The Global Socioeconomic Burden of Obstructive Sleep Apnea: A Comprehensive Review
Abstract
1. Introduction
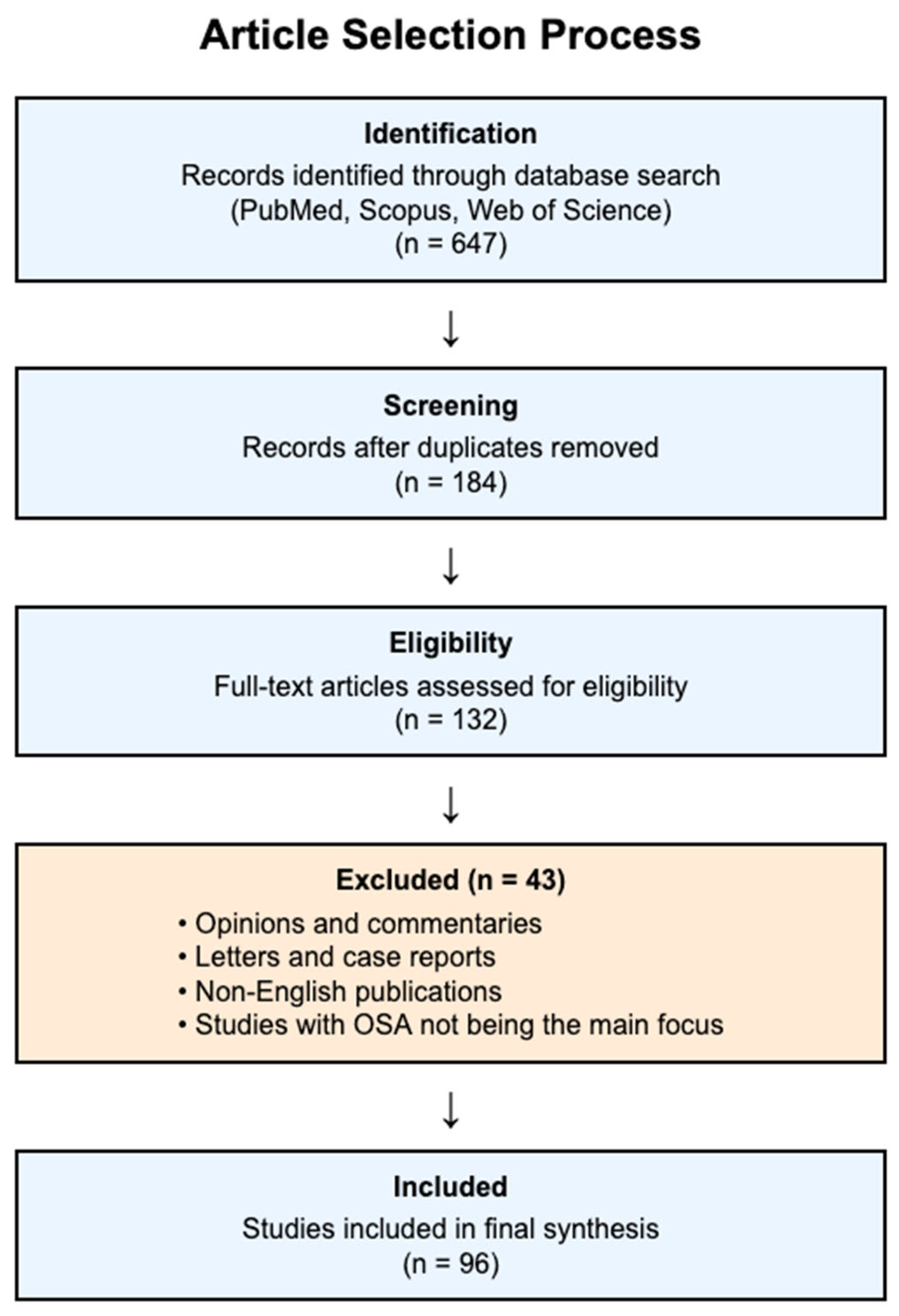
2. Materials and Methods
2.1. Search Strategy
- -
- Primary terms: “obstructive sleep apnea” OR “OSA” OR “sleep-disordered breathing”.
- -
- Combined with AND (“economic burden” OR “healthcare costs” OR “treatment access” OR “cost-effectiveness” OR “global health” OR “socioeconomic” OR “productivity loss” OR “absenteeism” OR “presenteeism” OR “quality of life” OR “healthcare utilization” OR “insurance coverage”).
2.2. Eligibility Criteria
- Original peer-reviewed research, review, or meta-analysis;
- Articles written in English;
- The article focuses on the economic burden, treatment, healthcare policy, or access to care associated with OSA.
- Official reports from major health organizations containing relevant data on OSA epidemiology, economic burden, or healthcare policy
- Opinions and commentaries, letters, and case reports;
- Non-English publications;
- Studies with OSA not being the main focus in the study, e.g., studies that did not specifically address economic or health system issues.
