Effects of a Multidimensional Exercise and Mindfulness Approach Targeting Physical, Psychological, and Functional Outcomes: Protocol for the BACKFIT Randomized Controlled Trial with an Active Control Group
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
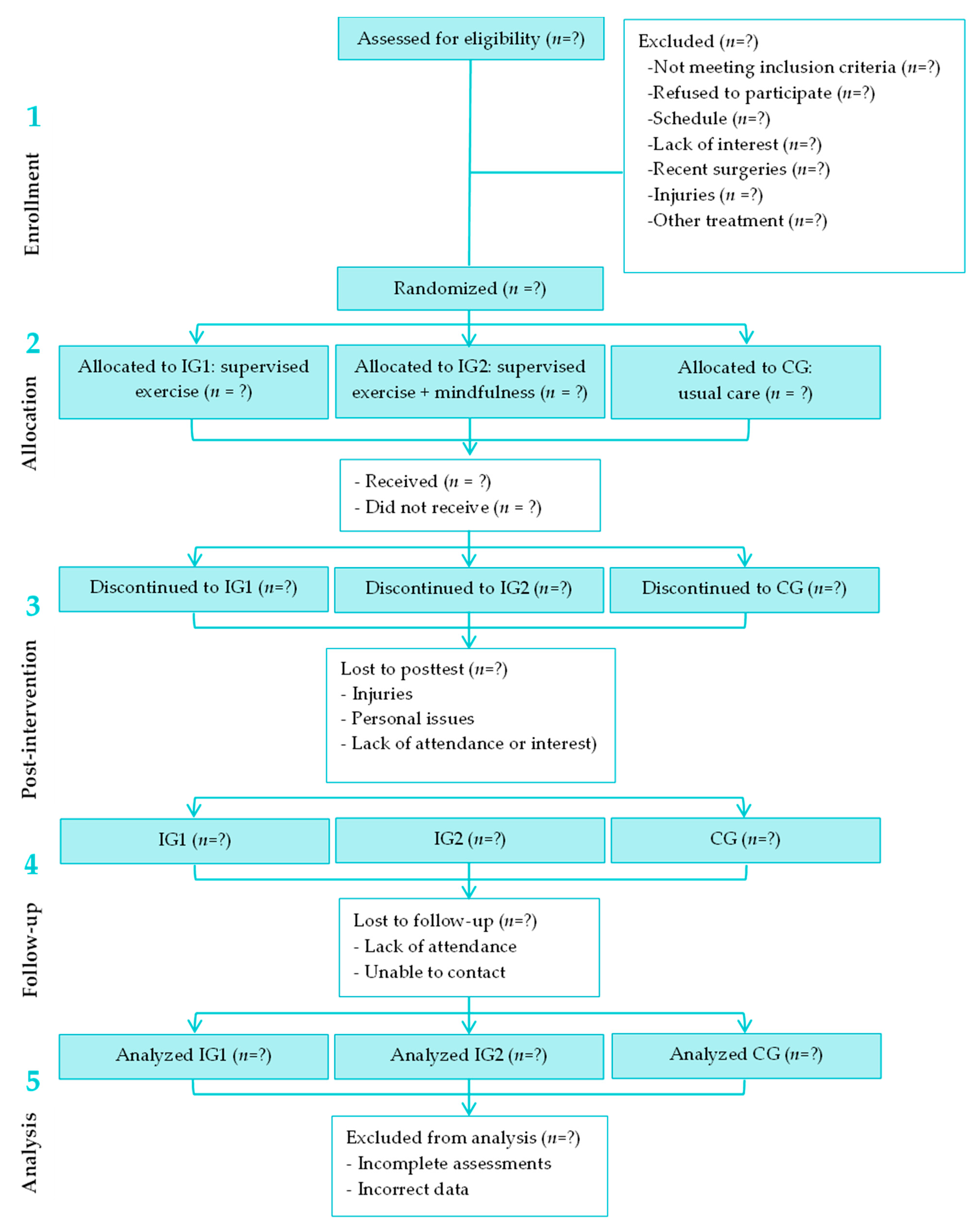
2.3. Recruitment and Randomization
2.4. Intervention
| Session | Content Description | Class Details | Session | Content Description | Class Details |
|---|---|---|---|---|---|
| 1 |
| Introduction session 2.5 h Individual practice Face-to-face Chairs | 2 |
| Introduction session 2.5 h Individual and group practice Face-to-face Chairs, mats |
| 3 |
| 2.5 h Individual and group practice Face-to-face Chairs, mats, notebook | 4 |
| 2.5 h Individual and group practice Face-to-face Chairs, mats, notebook |
| 5 |
| 2.5 h Individual and group practice Face-to-face Chairs, mats | 6 |
| 2.5 h Individual and group practice Face-to-face Chairs, mats |
| 7 |
| 2.5 h Individual and group practice Face-to-face Chairs, mats | 8 |
| 2.5 h Individual and group practice Face-to-face Chairs, mats, notebook |
3. Results
3.1. Primary Outcome Measures
3.1.1. Pain-Related Measures
Pressure Pain Threshold
Perceived Acute Pain
Pain Catastrophizing
Disability Due to Pain
3.2. Secondary Outcome Measures
3.2.1. Body Composition
3.2.2. Muscular Fitness
3.2.3. Motor Agility
3.2.4. Gait Parameters
3.2.5. Device-Measured Physical Activity and Sedentary Behaviour
3.2.6. Self-Reported Sedentary Behavior
3.2.7. Quality of Life and Mental Health
3.2.8. Sleep Quality
3.2.9. Central Sensitization
3.2.10. Rate of Perceived Exertion
4. Statistical Analysis
5. Discussion
5.1. Strengths
5.2. Limitations
5.3. Post-Trial Care and Dissemination Policy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Zundert, J.; Van Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- O’Sullivan, P. Diagnosis and classification of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline for Non-Surgical Management of Chronic Primary Low Back Pain in Adults in Primary and Community Care Settings; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Instituto Nacional de Estadística. Encuesta Europea de Salud en España 2023; Instituto Nacional de Estadística: Madrid, Spain, 2024. [Google Scholar]
- Hussain, S.M.; Urquhart, D.M.; Wang, Y.; Dunstan, D.; Shaw, J.E.; Magliano, D.J.; Wluka, A.E.; Cicuttini, F.M. Associations between television viewing and physical activity and low back pain in community-based adults. Medicine 2016, 95, e3963. [Google Scholar] [CrossRef]
- Dankaerts, W.; O’Sullivan, P.; Burnett, A.; Straker, L. Differences in Sitting Postures are Associated With Nonspecific Chronic Low Back Pain Disorders When Patients Are Subclassified. Spine (Phila Pa 1976) 2006, 31, 698–704. [Google Scholar] [CrossRef]
- Hodges, P.W.; Moseley, G.L. Pain and motor control of the lumbopelvic region: Effect and possible mechanisms. J. Electromyogr. Kinesiol. 2003, 13, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.; Franco, M.R.; Maher, C.G.; Tiedemann, A.; Silva, F.G.; Damato, T.M.; Nicholas, M.K.; Christofaro, D.G.D.; Pinto, R.Z. The efficacy of a multimodal physical activity intervention with supervised exercises, health coaching and an activity monitor on physical activity levels of patients with chronic, nonspecific low back pain (Physical Activity for Back Pain (PAyBACK) trial): Study protocol for a randomised controlled trial. Trials 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.G.; Margaret Grant, P.; Dall, P.M.; Gray, H.; Newton, M.; Granat, M.H. Individuals with chronic low back pain have a lower level, and an altered pattern, of physical activity compared with matched controls: An observational study. Aust. J. Physiother. 2009, 55, 53–58. [Google Scholar] [CrossRef]
- Berger, M.; Bertrand, A.M.; Robert, T.; Chèze, L. Measuring objective physical activity in people with chronic low back pain using accelerometers: A scoping review. Front. Sports Act Living 2023, 5, 1236143. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.-W.C.; Chenot, J.-F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Salman, D.; McGregor, A.H. Recent clinical practice guidelines for the management of low back pain: A global comparison. BMC Musculoskelet. Disord. 2024, 25, 344. [Google Scholar] [CrossRef]
