Therapeutic Plasma Exchange in Acute Liver Failure: A Real-World Study in Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Eligibility
2.3. Intervention
2.4. Clinical and Biochemical Evaluations
2.5. Statistical Analysis
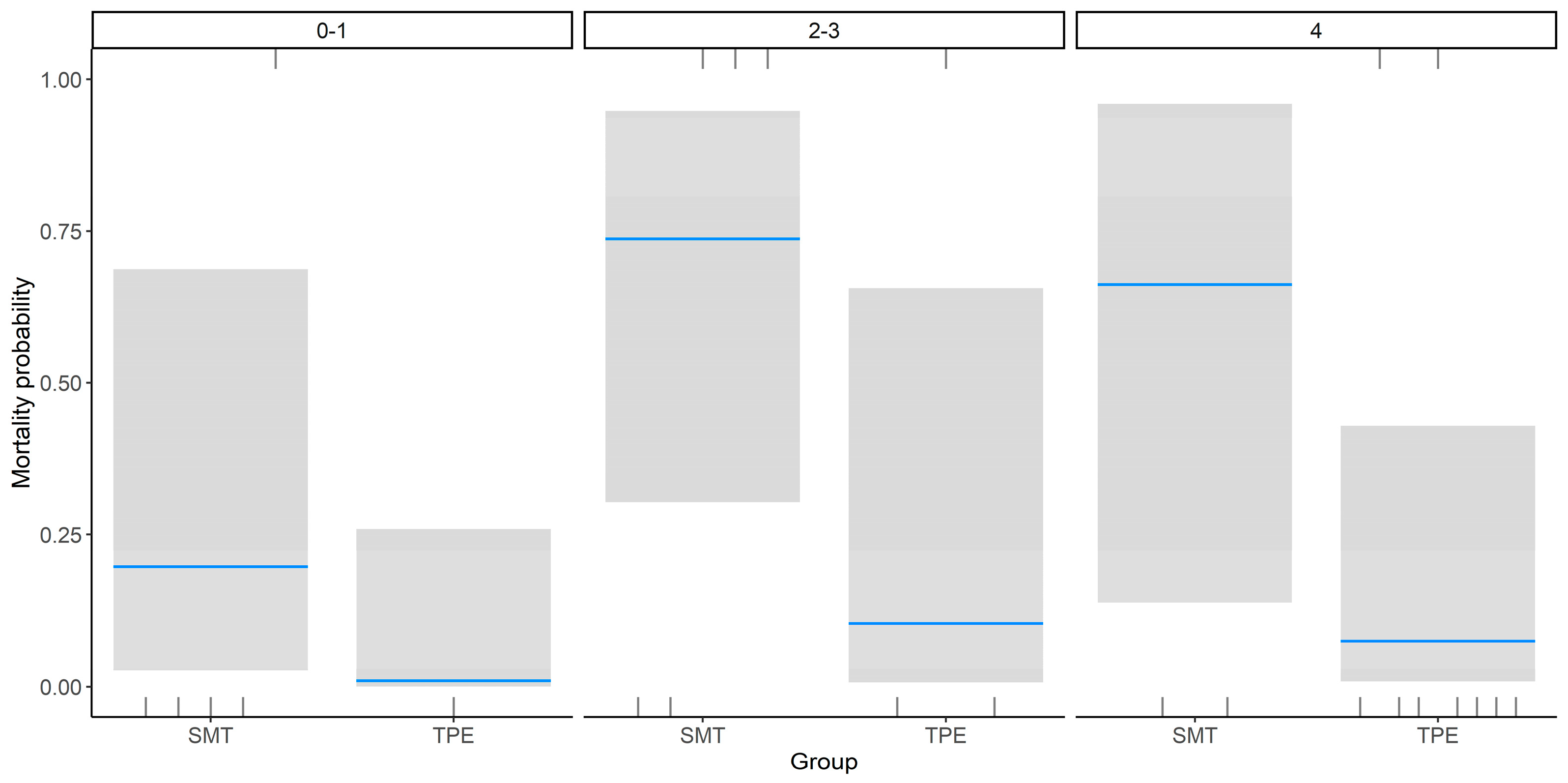
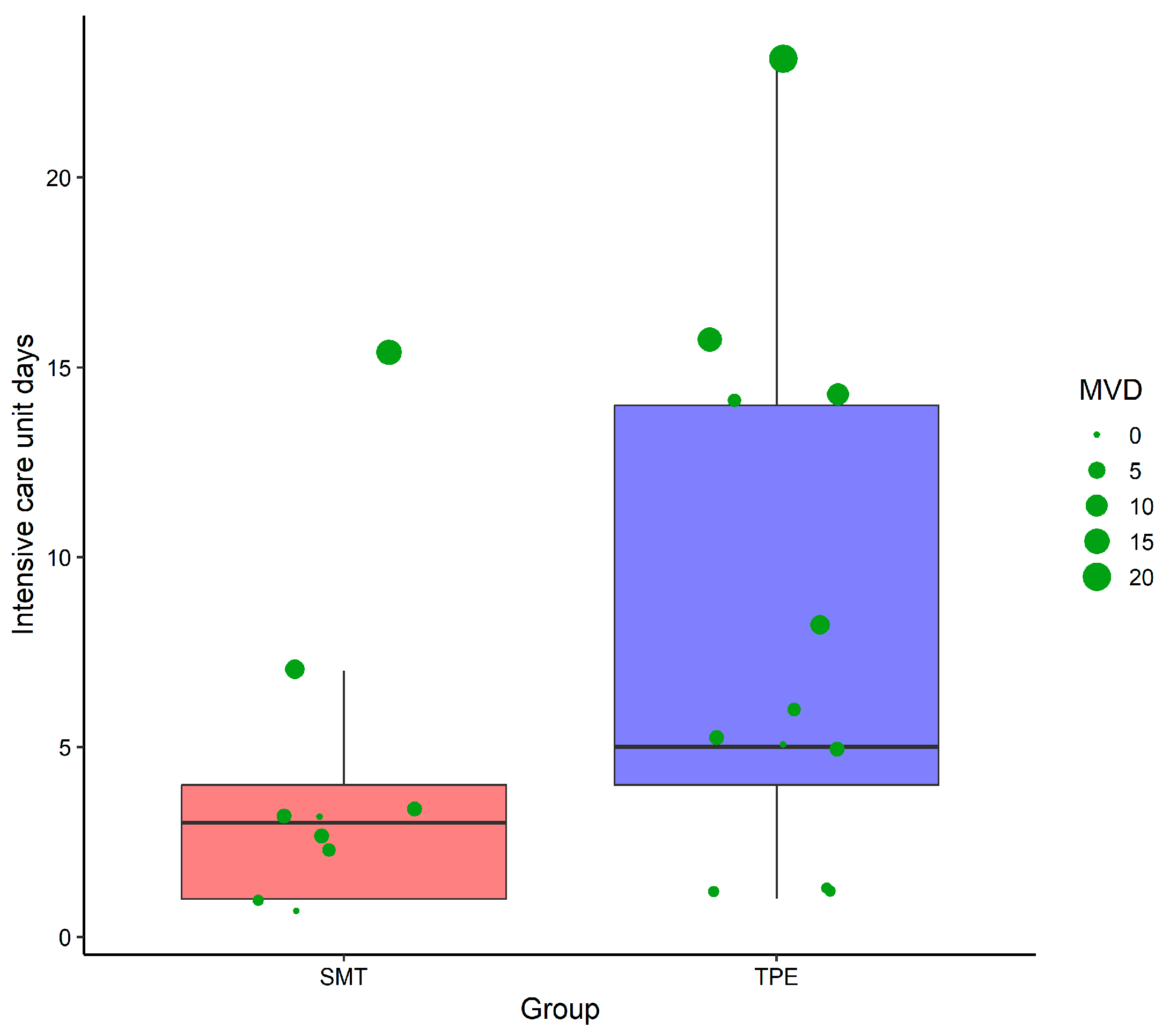
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALF | Acute Liver Failure |
| TPE | Therapeutic Plasma Exchange |
| SMT | Standard Medical Treatment |
| ICU | Intensive Care Unit |
| HV-TPE | High-Volume Therapeutic Plasma Exchange |
| SV-TPE | Standard-Volume Therapeutic Plasma Exchange |
| DILI | Drug-Induced Liver Injury |
| ALL | Acute Lymphoblastic Leukemia |
| AFLP | Acute Fatty Liver of Pregnancy |
| INR | International Normalized Ratio |
| PT | Prothrombin Time |
| ASAT | Aspartate Aminotransferase |
| ALAT | Alanine Aminotransferase |
| SOFA | Sequential Organ Failure Assessment |
| MELD | Model for End-Stage Liver Disease |
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| IgM | Immunoglobulin M |
| HIV | Human Immunodeficiency Virus |
| TBV | Total Blood Volume |
| TPV | Total Plasma Volume |
| DHL | Lactate Dehydrogenase |
| TGO | Transaminase Glutamic Oxaloacetic (AST/ASAT) |
| TGP | Transaminase Glutamic Pyruvic (ALT/ALAT) |
| MVDs | Mechanical Ventilation Days |
| R | Statistical Programming Language used for analysis |
References
- Shingina, A.; Mukhtar, N.; Wakim-Fleming, J.; Alqahtani, S.; Wong, R.J.; Limketkai, B.N.; Larson, A.M.; Grant, L. Acute Liver Failure Guidelines. Am. J. Gastroenterol. 2023, 118, 1128–1153. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J. Causes, Clinical Features, and Outcomes from a Prospective Study of Drug-Induced Liver Injury in the United States. Gastroenterology 2008, 135, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Schiodt, F.V.; Atillasoy, E.; Shakil, A.O.; Schiff, E.R.; Caldwell, C.; Kowdley, K.V.; Stribling, R.; Crippin, J.S.; Flamm, S.; Somberg, K.A.; et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant. Surg. 1999, 5, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Manka, P.; Verheyen, J.; Gerken, G.; Canbay, A. Liver failure due to acute viral hepatitis (A-E). Visc. Med. 2016, 32, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, N.B.; Herdan, E.; Saner, F.H.; Gurakar, A. A Comprehensive Review of the Diagnosis and Management of Acute Liver Failure. J. Clin. Med. 2023, 12, 7451. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Silva, S.F.; Frauches, D.O.; Almeida, A.L.; Mendonça, H.F.M.S.; Pereira, F.E.L. Acute liver failure in children: Observations in Vitória, Espírito Santo State, Brazil. Rev. Soc. Bras. Med. Trop. 2002, 35, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Santos Hepatology, O.; Santos, O.; Londoño, M.; Marín, J.; Muñoz, O.; Mena, A.; Guzmán, C.; Hoyos, S.; Restrepo, J.; Arbeláez, M.; et al. An Experience of Liver Transplantation in Latin America: A Medical Center in Colombia. Colomb. Med. 2015, 46, 8–13. [Google Scholar] [CrossRef]
- Mendizabal, M.; Marciano, S.; Videla, M.G.; Anders, M.; Zerega, A.; Balderramo, D.C.; Chan, D.; Barrabino, M.; Gil, O.; Mastai, R.; et al. Changing etiologies and outcomes of acute liver failure: Perspectives from 6 transplant centers in Argentina. Liver Transpl. 2014, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Tapia, N.C.; Barrientos-Gutiérrez, T.; Guerrero-López, C.M.; Santiago-Hernández, J.J.; Méndez-Sánchez, N.; Uribe, M. Acute liver failure in Mexico. Ann. Hepatol. 2012, 11, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Stravitz, R.T.; Kramer, D.J. Management of acute liver failure. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Lee, W.M.; Wendon, J.; Stolze Larsen, F.; Williams, R. Acute liver failure: A curable disease by 2024? J. Hepatol. 2015, 62, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Zachariah, U.; Daniel, D.; Eapen, C.E. Growing Evidence for Survival Benefit with Plasma Exchange to Treat Liver Failure. J. Clin. Exp. Hepatol. 2023, 13, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Karvellas, C.J.; Stravitz, R.T. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J. Hepatol. 