2.3. Study Selection Process
2.4. Quality Assessment and Context of Evidence
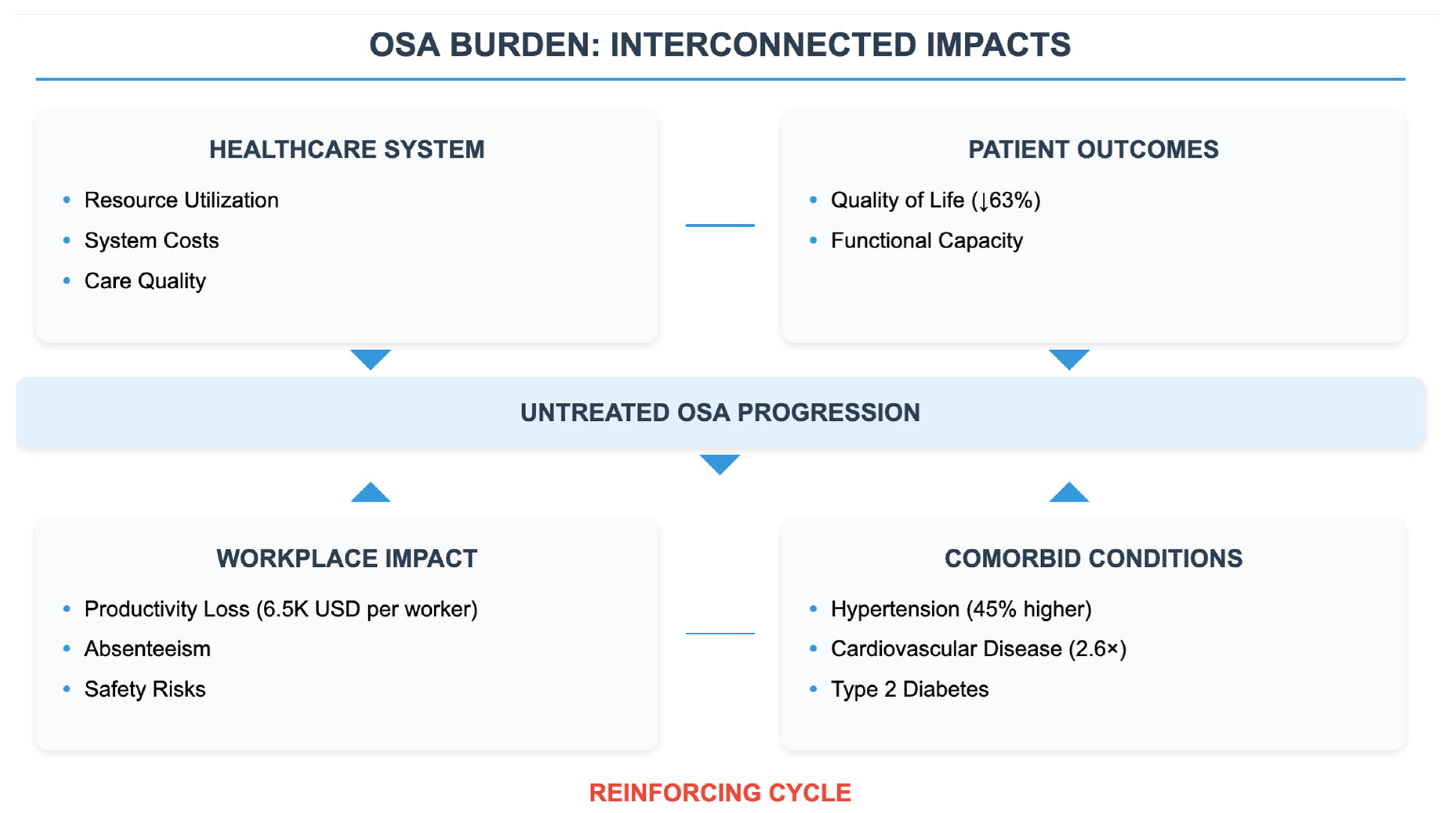
3. The Economic Burden of OSA: Direct and Indirect Costs
4. OSA Treatment Strategies: Cost-Effectiveness and Accessibility
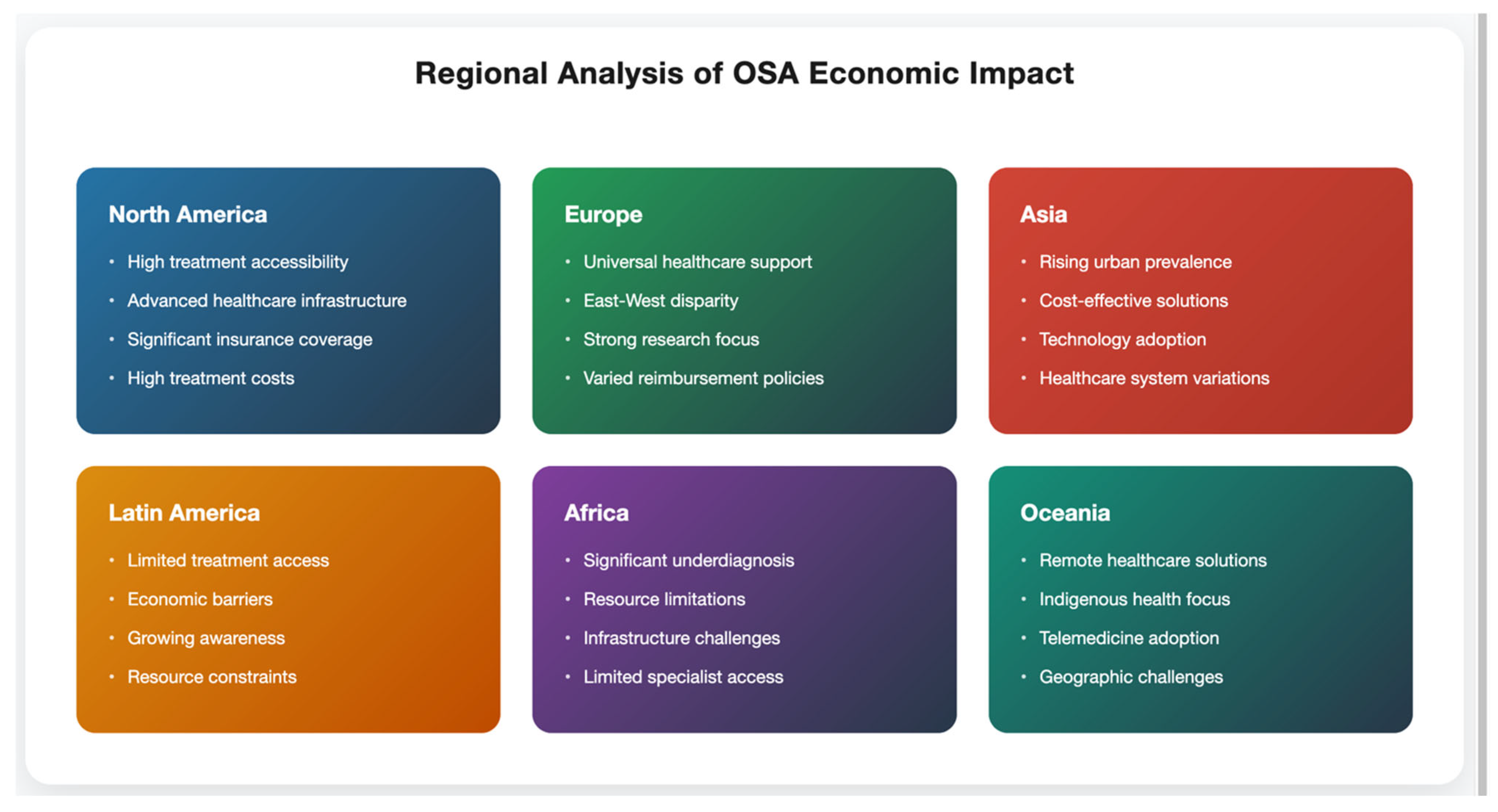
5. Regional Differences in OSA Burden and Treatment Impact
6. Policy Recommendations for Reducing the Socioeconomic Impact of OSA
6.1. Strategies to Improve Early Diagnosis and Reduce Long-Term Healthcare Costs
6.2. The Role of Insurance Reimbursement in Lowering the Economic Barrier to OSA Treatment
6.3. Interventions at the Workplace to Reduce Losses in Productivity and Costs
6.4. Contribution of Public Health Awareness Campaigns to Reducing the Burden
7. Future Directions in OSA Research and Global Healthcare Strategies
- -
- AI-based diagnostics and home-based screening programs in implementation science: There is a need for studies that move beyond efficacy in laboratory settings or under monitoring to effectiveness of these technologies in real life, especially in the underserved and in low-resource settings.
- -
- Development and assessment of models of integrated care: How management of OSA can be integrated effectively into extant chronic disease programs, cardiovascular disease, and diabetes to improve outcomes and resource use.
- -
- Full cost and benefit of innovative intervention: Long-term cost-effectiveness analysis of new pharmacologic treatments and minimally invasive surgical techniques is still needed to support the rational distribution of resources.
7.1. Research Needs in Low-Cost OSA Diagnostics and Treatments
7.2. Research Agenda for Telemedicine and AI-Based Solutions
7.3. Priorities for International Research Collaboration
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| OSAS | Obstructive Sleep Apnea Syndrome |
| CPAP | Continuous Positive Airway Pressure |
| MAD | Mandibular Advancement Devices |
| UPPP | Uvulopalatopharyngoplasty |
| ICER | Incremental Cost-Effectiveness Ratio |
| QALY | Quality-Adjusted Life Year |
| BMI | Body Mass Index |
| GDP | Gross Domestic Product |
| AI | Artificial Intelligence |
| USA | United States of America |
| RA | Rheumatoid Arthritis |
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Pan, L.; Meng, F.; Zhang, L.; Shen, H.; Kong, D.; Wang, W.; Kang, J. Global Research Trends of Obstructive Sleep Apnea from 2011 to 2020: A 10-Year Bibliometric Analysis. Ann. Palliat. Med. 2022, 11, 1671–1686. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112, Erratum in Physiol. Rev. 2010, 90, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Li, K.K.; Kushida, C.; Powell, N.B.; Riley, R.W.; Guilleminault, C. Obstructive sleep apnea syndrome: A comparison between Far-East Asian and white men. Laryngoscope 2000, 110 (10 Pt 1), 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Albrecht, J.S.; Towe, M.M.; Abariga, S.A.; Diaz-Abad, M.; Shipper, A.G.; Cooper, L.M.; Assefa, S.Z.; Tom, S.E.; Scharf, S.M. The impact of treatments for obstructive sleep apnea on monetized health economic outcomes: A systematic review. Chest 2019, 155, 947–961. [Google Scholar] [CrossRef]
- Huyett, P.; Bhattacharyya, N. Incremental healthcare costs for sleep disorders in the United States. J. Clin. Sleep Med. 2021, 17, 69–76. [Google Scholar] [CrossRef]
- Etindele Sosso, F.A.; Matos, E. Socioeconomic Disparities in Obstructive Sleep Apnea: A Systematic Review of Empirical Research. Sleep Breath. 2021, 25, 1729–1739. [Google Scholar] [CrossRef]
- Borsoi, L.; Facchin, F.; Gallo, F.; Casiraghi, M.; Perger, E.; Mazzotti, D.R.; Lombardi, C.; Parati, G. The socioeconomic burden of obstructive sleep apnea in Italy: A cost-of-illness analysis. PLoS ONE 2022, 17, e0262370. [Google Scholar] [CrossRef]
- Young, T.; Evans, L.; Finn, L.; Palta, M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997, 20, 705–706. [Google Scholar] [CrossRef]