- Matarán-Peñarrocha, G.A.; Lara Palomo, I.C.; Antequera Soler, E.; Gil-Martínez, E.; Fernández-Sánchez, M.; Aguilar-Ferrándiz, M.E.; Castro-Sánchez, A.M. Comparison of efficacy of a supervised versus non-supervised physical therapy exercise program on the pain, functionality and quality of life of patients with non-specific chronic low-back pain: A randomized controlled trial. Clin. Rehabil. 2020, 34, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.G.; Hodges, P.W.; Bostick, G.; Hancock, M.; Laberge, M.; Hanna, S.; Spadoni, G.; Gross, A.; Schneider, J. Which Exercise for Low Back Pain? (WELBack) trial predicting response to exercise treatments for patients with low back pain: A validation randomised controlled trial protocol. BMJ Open 2021, 11, e042792. [Google Scholar] [CrossRef]
- Verville, L.; Ogilvie, R.; Hincapié, C.A.; Southerst, D.; Yu, H.; Bussières, A.; Gross, D.P.; Pereira, P.; Mior, S.; Tricco, A.C.; et al. Systematic Review to Inform a World Health Organization (WHO) Clinical Practice Guideline: Benefits and Harms of Structured Exercise Programs for Chronic Primary Low Back Pain in Adults. J. Occup. Rehabil. 2023, 33, 636–650. [Google Scholar] [CrossRef]
- Li, Y.; Yan, L.; Hou, L.; Zhang, X.; Zhao, H.; Yan, C.; Li, X.; Li, Y.; Chen, X.; Ding, X. Exercise intervention for patients with chronic low back pain: A systematic review and network meta-analysis. Front. Public Health 2023, 11, 1155225. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Álvarez-Bueno, C.; Cavero-Redondo, I.; Torres-Costoso, A.; Pozuelo-Carrascosa, D.P.; Reina-Gutiérrez, S.; Pascual-Morena, C.; Martínez-Vizcaíno, V. Best Exercise Options for Reducing Pain and Disability in Adults With Chronic Low Back Pain: Pilates, Strength, Core-Based, and Mind-Body. A Network Meta-analysis. J. Orthop. Sports Phys. Ther. 2022, 52, 505–521. [Google Scholar] [CrossRef]
- Prat-Luri, A.; de los Rios-Calonge, J.; Moreno-Navarro, P.; Manresa-Rocamora, A.; Vera-Garcia, F.J.; Barbado, D. Effect of Trunk-Focused Exercises on Pain, Disability, Quality of Life, and Trunk Physical Fitness in Low Back Pain and How Potential Effect Modifiers Modulate Their Effects: A Systematic Review With Meta-analyses. J. Orthop. Sports Phys. Ther. 2023, 53, 64–93. [Google Scholar] [CrossRef]
- Owen, P.J.; Miller, C.T.; Mundell, N.L.; Verswijveren, S.J.J.M.; Tagliaferri, S.D.; Brisby, H.; Bowe, S.J.; Belavy, D.L. Infographic. What kinds of exercise are best for chronic low back pain? Br. J. Sports Med. 2020, 54, 1231–1232. [Google Scholar] [CrossRef]
- Deodato, M.; Saponaro, S.; Šimunič, B.; Martini, M.; Murena, L.; Buoite Stella, A. Trunk muscles’ characteristics in adolescent gymnasts with low back pain: A pilot study on the effects of a physiotherapy intervention including a postural reeducation program. J. Man. Manip. Ther. 2024, 32, 310–324. [Google Scholar] [CrossRef]
- Smith, S.; Langen, W. A systematic review of mindfulness practices for improving outcomes in chronic low back pain. Int. J. Yoga 2020, 13, 177. [Google Scholar] [CrossRef]
- Adler-Neal, A.L.; Zeidan, F. Mindfulness Meditation for Fibromyalgia: Mechanistic and Clinical Considerations. Curr. Rheumatol. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Gard, T.; Holzel, B.K.; Sack, A.T.; Hempel, H.; Lazar, S.W.; Vaitl, D.; Ott, U. Pain Attenuation through Mindfulness is Associated with Decreased Cognitive Control and Increased Sensory Processing in the Brain. Cereb. Cortex 2012, 22, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Palao-Tarrero, Á.; Torrijos-Zarcero, M.; Del Río, M.; Muñoz-Sanjosé, A.; Rodríguez-Vega, B. Intervenciones Basadas en Mindfulness y Compasión en Dolor Crónico. Rev. Investig. Educ. En Cienc. Salud 2019, 4, 112–122. [Google Scholar]
