opioid 1.17 (1.07–1.27). Continuing tramadol 1.02 (0.90–1.5) Continuing other opioid 1.19 (1.06–1.35) |
| Oh et al. [53] | 10,587,018 | 5 years | Chronic opioid prescription, weak vs. strong opioids | Chronic weak opioid use 0.92 (0.88–0.96) Chronic strong opioid use 1.35 (1.17–1.56) |
| Sjøgren et al. [54] | 2242 | (0–8 years) | Weak opioid Strong opioid No opioid (with chronic pain) | Chronic pain without opioids 1.21 (1.02–1.44) Chronic pain with weak opioids 1.07 0.65–1.76) Chronic pain with strong opioids 1.67 (1.03–2.70) |
| Song et al. [55] | 1,804,019 | 10 years | Long-term opioid | 1.21 (1.13–1.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orchard, J.W.; Tutt, L.E.; Hines, A.; Orchard, J.J. Associations Between Common Hip and Knee Osteoarthritis Treatments and All-Cause Mortality. Healthcare 2025, 13, 2229. https://doi.org/10.3390/healthcare13172229
Orchard JW, Tutt LE, Hines A, Orchard JJ. Associations Between Common Hip and Knee Osteoarthritis Treatments and All-Cause Mortality. Healthcare. 2025; 13(17):2229. https://doi.org/10.3390/healthcare13172229
Chicago/Turabian StyleOrchard, John W., L. Edward Tutt, Anna Hines, and Jessica J. Orchard. 2025. "Associations Between Common Hip and Knee Osteoarthritis Treatments and All-Cause Mortality" Healthcare 13, no. 17: 2229. https://doi.org/10.3390/healthcare13172229
APA StyleOrchard, J. W., Tutt, L. E., Hines, A., & Orchard, J. J. (2025). Associations Between Common Hip and Knee Osteoarthritis Treatments and All-Cause Mortality. Healthcare, 13(17), 2229. https://doi.org/10.3390/healthcare13172229