- Tan, A.; Yin, J.D.; Tan, L.W.; van Dam, R.M.; Cheung, Y.Y.; Lee, C.H. Using the Berlin Questionnaire to predict obstructive sleep apnea in the general population. J. Clin. Sleep Med. 2017, 13, 427–432. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Stroke Risk Factor Collaborators. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, S.R.; Kaambwa, B.; Kapur, V.K. The Cost Effectiveness of Sleep Apnea Management: A Critical Evaluation of the Impact of Therapy on Healthcare Costs. Chest 2024, 166, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Bhatt, A.N.; Sengupta, S.; Abolhassani, A.; Brower, D.; Forehand, C.; Keats, K.; Healy, W.J. Awakening Sleep Medicine: The Transformative Role of Artificial Intelligence in Sleep Health. Curr. Sleep Med. Rep. 2025, 11, 13. [Google Scholar] [CrossRef]
- Rotenberg, B.W.; Vicini, C.; Pang, E.B.; Pang, K.P. Reconsidering First-Line Treatment for Obstructive Sleep Apnea: A Systematic Review of the Literature. J. Otolaryngol. Head Neck Surg. 2016, 45, 23. [Google Scholar] [CrossRef]
- McDaid, C.; Griffin, S.; Weatherly, H.; Durée, K.; van der Burgt, M.; van Hout, S.; Akers, J.; Davies, R.J.; Sculpher, M.; Westwood, M. Continuous Positive Airway Pressure Devices for the Treatment of Obstructive Sleep Apnoea-Hypopnoea Syndrome: A Systematic Review and Economic Analysis. Health Technol. Assess. 2009, 13, iii–iv, xi–xiv, 1–119, 143–274. [Google Scholar] [CrossRef]
- Guest, J.F.; Panca, M.; Sladkevicius, E.; Taheri, S.; Stradling, J. Clinical Outcomes and Cost-Effectiveness of Continuous Positive Airway Pressure to Manage Obstructive Sleep Apnea in Patients with Type 2 Diabetes in the U.K. Diabetes Care 2014, 37, 1263–1271. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Léger, D.; Bayon, V.; Laaban, J.P.; Philip, P. Impact of Sleep Apnea on Economics. Sleep Med. Rev. 2012, 16, 455–462. [Google Scholar] [CrossRef]
- Philip, P.; Sagaspe, P.; Lagarde, E.; Léger, D.; Ohayon, M.M.; Bioulac, B.; Boussuge, J.; Taillard, J. Sleep Disorders and Accidental Risk in a Large Group of Regular Registered Highway Drivers. Sleep Med. 2010, 11, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Mulgrew, A.T.; Ryan, C.F.; Fleetham, J.A.; Cheema, R.; Fox, N.; Koehoorn, M.; Fitzgerald, J.M.; Marra, C.; Ayas, N.T. The Impact of Obstructive Sleep Apnea and Daytime Sleepiness on Work Limitation. Sleep Med. 2007, 9, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.; Wingenbach, D.D.; Kagey, A.N.; Schaneman, J.L.; Kasper, D. The Long-Term Health Plan and Disability Cost Benefit of Obstructive Sleep Apnea Treatment in a Commercial Motor Vehicle Driver Population. J. Occup. Environ. Med. 2010, 52, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Continuous Positive Airway Pressure Reduces Risk of Motor Vehicle Crash Among Drivers with Obstructive Sleep Apnea: Systematic Review and Meta-Analysis. Sleep 2010, 33, 1373–1380. [Google Scholar] [CrossRef]
- Jennum, P.; Ibsen, R.; Kjellberg, J. Social Consequences of Sleep-Disordered Breathing on Patients and Their Partners: A Controlled National Study. Eur. Respir. J. 2014, 43, 134–144. [Google Scholar] [CrossRef]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Obstructive Sleep Apnea and Risk of Motor Vehicle Crash: Systematic Review and Meta-Analysis. J. Clin. Sleep Med. 2009, 5, 573–581. [Google Scholar] [CrossRef]
- Gurubhagavatula, I.; Sullivan, S.; Meoli, A.; Patil, S.; Olson, R.; Berneking, M.; Watson, N.F. Management of Obstructive Sleep Apnea in Commercial Motor Vehicle Operators: Recommendations of the AASM Sleep and Transportation Safety Awareness Task Force. J. Clin. Sleep Med. 2017, 13, 745–758. [Google Scholar] [CrossRef]