- Day, M.A.; Ciol, M.A.; Mendoza, M.E.; Borckardt, J.; Ehde, D.M.; Newman, A.K.; Chan, J.F.; Drever, S.A.; Friedly, J.L.; Burns, J.; et al. The effects of telehealth-delivered mindfulness meditation, cognitive therapy, and behavioral activation for chronic low back pain: A randomized clinical trial. BMC Med. 2024, 22, 156. [Google Scholar] [CrossRef]
- Paschali, M.; Lazaridou, A.; Sadora, J.; Papianou, L.; Garland, E.L.; Zgierska, A.E.; Edwards, R.R. Mindfulness-based Interventions for Chronic Low Back Pain. Clin. J. Pain 2024, 40, 105–113. [Google Scholar] [CrossRef]
- Flynn, D. Chronic Musculoskeletal Pain: Nonpharmacologic, Noninvasive Treatments. Am. Fam. Physician 2020, 102, 465–477. [Google Scholar]
- Serrat, M.; Sanabria-Mazo, J.P.; Almirall, M.; Musté, M.; Feliu-Soler, A.; Méndez-Ulrich, J.L.; Sanz, A.; Luciano, J.V. Effectiveness of a Multicomponent Treatment Based on Pain Neuroscience Education, Therapeutic Exercise, Cognitive Behavioral Therapy, and Mindfulness in Patients With Fibromyalgia (FIBROWALK Study): A Randomized Controlled Trial. Phys. Ther. 2021, 101, pzab200. [Google Scholar] [CrossRef] [PubMed]
- In, T.-S.; Jung, J.-H.; Jung, K.-S.; Cho, H.-Y. Effects of the Multidimensional Treatment on Pain, Disability, and Sitting Posture in Patients with Low Back Pain: A Randomized Controlled Trial. Pain Res. Manag. 2021, 2021, 5581491. [Google Scholar] [CrossRef]
- Polaski, A.M.; Phelps, A.L.; Smith, T.J.; Helm, E.R.; Morone, N.E.; Szucs, K.A.; Kostek, M.C.; Kolber, B.J. Integrated Meditation and Exercise Therapy: A Randomized Controlled Pilot of a Combined Nonpharmacological Intervention Focused on Reducing Disability and Pain in Patients with Chronic Low Back Pain. Pain Med. 2021, 22, 444–458. [Google Scholar] [CrossRef]
- Deegan, O.; Fullen, B.M.; Casey, M.-B.; Segurado, R.; Hearty, C.; Doody, C. Mindfulness Combined With Exercise Online (MOVE) Compared With a Self-management Guide for Adults With Chronic Pain. Clin. J. Pain 2023, 39, 394–407. [Google Scholar] [CrossRef]
- McGill, S. Back Mechanic: The Secrets to a Healthy Spine Your Doctor Isn’t Telling You; Backfitpro Inc.: Waterloo, ON, Canada, 2015. [Google Scholar]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef]
- Baechle, T.; Earle, R. Essentials of Strength Training and Conditioning, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Tripepi, G.; Chesnaye, N.C.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Intention to treat and per protocol analysis in clinical trials. Nephrology 2020, 25, 513–517. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.E.M.; Ostelo, R.W.J.G.; Guzman, J.; van Tulder, M.W. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015, 350, h444. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F. Cuestionario de Aptitud para la Actividad Física (C-AAF), versión catalana/castellana del PAR-Q revisado. Apunts 1994, 31, 301–310. [Google Scholar]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical Activity and Public Health. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical Activity and Public Health in Older Adults. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef]
- McGill, S. El Mecánico de la Espalda; Editores de Argentina: Buenos Aires, Argentina, 2015. [Google Scholar]
- Grossman, P.; Niemann, L.; Schmidt, S.; Walach, H. Mindfulness-based stress reduction and health benefits. J. Psychosom. Res. 2004, 57, 35–43. [Google Scholar] [CrossRef]
- Mariño-Narvaez, C.; Romero-Gonzalez, B.; Puertas-Gonzalez, J.A.; Peralta-Ramírez, M.I.; Castellote-Caballero, Y. Mindfulness-Based Stress Reduction Program for reducing anxiety and depression in hospital staff during a pandemic: A randomized controlled trial. J. Psychiatr. Res. 2025, 181, 320–329. [Google Scholar] [CrossRef]
- Aoyagi, K.; He, J.; Nicol, A.L.; Clauw, D.J.; Kluding, P.M.; Jernigan, S.; Sharma, N.K. A Subgroup of Chronic Low Back Pain Patients With Central Sensitization. Clin. J. Pain 2019, 35, 869–879. [Google Scholar] [CrossRef]
- Christensen, K.S.; O’Sullivan, K.; Palsson, T.S. Conditioned Pain Modulation Efficiency Is Associated With Pain Catastrophizing in Patients With Chronic Low Back Pain. Clin. J. Pain 2020, 36, 825–832. [Google Scholar] [CrossRef]
