Characterization of Antidepressant Consumption in a Portuguese Inland Population
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterisation of the Population
3.1.1. Profile of the Studied Population
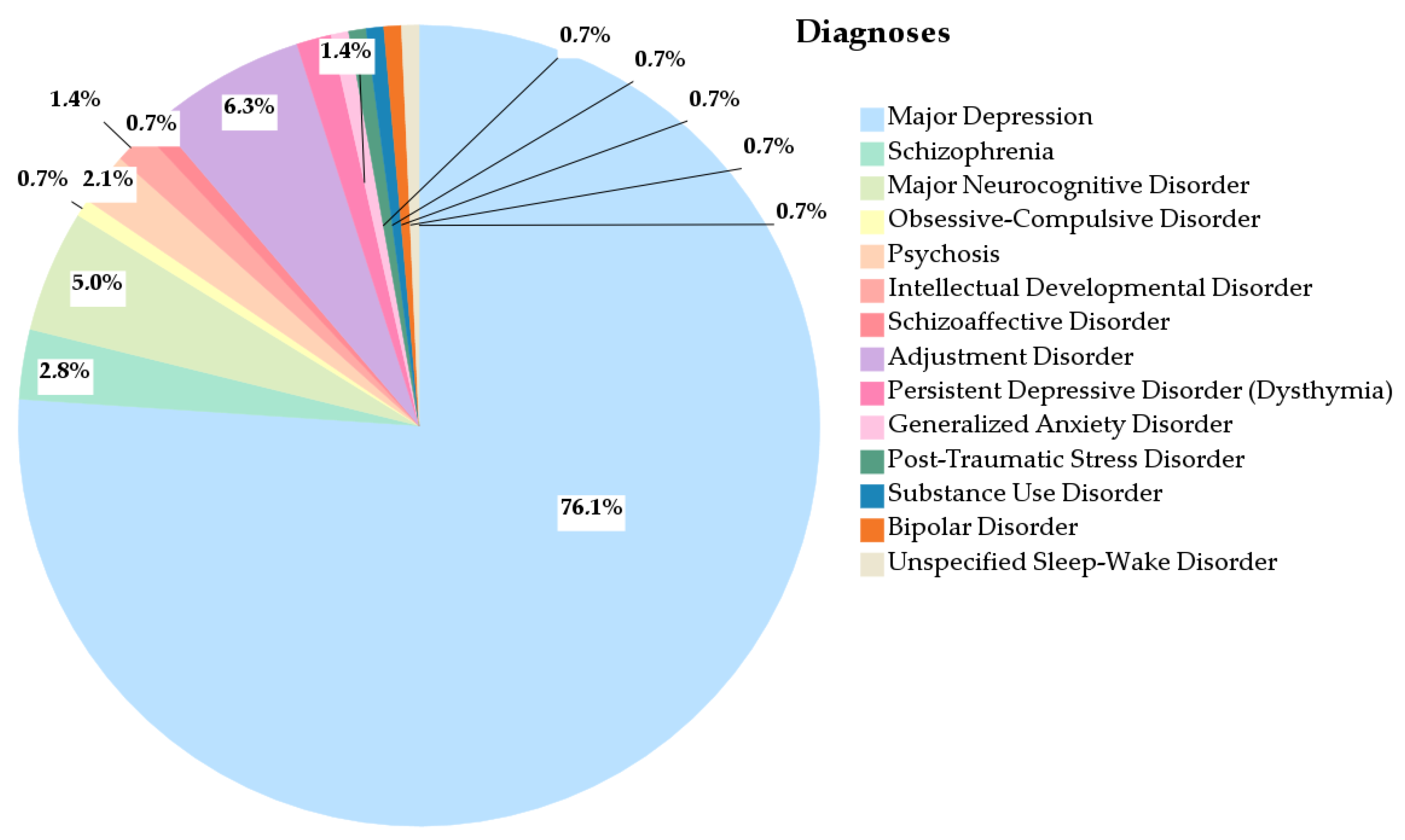
3.1.2. Diagnosis
3.1.3. Classes of Antidepressants
3.1.4. Other Classes of Medications
3.1.5. Health and Comorbidity Profile of the Population
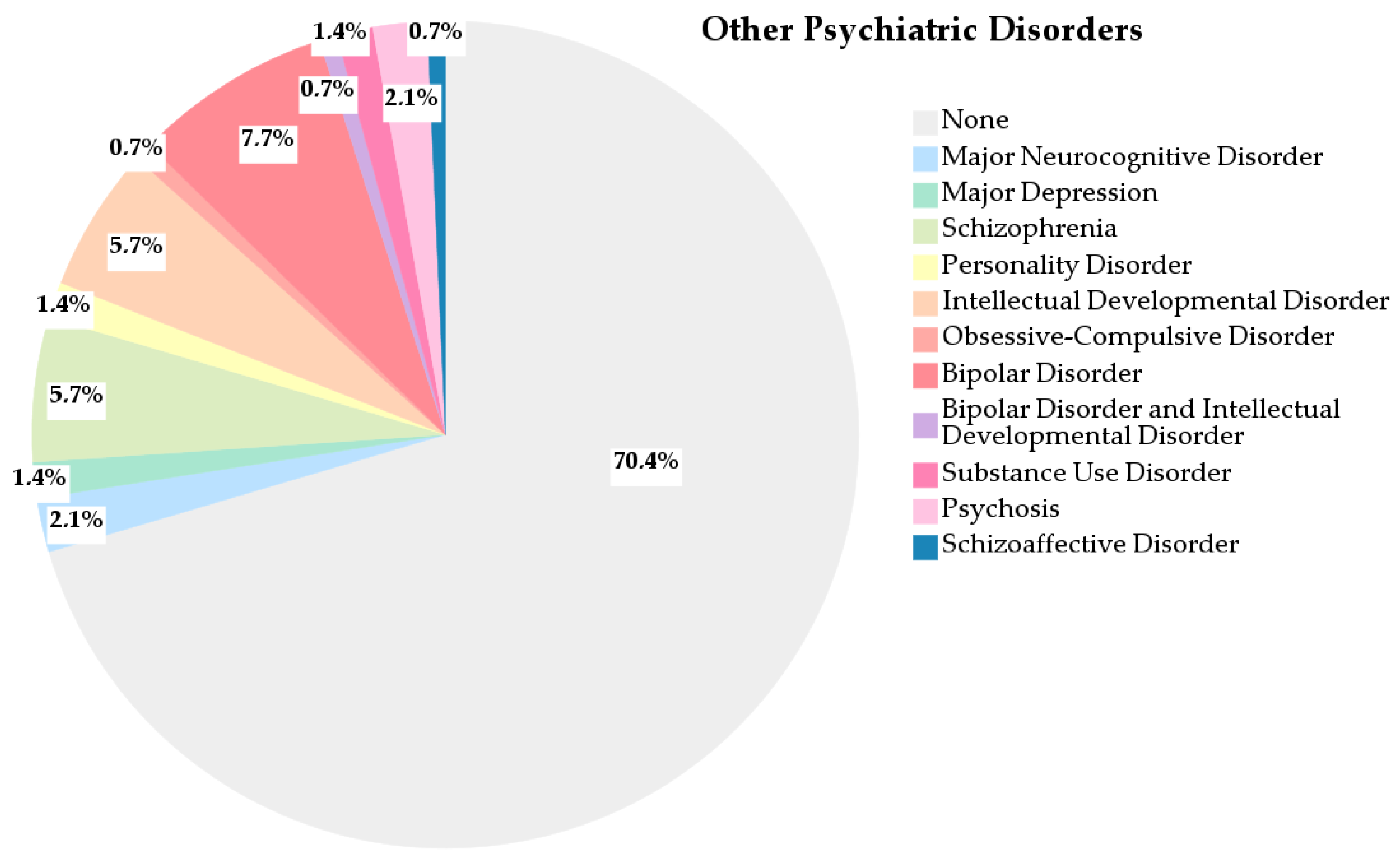
3.1.6. Other Psychiatric Disorders
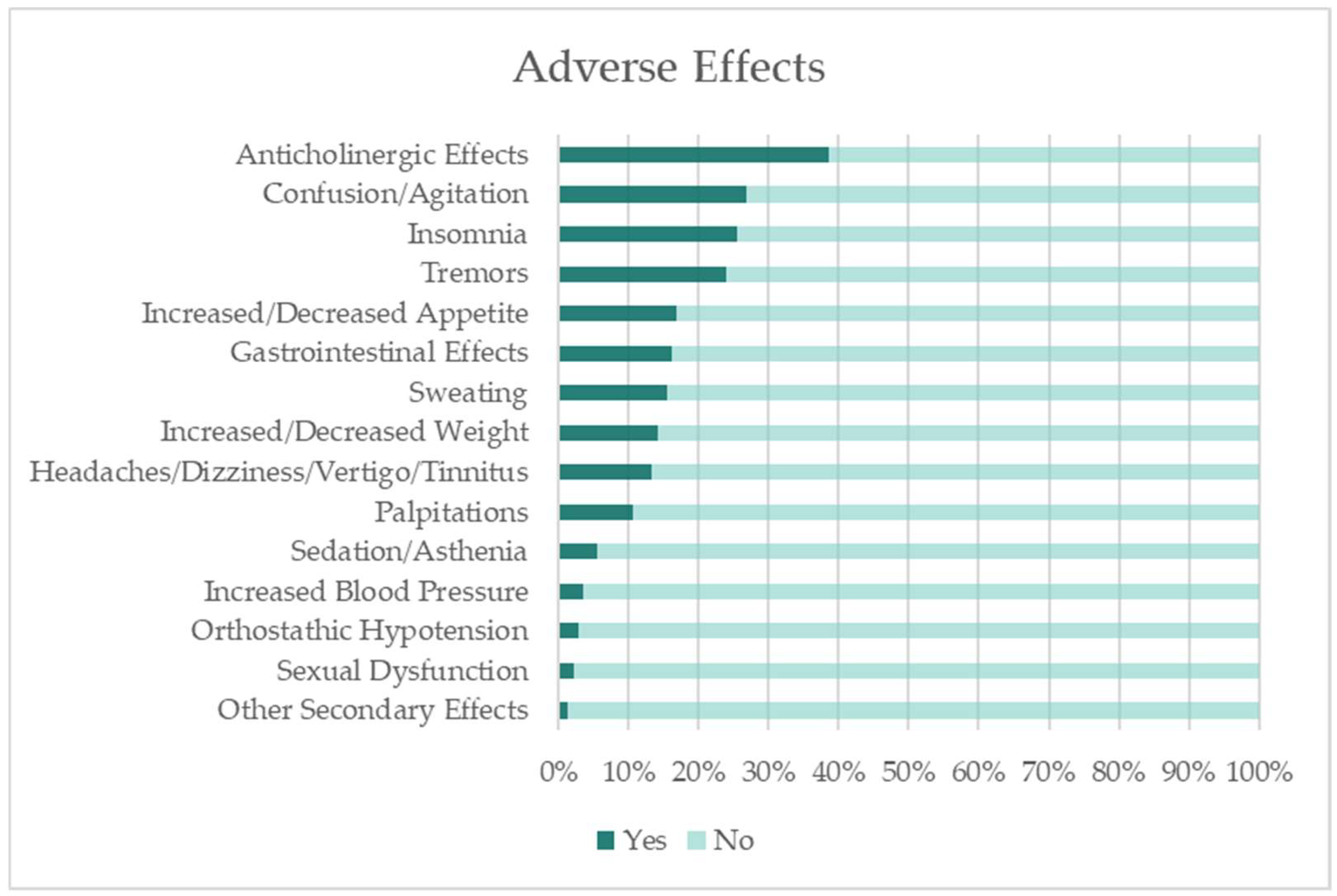
3.1.7. Adverse Effects
3.2. Association Between Sex and Antidepressants Consumption
3.3. Association Between Age and Antidepressants Consumption
3.4. Association Between Antidepressants Consumption and Adverse Effects
4. Study Limitations, Public Health Implications, and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT modulators | Serotonin Modulators |