- McMillan, A.; Bratton, D.J.; Faria, R.; Laskawiec-Szkonter, M.; Griffin, S.; Davies, R.J.; Nunn, A.J.; Stradling, J.R.; Riha, R.L.; Morrell, M.J. A Multicentre Randomised Controlled Trial and Economic Evaluation of Continuous Positive Airway Pressure for the Treatment of Obstructive Sleep Apnoea Syndrome in Older People: PREDICT. Health Technol. Assess. 2015, 19, 1–188. [Google Scholar] [CrossRef]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Sharples, L.; Glover, M.; Clutterbuck-James, A.; Bennett, M.; Jordan, J.; Chadwick, R.; Pittman, M.; East, C.; Cameron, M.; Davies, M.; et al. Clinical Effectiveness and Cost-Effectiveness Results from the Randomised Controlled Trial of Oral Mandibular Advancement Devices for Obstructive Sleep Apnoea-Hypopnoea (TOMADO) and Long-Term Economic Analysis of Oral Devices and Continuous Positive Airway Pressure. Health Technol. Assess. 2014, 18, 1–296. [Google Scholar] [CrossRef] [PubMed]
- Johal, A.; Fleming, P.S.; Manek, S.; Marinho, V.C. Mandibular Advancement Splint (MAS) Therapy for Obstructive Sleep Apnoea—An Overview and Quality Assessment of Systematic Reviews. Sleep Breath 2015, 19, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.S.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol. Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef]
- Zaghi, S.; Holty, J.E.; Certal, V.; Abdullatif, J.; Guilleminault, C.; Powell, N.B.; Riley, R.W.; Camacho, M. Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea: A Meta-Analysis. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 58–66. [Google Scholar] [CrossRef]
- Billings, M.E.; Kapur, V.K. Medicare Long-Term CPAP Coverage Policy: A Cost-Utility Analysis. J. Clin. Sleep Med. 2013, 9, 1023–1029. [Google Scholar] [CrossRef]
- Watson, N.F. Health Care Savings: The Economic Value of Diagnostic and Therapeutic Care for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2016, 12, 1075–1077. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Armitstead, J.; Pepin, J.L.; Woehrle, H.; Nunez, C.M.; Benjafield, A.; Malhotra, A. Short-Term CPAP Adherence in Obstructive Sleep Apnea: A Big Data Analysis Using Real World Data. Sleep Med. 2019, 59, 114–116. [Google Scholar] [CrossRef]
- Pack, A.I.; Magalang, U.J.; Singh, B.; Kuna, S.T.; Keenan, B.T.; Maislin, G. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: Understanding and overcoming bias. Sleep 2021, 44, zsaa229. [Google Scholar] [CrossRef]
- Masa Jiménez, J.F.; Barbé Illa, F.; Capote Gil, F.; Chiner Vives, E.; Díaz de Atauri, J.; Durán Cantolla, J.; López Ortiz, S.; Marín Trigo, J.M.; Montserrat Canal, J.M.; Rubio González, M.; et al. Recursos y demoras en el diagnóstico del síndrome de apneas-hipopneas durante el sueño (SAHS) [Resources and delays in the diagnosis of sleep apnea-hypopnea syndrome]. Arch. Bronconeumol. 2007, 43, 188–198. [Google Scholar] [CrossRef]
- Howarth, T.P.; Heraganahally, S.S.; Gentin, N.; Jonas, C.; Williamson, B.; Jing, M.X.; Suresh, S. Comparison of polysomnographic characteristics between low birthweight and normal birthweight children in the Northern Territory of Australia: A case-control study. Sleep Health 2022, 8, 625–631. [Google Scholar] [CrossRef]
- Pépin, J.L.; Tamisier, R.; Hwang, D.; Mereddy, S.; Parthasarathy, S. Does remote monitoring change OSA management and CPAP adherence? Respirology 2017, 22, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Badr, S.M.; Epstein, L.J.; Gay, P.C.; Gozal, D.; Kohler, M.; Lévy, P.; Malhotra, A.; Phillips, B.A.; Rosen, I.M.; et al. An official American Thoracic Society statement: Continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am. J. Respir. Crit. Care Med. 2013, 188, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Dhillon, G.; Grewal, H.; Prasad, V.; Munjal, R.S.; Sharma, P.; Buddhavarapu, V.; Devadoss, R.; Kashyap, R.; Surani, S. Artificial intelligence in sleep medicine: Present and future. World J. Clin. Cases 2023, 11, 8106–8110. [Google Scholar] [CrossRef] [PubMed]