- Fagundes Loss, J.; de Souza da Silva, L.; Ferreira Miranda, I.; Groisman, S.; Santiago Wagner Neto, E.; Souza, C.; Tarragô Candotti, C. Immediate effects of a lumbar spine manipulation on pain sensitivity and postural control in individuals with nonspecific low back pain: A randomized controlled trial. Chiropr. Man. Ther. 2020, 28, 25. [Google Scholar] [CrossRef]
- Kong, J.-T.; You, D.S.; Law, C.S.W.; Darnall, B.D.; Gross, J.J.; Manber, R.; Mackey, S. Association between temporal summation and conditioned pain modulation in chronic low back pain: Baseline results from 2 clinical trials. Pain Rep. 2021, 6, e975. [Google Scholar] [CrossRef]
- Kennedy, D.L.; Kemp, H.I.; Ridout, D.; Yarnitsky, D.; Rice, A.S.C. Reliability of conditioned pain modulation: A systematic review. Pain 2016, 157, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, B.S.; Shechtman, O.; Tuckey, L. Validity, Reliability, and Responsiveness of a Digital Version of the Visual Analog Scale. J. Hand Ther. 2011, 24, 356–364. [Google Scholar] [CrossRef]
- Alghadir, A.; Anwer, S.; Iqbal, A.; Iqbal, Z. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de la versión española de la escala de la catastrofización ante el dolor (Pain Catastrophizing Scale) en la fibromialgia. Med. Clin. (Barc.) 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Alcántara-Bumbiedro, S.; Flórez-García, M.T.; Echávarri-Pérez, C.; García-Pérez, F. Escala de incapacidad por dolor lumbar de Oswestry. Rehabilitacion (Madr) 2006, 40, 150–158. [Google Scholar] [CrossRef]
- Lim, J.S.; Hwang, J.S.; Lee, J.A.; Kim, D.H.; Park, K.D.; Jeong, J.S.; Cheon, G.J. Cross-calibration of multi-frequency bioelectrical impedance analysis with eight-point tactile electrodes and dual-energy X-ray absorptiometry for assessment of body composition in healthy children aged 6–18 years. Pediatr. Int. 2009, 51, 263–268. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Kroll, C.; Mastroeni, S.S.B.S.; Czarnobay, S.A.; Ekwaru, J.P.; Veugelers, P.J.; Mastroeni, M.F. The accuracy of neck circumference for assessing overweight and obesity: A systematic review and meta-analysis. Ann. Hum. Biol. 2017, 44, 667–677. [Google Scholar] [CrossRef]
- Biering-Sørensen, F. Physical Measurements as Risk Indicators for Low-Back Trouble Over a One-Year Period. Spine (Phila Pa 1976) 1984, 9, 106–119. [Google Scholar] [CrossRef]
- Kim, B.; Yim, J. Core Stability and Hip Exercises Improve Physical Function and Activity in Patients with Non-Specific Low Back Pain: A Randomized Controlled Trial. Tohoku J. Exp. Med. 2020, 251, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Nah, S.; Jang, H.; Moon, J.; Han, S. Association between Relative Handgrip Strength and Chronic Lower Back Pain: A Nationwide Cross-Sectional Analysis of the Korea National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public. Health 2021, 18, 10770. [Google Scholar] [CrossRef]
- Schellenberg, K.L.; Lang, J.M.; Chan, K.M.; Burnham, R.S. A Clinical Tool for Office Assessment of Lumbar Spine Stabilization Endurance. Am. J. Phys. Med. Rehabil. 2007, 86, 380–386. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Cruz, B.; Mocholi, M.H.; del Pozo-Cruz, J.; Parraca, J.A.; Adsuar, J.C.; Gusi, N. Reliability and validity of lumbar and abdominal trunk muscle endurance tests in office workers with nonspecific subacute low back pain. J. Back Musculoskelet. Rehabil. 2014, 27, 399–408. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Bohannon, R.W. Test-Retest Reliability of Measurements of Hand-Grip Strength Obtained by Dynamometry from Older Adults: A Systematic Review of Research in the PubMed Database. J. Frailty Aging 2017, 6, 83–87. [Google Scholar] [CrossRef]
- Jaén-Carrillo, D.; García-Pinillos, F.; Cartón-Llorente, A.; Almenar-Arasanz, A.J.; Bustillo-Pelayo, J.A.; Roche-Seruendo, L.E. Test–retest reliability of the OptoGait system for the analysis of spatiotemporal running gait parameters and lower body stiffness in healthy adults. Proc. Inst. Mech. Eng. P J. Sport. Eng. Technol. 2020, 234, 154–161. [Google Scholar] [CrossRef]