| INFARMED | Autoridade Nacional do Medicamento e Produtos de Saúde, I.P. |
| OECD | Organisation for Economic Cooperation and Development |
| OR | Odds Ratio |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| SNRIs | Serotonin and Norepinephrine Reuptake Inhibitors |
| TeCAs | Tetracyclic Antidepressants |
| TCAs | Tricyclic Antidepressants |
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Bessa, J.M.; Carvalho, S.; Cunha, I.B.; Fernandes, M.; Matos-Pires, A.; Neves, R.; Oliveira-Maia, A.J.; Santos, S.; Santos, V. Treatment-Resistant Depression in Portugal: Perspective From Psychiatry Experts. Front. Psychiatry 2022, 13, 824919. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.; Frasquilho, D.; Azeredo-Lopes, S.; Neto, D.; Silva, M.; Cardoso, G.; Caldas-de-Almeida, J.M. Disability and common mental disorders: Results from the World Mental Health Survey Initiative Portugal. Eur. Psychiatry 2018, 49, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Estrela, M.; Herdeiro, M.T.; Ferreira, P.L.; Roque, F. The Use of Antidepressants, Anxiolytics, Sedatives and Hypnotics in Europe: Focusing on Mental Health Care in Portugal and Prescribing in Older Patients. Int. J. Environ. Res. Public Health 2020, 17, 8612. [Google Scholar] [CrossRef] [PubMed]
- Madeira, L.; Queiroz, G.; Henriques, R. Psychotropic drugs in Portugal from 2016 to 2019: A nationwide pharmacoepidemiological profile. medRxiv 2022, 1–25. [Google Scholar] [CrossRef]
- World Health Organization. Management of Physical Health Conditions in Adults with Severe Mental Disorders: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/978-92-4-155038-3 (accessed on 7 August 2025).
- World Health Organization. Comprehensive Mental Health Action Plan 2013–2030; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240031029 (accessed on 7 August 2025).
- Alabaku, O.; Yang, A.; Tharmarajah, S.; Suda, K.; Vigod, S.; Tadrous, M. Global trends in antidepressant, atypical antipsychotic, and benzodiazepine use: A cross-sectional analysis of 64 countries. PLoS ONE 2023, 18, e0284389. [Google Scholar] [CrossRef]
- Lunghi, C.; Dugas, M.; Leclerc, J.; Poluzzi, E.; Martineau, C.; Carnovale, V.; Stéfan, T.; Blouin, P.; Lépine, J.; Jalbert, L.; et al. Global prevalence of antidepressant drug utilization in the community: Protocol for a systematic review. BMJ Open 2022, 12, e062197. [Google Scholar] [CrossRef]
- Gutiérrez-Abejón, E.; Herrera-Gómez, F.; Criado-Espegel, P.; Álvarez, F.J. Trends in Antidepressants Use in Spain between 2015 and 2018: Analyses from a Population-Based Registry Study with Reference to Driving. Pharmaceuticals 2020, 13, 61. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 7 August 2025).