- Schiza, S.; Simonds, A.; Randerath, W.; Fanfulla, F.; Testelmans, D.; Grote, L.; Montserrat, J.M.; Pépin, J.L.; Verbraecken, J.; Ersu, R.; et al. Sleep laboratories reopening and COVID-19: A European perspective. Eur. Respir. J. 2021, 57, 2002722. [Google Scholar] [CrossRef]
- Araújo, T.; Jarrin, D.C.; Leanza, Y.; Vallières, A.; Morin, C.M. Qualitative studies of insomnia: Current state of knowledge in the field. Sleep Med. Rev. 2017, 31, 58–69. [Google Scholar] [CrossRef]
- Malhotra, A.; Crocker, M.E.; Willes, L.; Kelly, C.; Lynch, S.; Benjafield, A.V. Patient Engagement Using New Technology to Improve Adherence to Positive Airway Pressure Therapy: A Retrospective Analysis. Chest 2018, 153, 843–850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penzel, T.; Schöbel, C.; Fietze, I. New technology to assess sleep apnea: Wearables, smartphones, and accessories. F1000Research 2018, 7, 413. [Google Scholar] [CrossRef]
- Martinez-Beneyto, P.; Soria Checa, C.E.; Botella-Rocamora, P.; Rincon-Piedrahita, I.; Garcia Callejo, F.J.; Algarra, J.M. Lessons from healthcare utilization in children with obstructive sleep apnoea syndrome. Acta Otorrinolaringol. Esp. (Engl. Ed.) 2017, 68, 336–343. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Subramanian, S.; Quan, S.F. A multicenter prospective comparative effectiveness study of the effect of physician certification and center accreditation on patient-centered outcomes in obstructive sleep apnea. J. Clin. Sleep Med. 2014, 10, 243–249. [Google Scholar] [CrossRef]
- Grandner, M.A.; Petrov, M.E.; Rattanaumpawan, P.; Jackson, N.; Platt, A.; Patel, N.P. Sleep symptoms, race/ethnicity, and socioeconomic position. J. Clin. Sleep Med. 2013, 9, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Whinnery, J.; Jackson, N.; Rattanaumpawan, P.; Grandner, M.A. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep 2014, 37, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Grote, L.; McNicholas, W.T.; Hedner, J.; ESADA Collaborators. Sleep apnoea management in Europe during the COVID-19 pandemic: Data from the European Sleep Apnoea Database (ESADA). Eur. Respir. J. 2020, 55, 2001323. [Google Scholar] [CrossRef]
- Escourrou, P.; Grote, L.; Penzel, T.; McNicholas, W.T.; Verbraecken, J.; Tkacova, R.; Riha, R.L.; Hedner, J.; ESADA Study Group. The diagnostic method has a strong influence on classification of obstructive sleep apnea. J. Sleep Res. 2015, 24, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Pływaczewski, R.; Brzecka, A.; Bielicki, P.; Czajkowska-Malinowska, M.; Cofta, S.; Jonczak, L.; Radliński, J.; Tażbirek, M.; Wasilewska, J.; Polish Society of Lung Diseases. Sleep related breathing disorders in adults—Recommendations of Polish Society of Lung Diseases. Pneumonol. Alergol. Pol. 2013, 81, 221–258. [Google Scholar]
- Yu, X.; Fujimoto, K.; Urushibata, K.; Matsuzawa, Y.; Kubo, K. Cephalometric analysis in obese and nonobese patients with obstructive sleep apnea syndrome. Chest 2003, 124, 212–218. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Wang, Z.; Zhao, G.; Liu, L.; Bi, Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 169, 207–214. [Google Scholar] [CrossRef]
- Sadeh, A. Sleep assessment methods. Monogr. Soc. Res. Child Dev. 2015, 80, 33–48. [Google Scholar] [CrossRef]
- Chen, Z.; Foo, Z.S.T.; Tang, J.Y.; Sim, M.W.C.; Lim, B.L.; Fong, K.Y.; Tan, K.H. Sleep quality and burnout: A Singapore study. Sleep Med. 2023, 102, 205–212. [Google Scholar] [CrossRef]
- Bouscoulet, L.T.; Vázquez-García, J.C.; Muiño, A.; Márquez, M.; López, M.V.; de Oca, M.M.; Talamo, C.; Valdivia, G.; Pertuze, J.; Menezes, A.M.; et al. Prevalence of sleep related symptoms in four Latin American cities. J. Clin. Sleep Med. 2008, 4, 579–585. [Google Scholar] [CrossRef]
- Adewole, O.O.; Hakeem, A.; Fola, A.; Anteyi, E.; Ajuwon, Z.; Erhabor, G. Obstructive sleep apnea among adults in Nigeria. J. Natl. Med. Assoc. 2009, 101, 720–725. [Google Scholar] [CrossRef]