- Weart, A.N.; Miller, E.M.; Freisinger, G.M.; Johnson, M.R.; Goss, D.L. Agreement Between the OptoGait and Instrumented Treadmill System for the Quantification of Spatiotemporal Treadmill Running Parameters. Front. Sports Act. Living 2020, 2, 571385. [Google Scholar] [CrossRef]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Montoye, A.H.K.; Clevenger, K.A.; Pfeiffer, K.A.; Nelson, M.B.; Bock, J.M.; Imboden, M.T.; Kaminsky, L.A. Development of cut-points for determining activity intensity from a wrist-worn ActiGraph accelerometer in free-living adults. J. Sports Sci. 2020, 38, 2569–2578. [Google Scholar] [CrossRef]
- Doherty, A.; Jackson, D.; Hammerla, N.; Plötz, T.; Olivier, P.; Granat, M.H.; White, T.; van Hees, V.T.; Trenell, M.I.; Owen, C.G.; et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS ONE 2017, 12, e0169649. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of Accelerometer Wear and Nonwear Time Classification Algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Bammann, K.; Thomson, N.K.; Albrecht, B.M.; Buchan, D.S.; Easton, C. Generation and validation of ActiGraph GT3X+ accelerometer cut-points for assessing physical activity intensity in older adults. The OUTDOOR ACTIVE validation study. PLoS ONE 2021, 16, e0252615. [Google Scholar] [CrossRef]
- Munguia-Izquierdo, D.; Segura-Jiménez, V.; Camiletti-Moirón, D.; Alvarez-Gallardo, I.C.; Estévez-López, F.; Romero, A.; Chillon, P.; Carbonell-Baeza, A.; Ortega, F.B.; Ruiz, J.R.; et al. Spanish adaptation and psychometric properties of the Sedentary Behaviour Questionnaire for fibromyalgia patients: The al-Andalus study. Clin. Exp. Rheumatol. 2013, 31, S22–S33. [Google Scholar]
- Alonso, J.; Prieto, L.; Antó, J.M. La versión española del SF-36 Health Survey (Cuestionario de Salud SF-36): Un instrumento para la medida de los resultados clínicos [The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): An instrument for measuring clinical results]. Med. Clin. (Barc.) 1995, 104, 771–776. [Google Scholar]
- Beck, A.T. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory. In The Corsini Encyclopedia of Psychology; Wiley: Hoboken, NJ, USA, 2010; p. 1. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Bigatti, S.M.; Hernandez, A.M.; Cronan, T.A.; Rand, K.L. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Care Res. 2008, 59, 961–967. [Google Scholar] [CrossRef]
- Neblett, R.; Hartzell, M.M.; Mayer, T.G.; Cohen, H.; Gatchel, R.J. Establishing Clinically Relevant Severity Levels for the Central Sensitization Inventory. Pain Pract. 2017, 17, 166–175. [Google Scholar] [CrossRef]
- Scerbo, T.; Colasurdo, J.; Dunn, S.; Unger, J.; Nijs, J.; Cook, C. Measurement Properties of the Central Sensitization Inventory: A Systematic Review. Pain Pract. 2018, 18, 544–554. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand. J. Work. Environ. Health 1990, 16, 55–58. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Ruiz, J.R.; Álvarez-Gallardo, I.C.; Segura-Jiménez, V.; Santalla, A.; Munguía-Izquierdo, D. Validity and reliability of rating perceived exertion in women with fibromyalgia: Exertion-pain discrimination. J. Sports Sci. 2015, 33, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, F.; Vago, D.R. Mindfulness meditation–based pain relief: A mechanistic account. Ann. N. Y Acad. Sci. 2016, 1373, 114–127. [Google Scholar] [CrossRef]
- Dubois, J.-D.; Piché, M.; Cantin, V.; Descarreaux, M. Effect of experimental low back pain on neuromuscular control of the trunk in healthy volunteers and patients with chronic low back pain. J. Electromyogr. Kinesiol. 2011, 21, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Niederer, D. Dose-response-relationship of stabilisation exercises in patients with chronic non-specific low back pain: A systematic review with meta-regression. Sci. Rep. 2020, 10, 16921. [Google Scholar] [CrossRef] [PubMed]
- Remskar, M.; Western, M.J.; Osborne, E.L.; Maynard, O.M.; Ainsworth, B. Effects of combining physical activity with mindfulness on mental health and wellbeing: Systematic review of complex interventions. Ment. Health Phys. Act. 2024, 26, 100575. [Google Scholar] [CrossRef]