- Estrela, M.; Silva, T.M.; Gomes, E.R.; Piñeiro, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. Prescription of anxiolytics, sedatives, hypnotics and antidepressants in outpatient, universal care during the COVID-19 pandemic in Portugal: A nationwide, interrupted time-series approach. J. Epidemiol. Community Health 2022, 76, 335–340. [Google Scholar] [CrossRef]
- Yao, H.; Chen, J.H.; Xu, Y.F. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry 2020, 7, e21. [Google Scholar] [CrossRef]
- Negrão, L.G.; Coelho, C.; Castel-Branco, M.M.; Figueiredo, I.V.; Fernandez-Llimos, F. Impact of the COVID-19 pandemic on antidepressant consumption in the Central region of Portugal: Interrupted time series. Soc. Psychiatry Psychiatr. Epidemiol. 2024, 60, 621–629. [Google Scholar] [CrossRef]
- Madeira, L.; Queiroz, G.; Henriques, R. Prepandemic psychotropic drug status in Portugal: A nationwide pharmacoepidemiological profile. Sci. Rep. 2023, 13, 6912. [Google Scholar] [CrossRef] [PubMed]
- Autorização de Introdução No Mercado—INFARMED, I.P. 2024. Available online: https://www.infarmed.pt/web/infarmed/entidades/medicamentos-uso-humano/autorizacao-de-introducao-no-mercado (accessed on 7 August 2025).
- Afonso, R.M.; Ribeiro, O.; Vaz Patto, M.; Loureiro, M.; Loureiro, M.J.; Castelo-Branco, M.; Patrício, S.; Alvarinhas, S.; Tomáz, T.; Rocha, C.; et al. Reaching 100 in the Countryside: Health Profile and Living Circumstances of Portuguese Centenarians from the Beira Interior Region. Curr. Gerontol. Geriatr. Res. 2018, 2018, 8450468. [Google Scholar] [CrossRef] [PubMed]
- Naslund, J.A.; Deng, D. Addressing mental health stigma in low-income and middle-income countries: A new frontier for digital mental health. Ethics Med. Public Health 2021, 19, 100719. [Google Scholar] [CrossRef]
- Gomes, D.; Placido, A.I.; Mó, R.; Simões, J.L.; Amaral, O.; Fernandes, I.; Lima, F.; Morgado, M.; Figueiras, A.; Herdeiro, M.T.; et al. Daily Medication Management and Adherence in the Polymedicated Elderly: A Cross-Sectional Study in Portugal. Int. J. Environ. Res. Public Health 2019, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- de Simoes, J.A.; Augusto, G.F.; Fronteira, I.; Hernandez-Quevedo, C. Portugal: Health System Review. Health Syst. Transit. 2017, 19, 1–184. [Google Scholar]
- Boyd, A.; Van de Velde, S.; Pivette, M.; Have, M.T.; Florescu, S.; O’Neill, S.; Caldas-de-Almeida, J.M.; Vilagut, G.; Haro, J.M.; Alonso, J.; et al. Gender differences in psychotropic use across Europe: Results from a large cross-sectional, population-based study. Eur. Psychiatry 2015, 30, 778–788. [Google Scholar] [CrossRef]
- Sundbom, L.T.; Bingefors, K.; Hedborg, K.; Isacson, D. Are men under-treated and women over-treated with antidepressants? Findings from a cross-sectional survey in Sweden. BJPsych Bull. 2017, 41, 145–150. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; pp. 67–758. [Google Scholar]
- Simon, G.E.; Stewart, C.; Beck, A.; Ahmedani, B.K.; Coleman, K.J.; Whitebird, R.R.; Lynch, F.; Owen-Smith, A.A.; Waitzfelder, B.E.; Soumerai, S.B.; et al. National prevalence of receipt of antidepressant prescriptions by persons without a psychiatric diagnosis. Psychiatr. Serv. 2014, 65, 944–946. [Google Scholar] [CrossRef]
- Vanderah, T.W. Drugs that Act in the Central Nervous System: Antidepressant Agents. In Katzung’s Basic & Clinical Pharmacology, 16th ed.; Vanderah, T.W., Ed.; McGraw Hill: New York, NY, USA, 2023; pp. 833–868. [Google Scholar]
- Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef]
- Bushnell, G.A.; Stürmer, T.; Gaynes, B.N.; Pate, V.; Miller, M. Simultaneous Antidepressant and Benzodiazepine New Use and Subsequent Long-term Benzodiazepine Use in Adults With Depression, United States, 2001–2014. JAMA Psychiatry 2017, 74, 747–755. [Google Scholar] [CrossRef]
- Mulder, R.; Hamilton, A.; Irwin, L.; Boyce, P.; Morris, G.; Porter, R.J.; Malhi, G.S. Treating depression with adjunctive antipsychotics. Bipolar Disord. 2018, 20 (Suppl. S2), 17–24. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.; Gutierrez, G.; Llavero, M.; Gargallo, J.; Escalada, J.; López, J. Endocrine Disorders and Psychiatric Manifestations. In Endocrinology and Systemic Diseases; Portincasa, P., Frühbeck, G., Nathoe, H.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 311–345. [Google Scholar]
- Li, X.; Zhou, J.; Wang, M.; Yang, C.; Sun, G. Cardiovascular disease and depression: A narrative review. Front. Cardiovasc. Med. 2023, 10, 1274595. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Nestler, E.J. The neurobiology of stress: Vulnerability, resilience, and major depression. Proc. Natl. Acad. Sci. USA 2023, 120, e2312662120. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Sălcudean, A.; Popovici, R.A.; Pitic, D.E.; Sârbu, D.; Boroghina, A.; Jomaa, M.; Salehi, M.A.; Kher, A.A.M.; Lica, M.M.; Bodo, C.R.; et al. Unraveling the Complex Interplay Between Neuroinflammation and Depression: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 1645. [Google Scholar] [CrossRef]
- Tune, L.E. Anticholinergic effects of medication in elderly patients. J. Clin. Psychiatry 2001, 62 (Suppl. S2), 11–14. [Google Scholar]
- Cohen, Z.L.; Eigenberger, P.M.; Sharkey, K.M.; Conroy, M.L.; Wilkins, K.M. Insomnia and Other Sleep Disorders in Older Adults. Psychiatr. Clin. N. Am. 2022, 45, 717–734. [Google Scholar] [CrossRef]
- Food and Drug Administration. Highlights of Prescribing Information: Wellbutrin (Bupropion Hydrochloride); Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Prescribing Information: Maprotiline Hydrochloride; Food and Drug Administration: Silver Spring, MD, USA, 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/072285s021lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Highlights of Prescribing Information: Remeron (Mirtazapine); Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Highlights of Prescribing Information: Citalopram; Food and Drug Administration: Silver Spring, MD, USA, 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215428s002lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Highlights of Prescribing Information: Prozac (Fluoxetine Hydrochloride); Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Highlights of Prescribing Information: Luvox CR (Fluvoxamine Maleate); Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022033s011lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Highlights of Prescribing Information: Paroxetine; Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207139orig1s000lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Highlights of Prescribing Information: Zoloft (Sertraline Hydrochloride); Food and Drug Administration: Silver Spring, MD, USA, 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf (accessed on 7 August 2025).
- Food and Drug Administration. Highlights of Prescribing Information: Desyrel (Trazodone Hydrochloride); Food and Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf (accessed on 7 August 2025).