- Knauert, M.; Naik, S.; Gillespie, M.B.; Kryger, M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 17–27. [Google Scholar] [CrossRef]
- Tarasiuk, A.; Reuveni, H. The economic impact of obstructive sleep apnea. Curr. Opin. Pulm. Med. 2013, 19, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Malan, D.P. Public-private partnerships in global health. N. Engl. J. Med. 2019, 381, 193. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.E.; McPherson, K.; Tikoft, E.; Usher, K.; Hosseini, F.; Ferns, J.; Jersmann, H.; Antic, R.; Maguire, G.P. Sleep disorders in Aboriginal and Torres Strait Islander people and residents of regional and remote Australia. J. Clin. Sleep Med. 2015, 11, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Gander, P.; Scott, G.; Mihaere, K.; Scott, H. Societal costs of obstructive sleep apnoea syndrome. N. Z. Med. J. 2010, 123, 13–23. [Google Scholar]
- Szily, M.; Tarnoki, A.D.; Tarnoki, D.L.; Kovacs, D.T.; Forgo, B.; Lee, J.; Kim, E.; Sung, J.; Kunos, L.; Meszaros, M.; et al. Genetic influences on the onset of obstructive sleep apnoea and daytime sleepiness: A twin study. Respir. Res. 2019, 20, 125. [Google Scholar] [CrossRef]
- Toll, K.; Robinson, S.; Andrew, S.; Williams, A.; Yeung, J.; Varhol, R.; Moullin, J.C. Implementation of rural provider-to-provider telehealth in country Western Australia: A retrospective observational analysis via the RE-AIM framework. BMC Health Serv. Res. 2025, 25, 189. [Google Scholar] [CrossRef]
- Chai-Coetzer, C.L.; Antic, N.A.; McEvoy, R.D. Ambulatory models of care for obstructive sleep apnoea: Diagnosis and management. Respirology 2013, 18, 605–615. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Barbé, F.; Gozal, D.; Agustí, A. Precision medicine in obstructive sleep apnoea. Lancet Respir. Med. 2019, 7, 456–464. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Goldstein, C. Clinical applications of artificial intelligence in sleep medicine: A sleep clinician’s perspective. Sleep Breath. 2023, 27, 39–55. [Google Scholar] [CrossRef]
- Kim, R.D.; Kapur, V.K.; Redline-Bruch, J.; Rueschman, M.; Auckley, D.H.; Benca, R.M.; Foldvary-Schafer, N.R.; Iber, C.; Zee, P.C.; Rosen, C.L.; et al. An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea. Sleep 2015, 38, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Tedford, S.E.; Romano, L.; Gozal, D.; Medalie, L. Digital solutions for sleep problems in children: A pilot study. Pediatr. Pulmonol. 2022, 57, 1914–1920. [Google Scholar] [CrossRef]
- Chang, M.S.; Park, S.; Lim, J.; Lee, J.H. Impact of High Risk of Obstructive Sleep Apnea on Health-Related Quality of Life: The Korean National Health and Nutrition Survey 2019–2021. J Clin Med. 2024, 13, 4360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pépin, J.L.; Baillieul, S.; Tamisier, R. Reshaping sleep apnea care: Time for value-based strategies. Ann. Am. Thorac. Soc. 2019, 16, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Calvo, D.; Brennan, E.M.; Reljic, T.; Drasher-Phillips, L.; Schwartz, D.J.; Kumar, A.; Cotner, B.A.; Taylor, D.J.; Nakase-Richardson, R. Incidence and predictors of adherence to sleep apnea treatment in rehabilitation inpatients with acquired brain injury. Sleep Med. 2020, 69, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.W.J.; Adams, R.J.; Wanstall, S.; Crowther, M.E.; Rawson, G.; Vakulin, A.; Rayner, T.; McEvoy, R.D.; Eastwood, P.; Reynolds, A.C. Introducing a sleep disorder screening and management strategy for workers with future shift work requirements: A feasibility and acceptability study. Sci. Rep. 2024, 14, 19964. [Google Scholar] [CrossRef]
- van der Kleij, S.; de Backer, I.; Hanraets, B.; Verbraecken, J.; Asin, J. Effectiveness of remote monitoring in improving CPAP compliance and the impact of preexisting organisation of standard care: A randomised controlled trial. Sleep Breath. 2024, 28, 1715–1721. [Google Scholar] [CrossRef]