- Payne, J.E.; Chambers, R.; Liknaitzky, P. Combining Psychedelic and Mindfulness Interventions: Synergies to Inform Clinical Practice. ACS Pharmacol. Transl. Sci. 2021, 4, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Tucker, R.P.; Greene, P.A.; Davidson, R.J.; Wampold, B.E.; Kearney, D.J.; Simpson, T.L. Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clin. Psychol. Rev. 2018, 59, 52–60. [Google Scholar] [CrossRef]
- Banth, S.; Ardebil, M. Effectiveness of mindfulness meditation on pain and quality of life of patients with chronic low back pain. Int. J. Yoga 2015, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Reiner, K.; Tibi, L.; Lipsitz, J.D. Do Mindfulness-Based Interventions Reduce Pain Intensity? A Critical Review of the Literature. Pain Med. 2013, 14, 230–242. [Google Scholar] [CrossRef] [PubMed]
| Enrolment | Baseline | Allocation | Intervention | After Intervention | Follow-Up | |
|---|---|---|---|---|---|---|
| Timepoint | −t1 | t0 | 0 | 0 | t1 | t2 |
| Enrolment | ||||||
| Preliminary contact | X | |||||
| Informative session | X | |||||
| Informed consent | X | |||||
| Randomization | X | |||||
| Allocation | X | |||||
| Interventions | ||||||
| Supervised exercise | X | |||||
| Supervised exercise + mindfulness | X | |||||
| Control group | X | |||||
| Assessments | ||||||
| Clinical information survey | X | X | ||||
| C-AAF | X | |||||
| Blood pressure | X | X | X | |||
| Resting heart rate | X | X | X | |||
| Body composition | X | X | X | |||
| Pain related measures | ||||||
| Pain threshold (algometry) | X | X | X | |||
| Perceived acute pain (VAS, NRS) | X | X | X | X | ||
| Pain catastrophizing (PCS) | X | X | X | |||
| Disability due to pain (ODI) | X | X | X | |||
| Central Sensitization (CSI) | X | X | X | |||
| Physical fitness tests | X | X | X | |||
| Biering–Sørensen | X | X | X | |||
| Prone Bridging | X | X | X | |||
| 30-sec chair stand | X | X | X | |||
| Hand dynamometry | X | X | X | |||
| 8-foot up-and-go | X | X | X | |||
| Gait parameters | X | X | X | |||
| Accelerometry | X | X | X | |||
| Self-reported sedentary behavior (SBQ-S) | X | X | X | |||
| Quality of life and mental health | ||||||
| Health-related quality of life (SF-36) | X | X | X | |||
| Depression (BDI-II) | X | X | X | |||
| Anxiety (STAI) | X | X | X | |||
| Sleep quality (PSQI) | X | X | X | |||
| Rate of perceived exertion (RPE) | X | X | X | X |
| Inclusion Criteria |
|
|
|
|
|
| Exclusion Criteria |
|
|
|
|
|
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | |
| Session 1/2 | Session 3/4 | Session 5/6 | Session 7/8 | Session 9/10 | Session 11/12 | Session 13/14 | Session 15/16 | |
| Equipment | Mats | Mats, Resistance Bands | Mats, 2-kg Dumbbells | Mats, Foam Rollers, Stability Balls and 2-kg Dumbbells | ||||
| IG1 | 5′ warm-up | 5′ warm-up | 5′ warm-up | 5′ warm-up | ||||
| 35′ mobility, isometric and motor control exercises | 35′ co-contraction exercises | 35′ functional exercises with load | 35′ functional exercises in unstable surface | |||||
| 5′ cool-down | 5′ cool-down | 5′ cool-down | 5′ cool-down | |||||
| IG2 | 5′ warm-up | 5′ warm-up | 5′ warm-up | 5′ warm-up | ||||
| 35′ mobility, isometric and motor control exercises | 35′ co-contractions exercises | 35′ functional exercises with load | 35′ functional exercises on unstable surface | |||||
| 5′ cool-down | 5′ cool-down | 5′ cool-down | 5′ cool-down | |||||
| +2.5-h weekly mindfulness session | ||||||||
| Sections: topic presentation, group dialogue, exploration and mindfulness practice | ||||||||
| CG | 45′ of stretching, breathing and motor control exercises | |||||||
| Warm up: postural awareness, diaphragmatic and costal breathing, transversus abdominis contraction | ||||||||
| Main activities: inferior and superior abdominal, hip abduction with knee extension, cat-camel and bird-dog exercises | ||||||||
| Stretching: lumbosacral, psoas, hamstring and pyramidal muscle stretching in sitting and supine position | ||||||||