| Variable | n | % |
|---|---|---|
| Sex | ||
| Female | 115 | 81.0 |
| Male | 27 | 19.0 |
| Age (in years) | ||
| 18–64 | 98 | 69.0 |
| >64 | 44 | 31.0 |
| Number of antidepressants | ||
| 1 | 97 | 68.3 |
| >1 | 45 | 31.7 |
| Therapy duration | ||
| <1 month | 9 | 6.3 |
| <6 months | 19 | 13.4 |
| <1 year | 12 | 8.5 |
| ≥1 year | 85 | 59.9 |
| ≥5 years | 14 | 9.9 |
| Not specified | 3 | 2.1 |
| Classes | Antidepressants | Count n (%) | Classes of Antidepressants n (%) |
|---|---|---|---|
| TCAs | Amitriptyline | 3 (1.5) | 5 (2.6) |
| Clomipramine | 2 (1.0) | ||
| TeCAs and unicyclic | Bupropion | 5 (2.6) | 31 (16.0) |
| Maprotiline | 1 (0.5) | ||
| Mirtazapine | 25 (12.9) | ||
| SSRIs | Citalopram | 12 (6.2) | 92 (47.4) |
| Fluoxetine | 22 (11.3) | ||
| Fluvoxamine | 3 (1.5) | ||
| Paroxetine | 16 (8.2) | ||
| Sertraline | 39 (20.1) | ||
| SNRIs | Duloxetine | 4 (2.1) | 12 (6.2) |
| Venlafaxine | 8 (4.1) | ||
| 5-HT modulators | Trazodone | 54 (27.8) | 54 (27.8) |
| Variable | n (Yes) | n (No) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| TCAs | ||||
| Female Male Total | 505 | 110 27 137 | 0.583 | 1.25 (1.15–1.35) a |
| TeCAs and unicyclic | ||||
| Female Male Total | 26 5 31 | 89 22 111 | 0.643 | 1.29 (0.44–3.73) a |
| SSRIs | ||||
| Female Male Total | 70 21 91 | 45 6 51 | 0.099 | 2.25 (0.84–6.00) b |
| SNRIs | ||||
| Female Male Total | 11 1 12 | 104 26 130 | 0.463 | 2.75 (0.34–22.27) a |
| 5-HT modulators | ||||
| Female Male Total | 49 5 54 | 66 22 88 | 0.020 | 3.27 (1.16–9.23) a |
| Variable | n(>1) | n(1) | p-Value | OR (95% CI) |
| Number of antidepressants | ||||
| Female Male Total | 39 6 45 | 76 21 97 | 0.240 | 1.80 (0.67–4.81) c |
| Variable | n (Yes) | n (No) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| TCAs | ||||
| 18–64 >64 Total | 2 3 5 | 96 41 137 | 0.173 | 3.51 (0.57–21.81) a |
| TeCAs and unicyclic | ||||
| 18–64 >64 Total | 15 16 31 | 83 28 111 | 0.005 | 3.16 (1.39–7.21) a |
| SSRIs | ||||
| 18–64 >64 Total | 67 24 91 | 31 20 51 | 0.112 | 1.80 (0.87–3.74) b |
| SNRIs | ||||
| 18–64 >64 Total | 9 3 12 | 89 41 130 | 0.754 | 1.38 (0.36–5.37) b |
| 5-HT modulators | ||||
| 18–64 >64 Total | 31 23 54 | 67 21 88 | 0.019 | 2.37 (1.14–4.91) a |
| Variable | n(>1) | n(1) | p-Value | OR (95% CI) |
| Number of antidepressants | ||||
| 18–64 >64 Total | 23 22 45 | 75 22 97 | 0.002 | 3.26 (1.54–6.93) c |
| Variable 1 | Variable 2 | n (Yes) | n (No) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|
| TeCAs and unicyclic | Anticholinergic effects | ||||
| Yes No Total | 17 14 31 | 38 73 111 | 0.037 | 2.33 (1.04–5.28) a | |
| Tremors | |||||
| Yes No Total | 12 19 31 | 22 89 111 | 0.029 | 2.56 (1.08–6.04) a | |
| Gastrointestinal effects | |||||
| Yes No Total | 10 21 31 | 13 98 111 | 0.006 | 3.59 (1.39–9.28) a | |
| Orthostatic hypotension | |||||
| Yes No Total | 3 28 31 | 1 110 111 | 0.033 | 11.79 (1.18–117.66) a | |
| SSRIs | Anticholinergic effects | ||||
| Yes No Total | 25 66 91 | 30 21 51 | <0.001 | 3.77 (1.83–7.77) b | |