- Urbanová, L.; Vaníček, O.; Červená, K.; Bartoš, A.; Evansová, K. The impact of sleep education, light intervention and relaxation on sleep and mood in the elderly. Chronobiol. Int. 2024, 41, 567–576. [Google Scholar] [CrossRef]
- Seixas, A.A.; Moore, J.; Chung, A.; Robbins, R.; Grandner, M.; Rogers, A.; Williams, N.J.; Jean-Louis, G. Benefits of community-based approaches in assessing and addressing sleep health and sleep-related cardiovascular disease risk: A precision and personalized population health approach. Curr. Hypertens. Rep. 2020, 22, 52. [Google Scholar] [CrossRef]
- Drager, L.F.; Malhotra, A.; Yan, Y.; Pépin, J.L.; Armitstead, J.P.; Woehrle, H.; Nunez, C.M.; Cistulli, P.A.; Benjafield, A.V.; medXcloud group. Adherence with positive airway pressure therapy for obstructive sleep apnea in developing vs. developed countries: A big data study. J. Clin. Sleep Med. 2021, 17, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Tondo, P.; Scioscia, G.; Bailly, S.; Sabato, R.; Campanino, T.; Soccio, P.; Foschino Barbaro, M.P.; Gallo, C.; Pépin, J.L.; Lacedonia, D. Exploring phenotypes to improve long-term mortality risk stratification in obstructive sleep apnea through a machine learning approach: An observational cohort study. Eur. J. Intern. Med. 2025, 133, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, Z.; Wu, Y.; Liu, Y.; Li, Z.; Jia, Y.; Xiang, H. Sleep disorders: Pathogenesis and therapeutic interventions. MedComm 2025, 6, e70130. [Google Scholar] [CrossRef] [PubMed]
- Hossain, J.L.; Shapiro, C.M. The prevalence, cost implications, and management of sleep disorders: An overview. Sleep Breath. 2002, 6, 85–102. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Shaya, F.T.; Scharf, S.M. Health economics of insomnia treatments: The return on investment for a good night’s sleep. Sleep Med. Rev. 2016, 30, 72–82. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Zhang, J.; Ho, K.W.; Wei, Y.; Cheng, H. Validation of a wearable forehead sleep recorder against polysomnography in sleep staging and desaturation events in a clinical sample. J. Clin. Sleep Med. 2023, 19, 711–718. [Google Scholar] [CrossRef]
- Reuven, H.; Schweitzer, E.; Tarasiuk, A. A cost-effectiveness analysis of alternative at-home or in-laboratory technologies for the diagnosis of obstructive sleep apnea syndrome. Med. Decis. Mak. 2001, 21, 451–458. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Kim, J.; Goldbart, A.D.; Gozal, D. Novel pharmacological approaches for treatment of obstructive sleep apnea in children. Expert Opin. Investig. Drugs 2013, 22, 71–85. [Google Scholar] [CrossRef]
- Ronsivalle, V.; Gastaldi, G.; Fiorillo, G.; Amato, A.; Loreto, C.; Leonardi, R.; Lo Giudice, A. Customized facial orthopedics: Proof of concept for generating 3D-printed extra-oral appliance for early intervention in class III malocclusion. Prosthesis 2024, 6, 135–145. [Google Scholar] [CrossRef]
- Singh, A.B.; Khandelwal, C.; Dangayach, G.S. Revolutionizing healthcare materials: Innovations in processing, advancements, and challenges for enhanced medical device integration and performance. J. Micromanuf. 2024, 25165984241256234. [Google Scholar] [CrossRef]
- Peng, K.; He, H.; Liu, J.; Li, T.; Hou, S.; Qiao, S. EdgeGAN: Enhancing sleep quality monitoring in medical IoT through generative AI at the edge. IEEE Internet Things Mag. 2024, 7, 16–21. [Google Scholar] [CrossRef]
- Pépin, J.L.; Baillieul, S.; Bailly, S.; Tamisier, R. New management pathways for follow-up of CPAP-treated sleep apnoea patients including digital medicine and multimodal telemonitoring. Thorax 2024, 80, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Li, Y.; Wang, S.; Duan, Y.; Xu, L.-Q.; Gao, H. A pilot study of the ‘PSGCloud’—A cloud-based care service delivery and sleep disorders diagnosis system. Part I: Sleep structure and arousal analysis. Clin. eHealth 2020, 3, 23–30. [Google Scholar] [CrossRef]