| PHASE 1 (≥5 RPE) | |||||||||||||
| Warm-up | 1. Lumbo-pelvic movement with breathing control; 2. Transversus abdominis activation; 3. Plank on the wall; 4. Hip rotation; 5. Floor slides with foam roller decubitus and lateral position) | ||||||||||||
| Main part | 1st session | 2nd session | 3rd session | ||||||||||
| N | D | E | D | E | D | E | |||||||
| 1 | Good morning | Mat | The lunge | Mat | Static wall squat | Mat | |||||||
| 2 | Lumbo-pelvic movement in sitting position | Mat | Tie your shoelaces | Mat | Good morning | Mat | |||||||
| 3 | Left lifting progression | Mat | Adapted crunch | Mat | Monster Walk initiation | Mat | |||||||
| 4 | The cat | Mat | Adapted hollow rock | Mat | Bird dog initiation | Mat | |||||||
| 5 | Sun salute | Mat | Open yourself “like a book” | Mat | Adapted crunch | Mat | |||||||
| 6 | Lateral greeting | Mat | Lying on your side | Mat | Reversed bird dog | Mat | |||||||
| 7 | Bird dog initiation | Mat | Modified side plank | Mat | Glute bridge | Mat | |||||||
| 8 | - | - | - | - | Modified side plank | Mat | |||||||
| Cool-down | Stretching of the dorsal, abdominal, hamstrings, oblique and psoas muscles | ||||||||||||
| PHASE 2 (≥5 RPE) | |||||||||||||
| Warm-up | 1. Lumbo-pelvic movement with breathing control; 2. Transversus abdominis activation; 3. Plank on the wall; 4. Hip rotation; 5. Floor slides with foam roller decubitus and lateral position | ||||||||||||
| Main part | 1st session | 2nd session | 3rd session | 4th session | |||||||||
| D | E | D | E | D | E | D | E | ||||||
| 1 | Stading kick back | - | Deep breathing | Mat | Upper body mobility | Foam roller | Window washing at the wall | - | |||||
| 2 | Adapted flamingo | - | Glute bridge | Mat | Lunge on the floor | Mat | Draw crosses at the wall | - | |||||
| 3 | Adapted bird dog | - | Prone plank with leg raises | Mat | Lateral greeting | Mat | Clamshell | Resistance band | |||||
| 4 | Hedgehog | Stability ball | Bird dog initiation | Mat | Arms and legs coordination | Mat | Leg lifts | Mat | |||||
| 5 | Shoulder movement | Mat | Side plank rotations | Mat | Hypopressives in supine position | Mat | Leg opening | Mat | |||||
| 6 | Lying on the beach | Mat | Wall climb | - | Unilateral leg lifts | Mat | Bicycle | Mat | |||||
| 7 | Child pose | Mat | Catching object | - | Clamshell | Mat | Side Lying Hip Adduction | Mat | |||||
| 8 | Side hug | Mat | Lying crab | - | Horse kick | Mat | Triceps extension | Chair | |||||
| Cool-down | Stretching of the dorsal, abdominal, hamstrings, oblique and psoas muscles | ||||||||||||
| PHASE 3 (≥6 RPE) | |||||||||||||
| Warm-up | 1. Lumbo-pelvic movement with breathing control; 2. Transversus abdominis activation; 3. Plank on the wall; 4. Lateral greeting; 5. Bird-dog; 6. Adapted frog pose; 7. Floor slides with foam roller (decubitus and lateral position) | ||||||||||||
| Main part | 1st session | 2nd session | 3rd session | 4th session | |||||||||
| N | D | E | D | E | D | E | D | E | |||||
| 1 | Activation of the spinal, hamstring and abdominal muscles | Mat, dumbbell | Activation of the spinal, hamstring and abdominal muscles | Mat, dumbbell | Dumbbell plank pull through (sliding) | Mat, dumbbell | Reverse crunch | Stick | |||||
| 2 | Glute bridge | Mat, dumbbell | Infinity | Dumbbell | Infinity | Dumbbell | Glute bridge | Mat, dumbbell | |||||
| 3 | Bird-dog | Mat, dumbbell | Stride | Dumbbell | Shoulder flexion | Dumbbell | Adapted shoulder tap | Mat | |||||
| 4 | Squat | Dumbbell | Squat with shoulder flexion | Dumbbell | Squat | Resistance band | Crawling | - | |||||
| 5 | Squats with shoulder flexion | Dumbbell | Dead bug | Mat, dumbbell | Starfish | Mat | Squat | Dumbbell | |||||
| 6 | Shoulder flexions | Dumbbell | Glute bridge | Mat, dumbbell | Activation of the spinal, hamstring and abdominal muscles | Mat | Shoulder and hip mobility | Mat | |||||
| 7 | Dumbbell one | Dumbbell | Window washing with feet | Mat | Dumbbell squats | Dumbbell | Dumbbell squats | Dumbbell | |||||
| 8 | Half squat | Resistance band | Swing | Mat, dumbbell | Adapted stair climbing | Mat | Adapted stair climbing | Mat | |||||