| Confusion/Agitation | |||||
| Yes No Total | 18 73 91 | 20 31 51 | 0.012 | 2.62 (1.22–5.61) b | |
| Insomnia | |||||
| Yes No Total | 16 75 91 | 20 31 51 | 0.004 | 3.02 (1.39–6.59) b | |
| Tremors | |||||
| Yes No Total | 14 77 91 | 20 31 51 | 0.001 | 3.55 (1.59–7.90) b | |
| Increased/Decreased appetite | |||||
| Yes No Total | 9 82 91 | 15 36 51 | 0.003 | 3.80 (1.52–9.48) b | |
| Gastrointestinal effects | |||||
| Yes No Total | 9 82 91 | 14 37 51 | 0.006 | 3.45 (1.37–8.68) b | |
| Increased/Decreased weight | |||||
| Yes No Total | 8 83 91 | 12 39 51 | 0.015 | 3.19 (1.21–8.44) b | |
| 5-HT modulators | Anticholinergic effects | ||||
| Yes No Total | 35 19 54 | 20 68 88 | <0.001 | 6.26 (2.96–13.24) a | |
| Confusion/Agitation | |||||
| Yes No Total | 23 31 54 | 15 73 88 | 0.001 | 3.61 (1.67–7.83) a | |
| Insomnia | |||||
| Yes No Total | 21 33 54 | 15 73 88 | 0.004 | 3.10 (1.42–6.75) a | |
| Tremors | |||||
| Yes No Total | 21 33 54 | 13 75 88 | 0.001 | 3.67 (1.64–8.20) a | |
| Increased/Decreased appetite | |||||
| Yes No Total | 16 38 54 | 8 80 88 | 0.002 | 4.21 (1.66–10.70) a | |
| Gastrointestinal effects | |||||
| Yes No Total | 13 41 54 | 10 78 88 | 0.046 | 2.47 (1.00–6.13) a | |
| Sweating | |||||
| Yes No Total | 14 40 54 | 8 80 88 | 0.007 | 3.50 (1.36–9.03) a | |
| Increased/Decreased weight | |||||
| Yes No Total | 14 40 54 | 6 82 88 | 0.001 | 4.78 (1.71–13.38) a | |
| Headaches/Dizziness/Vertigo/Tinnitus | |||||
| Yes No Total | 13 41 54 | 6 82 88 | 0.003 | 4.33 (1.54–12.23) a | |
| Palpitations | |||||
| Yes No Total | 11 43 54 | 4 84 88 | 0.003 | 5.37 (1.62–17.87) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, S.; Rosado, T.; Santos, V.H.; Rei, C.; Amantegui, P.; Pissarra da Costa, A.; Chaves, T.; Valente, R.; Duarte, F.; Pacheco, S.; et al. Characterization of Antidepressant Consumption in a Portuguese Inland Population. Healthcare 2025, 13, 2177. https://doi.org/10.3390/healthcare13172177
Soares S, Rosado T, Santos VH, Rei C, Amantegui P, Pissarra da Costa A, Chaves T, Valente R, Duarte F, Pacheco S, et al. Characterization of Antidepressant Consumption in a Portuguese Inland Population. Healthcare. 2025; 13(17):2177. https://doi.org/10.3390/healthcare13172177
Chicago/Turabian StyleSoares, Sofia, Tiago Rosado, Vítor Hugo Santos, Cristina Rei, Patricia Amantegui, António Pissarra da Costa, Telma Chaves, Rita Valente, Fábio Duarte, Susana Pacheco, and et al. 2025. "Characterization of Antidepressant Consumption in a Portuguese Inland Population" Healthcare 13, no. 17: 2177. https://doi.org/10.3390/healthcare13172177
APA StyleSoares, S., Rosado, T., Santos, V. H., Rei, C., Amantegui, P., Pissarra da Costa, A., Chaves, T., Valente, R., Duarte, F., Pacheco, S., Martins, M., Dias, K., Costa, P., Costa, R., Castro, S., Sousa, D., Figueiredo, D., Soares, I., Mouta, S., ... Gallardo, E. (2025). Characterization of Antidepressant Consumption in a Portuguese Inland Population. Healthcare, 13(17), 2177. https://doi.org/10.3390/healthcare13172177