- Bjornsdottir, E.; Keenan, B.T.; Eysteinsdottir, B.; Arnardottir, E.S.; Janson, C.; Gislason, T.; Sigurdsson, J.F.; Kuna, S.T.; Pack, A.I.; Benediktsdottir, B. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. J. Sleep Res. 2015, 24, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Mkuu, R.; Andreadis, K.; Muellers, K.A.; Ancker, J.S.; Horowitz, C.; Kaushal, R.; Lin, J.J. Examining and Addressing Telemedicine Disparities Through the Lens of the Social Determinants of Health: A Qualitative Study of Patient and Provider During the COVID-19 Pandemic. AMIA Annu Symp Proc. 2024, 2023, 1287–1296. [Google Scholar] [PubMed] [PubMed Central]
- Ssegonja, R.; Ljunggren, M.; Sampaio, F.; Tegelmo, T.; Theorell-Haglöw, J. Economic Evaluation of Telemonitoring as a Follow-Up Approach for Patients with Obstructive Sleep Apnea Syndrome Starting Treatment with Continuous Positive Airway Pressure. J. Sleep Res. 2024, 33, e13968. [Google Scholar] [CrossRef]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain Charts for the Human Lifespan. Nature 2022, 604, 525–533. [Google Scholar] [CrossRef]
- Chaput, J.P.; Gariépy, G.; Pendharkar, S.R.; Ayas, N.T.; Samuels, C.; Vallières, A.; Davidson, J.R.; Morin, C.M.; Simonelli, G.; Bourguinat, C.; et al. National Strategy on the Integration of Sleep and Circadian Rhythms into Public Health Research and Policies: Report from the Canadian Sleep and Circadian Network. Sleep Health 2022, 8, 551–563. [Google Scholar] [CrossRef]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef]
- Chee, M.W.; Baumert, M.; Scott, H.; Cellini, N.; Goldstein, C.; Baron, K.; Imtiaz, S.A.; Penzel, T.; Kushida, C.A.; World Sleep Society Sleep Tracker Task Force. World Sleep Society Recommendations for the Use of Wearable Consumer Health Trackers That Monitor Sleep. Sleep Med. 2025, 131, 106506. [Google Scholar] [CrossRef]
- Streatfeild, J.; Smith, J.; Mansfield, D.; Pezzullo, L.; Hillman, D. The Social and Economic Cost of Sleep Disorders. Sleep 2021, 44, zsab132. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, P.; Annett, H.; Cibulskis, R. What Can Information Systems Do for Primary Health Care? An International Perspective. Soc. Sci. Med. 1992, 34, 1077–1087. [Google Scholar] [CrossRef]
- Iannella, G.; Pace, A.; Bellizzi, M.G.; Magliulo, G.; Greco, A.; De Virgilio, A.; Croce, E.; Gioacchini, F.M.; Re, M.; Costantino, A.; et al. The Global Burden of Obstructive Sleep Apnea. Diagnostics 2025, 15, 1088. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.M.; Jahanshad, N.; Ching, C.R.K.; Salminen, L.E.; Thomopoulos, S.I.; Bright, J.; Baune, B.T.; Bertolín, S.; Bralten, J.; Bruin, W.B.; et al. ENIGMA and Global Neuroscience: A Decade of Large-Scale Studies of the Brain in Health and Disease Across More than 40 Countries. Transl. Psychiatry 2020, 10, 100. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappalà, P.; Lentini, M.; Ronsivalle, S.; Lavalle, S.; La Via, L.; Maniaci, A. The Global Socioeconomic Burden of Obstructive Sleep Apnea: A Comprehensive Review. Healthcare 2025, 13, 2115. https://doi.org/10.3390/healthcare13172115
Zappalà P, Lentini M, Ronsivalle S, Lavalle S, La Via L, Maniaci A. The Global Socioeconomic Burden of Obstructive Sleep Apnea: A Comprehensive Review. Healthcare. 2025; 13(17):2115. https://doi.org/10.3390/healthcare13172115
Chicago/Turabian StyleZappalà, Paolo, Mario Lentini, Salvatore Ronsivalle, Salvatore Lavalle, Luigi La Via, and Antonino Maniaci. 2025. "The Global Socioeconomic Burden of Obstructive Sleep Apnea: A Comprehensive Review" Healthcare 13, no. 17: 2115. https://doi.org/10.3390/healthcare13172115
APA StyleZappalà, P., Lentini, M., Ronsivalle, S., Lavalle, S., La Via, L., & Maniaci, A. (2025). The Global Socioeconomic Burden of Obstructive Sleep Apnea: A Comprehensive Review. Healthcare, 13(17), 2115. https://doi.org/10.3390/healthcare13172115