| Cool-down | 1.Stretching of the dorsal, abdominal, hamstrings, oblique and psoas muscles | ||||||||||||
| PHASE 4 (≥6 RPE) | |||||||||||||
| Warm-up | 1. Lumbo-pelvic movement with breathing control; 2. Transversus abdominis activation; 3. Adapted skipping; 4. Adapted Jumping Jack; 5. Plank on the floor; 6. Adapted mountain climber at the wall; 7. Floor slides with foam roller (decubitus and lateral position) | ||||||||||||
| Main part | 1st session | 2nd session | 3rd session | 4th session | 5th session | ||||||||
| N | D | E | D | E | D | E | D | E | D | E | |||
| 1 | Mountain climber | Mat | Side steps | Mat | Bird-dog | Stability ball | Planks | Stability ball | Crunches | Stability ball | |||
| 2 | Single-leg balance with arms extended | Mat | Standing lateral crunches | Mat | Mountain climber | Mat | Raising legs | Stability ball | Bird-dog | Stability ball | |||
| 3 | Balance drill | Mat | Bulgarian squats | Stability ball | Standing lateral crunches | Mat | Hip thrust | Stability ball | Adapted shoulder tap | Balance trainer | |||
| 4 | Raising legs | Stability ball | Raising legs | Stability ball | Side steps with overhead press | Balance trainer, dumbbell | Bird-dog | Stability ball | Single-leg balance with arms extended | Mat | |||
| 5 | Hip anteversion and retroversion | Stability ball | Bird-dog | Stability ball | Raising legs | Stability ball | Adapted skipping | Balance trainer | Standing lateral crunches | Mat | |||
| 6 | Initiation to skipping | Balance trainer | Side steps | Balance trainer | Squats | Balance trainer | Bulgarian squats | Balance trainer | Side steps with overhead press | Balance trainer, dumbbell | |||
| 7 | Squats | Balance trainer | Plank leg raises | Balance trainer | Adapted skipping | Balance trainer | Squats | Balance trainer | Squats | Balance trainer | |||
| Cool-down | Stretching of the dorsal, abdominal, hamstrings, oblique and psoas muscles | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoso, B.; Tsiarleston, G.; Castellote-Caballero, Y.; Villegas-Fuentes, A.; Gil-Gutiérrez, Y.M.; Fernández-Álvarez, J.E.; Montes, S.; Delgado-Fernández, M.; Mesa-Ruíz, A.M.; Molina-García, P.; et al. Effects of a Multidimensional Exercise and Mindfulness Approach Targeting Physical, Psychological, and Functional Outcomes: Protocol for the BACKFIT Randomized Controlled Trial with an Active Control Group. Healthcare 2025, 13, 2065. https://doi.org/10.3390/healthcare13162065
Donoso B, Tsiarleston G, Castellote-Caballero Y, Villegas-Fuentes A, Gil-Gutiérrez YM, Fernández-Álvarez JE, Montes S, Delgado-Fernández M, Mesa-Ruíz AM, Molina-García P, et al. Effects of a Multidimensional Exercise and Mindfulness Approach Targeting Physical, Psychological, and Functional Outcomes: Protocol for the BACKFIT Randomized Controlled Trial with an Active Control Group. Healthcare. 2025; 13(16):2065. https://doi.org/10.3390/healthcare13162065
Chicago/Turabian StyleDonoso, Belén, Gavriella Tsiarleston, Yolanda Castellote-Caballero, Alba Villegas-Fuentes, Yolanda María Gil-Gutiérrez, José Enrique Fernández-Álvarez, Santiago Montes, Manuel Delgado-Fernández, Antonio Manuel Mesa-Ruíz, Pablo Molina-García, and et al. 2025. "Effects of a Multidimensional Exercise and Mindfulness Approach Targeting Physical, Psychological, and Functional Outcomes: Protocol for the BACKFIT Randomized Controlled Trial with an Active Control Group" Healthcare 13, no. 16: 2065. https://doi.org/10.3390/healthcare13162065
APA StyleDonoso, B., Tsiarleston, G., Castellote-Caballero, Y., Villegas-Fuentes, A., Gil-Gutiérrez, Y. M., Fernández-Álvarez, J. E., Montes, S., Delgado-Fernández, M., Mesa-Ruíz, A. M., Molina-García, P., Pozuelo-Calvo, R., Membrilla-Mesa, M. D., & Segura-Jiménez, V. (2025). Effects of a Multidimensional Exercise and Mindfulness Approach Targeting Physical, Psychological, and Functional Outcomes: Protocol for the BACKFIT Randomized Controlled Trial with an Active Control Group. Healthcare, 13(16), 2065. https://doi.org/10.3390/healthcare13162065