2016, 64, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Maiwall, R.; Bajpai, M.; Singh, A.; Agarwal, T.; Kumar, G.; Bharadwaj, A.; Nautiyal, N.; Tevethia, H.; Jagdish, R.K.; Vijayaraghavan, R.; et al. Standard-Volume Plasma Exchange Improves Outcomes in Patients With Acute Liver Failure: A Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2022, 20, e831–e854. [Google Scholar] [CrossRef] [PubMed]
- Vento, S.; Cainelli, F. Acute liver failure in low-income and middle-income countries. Lancet Gastroenterol. Hepatol. 2023, 8, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G.; De Mendonga, A.; et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Craig, N.; Ford, A.; Hayes, P.C.; Simpson, J. Systematic review and meta-analysis: Prognostic tests of paracetamol-induced acute liver failure. Aliment. Pharmacol. Ther. 2010, 10, 1064–1076. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M. Package “Survival” 2024. Available online: https://cran.r-project.org/web/packages/survival/index.html (accessed on 17 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Kulkarni, A.V.; Venishetty, S.; Vora, M.; Naik, P.; Chouhan, D.; Iyengar, S.; Karandikar, P.; Gupta, A.; Gahra, A.; Rakam, K.; et al. Standard-Volume Is As Effective As High-Volume Plasma Exchange for Patients With Acute Liver Failure. J. Clin. Exp. Hepatol. 2024, 14, 101354. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.; Bernal, W.; Pirani, T.; Agarwal, B.; Jalan, R.; Ryan, J.; Bangash, M.N.; El-Dalil, P.; Murphy, N.; Donnelly, M.; et al. Plasma exchange does not improve overall survival in patients with acute liver failure in a real-world cohort. J. Hepatol. 2024, 82, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Karvellas, C.J.; Subramanian, R.M. Current Evidence for Extracorporeal Liver Support Systems in Acute Liver Failure and Acute-on-Chronic Liver Failure. Crit. Care Clin. 2016, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Stravitz, R.T.; Fontana, R.J.; Karvellas, C.; Durkalski, V.; McGuire, B.; Rule, J.A.; Tujios, S.; Lee, W.M. Future directions in acute liver failure. Hepatology 2023, 78, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
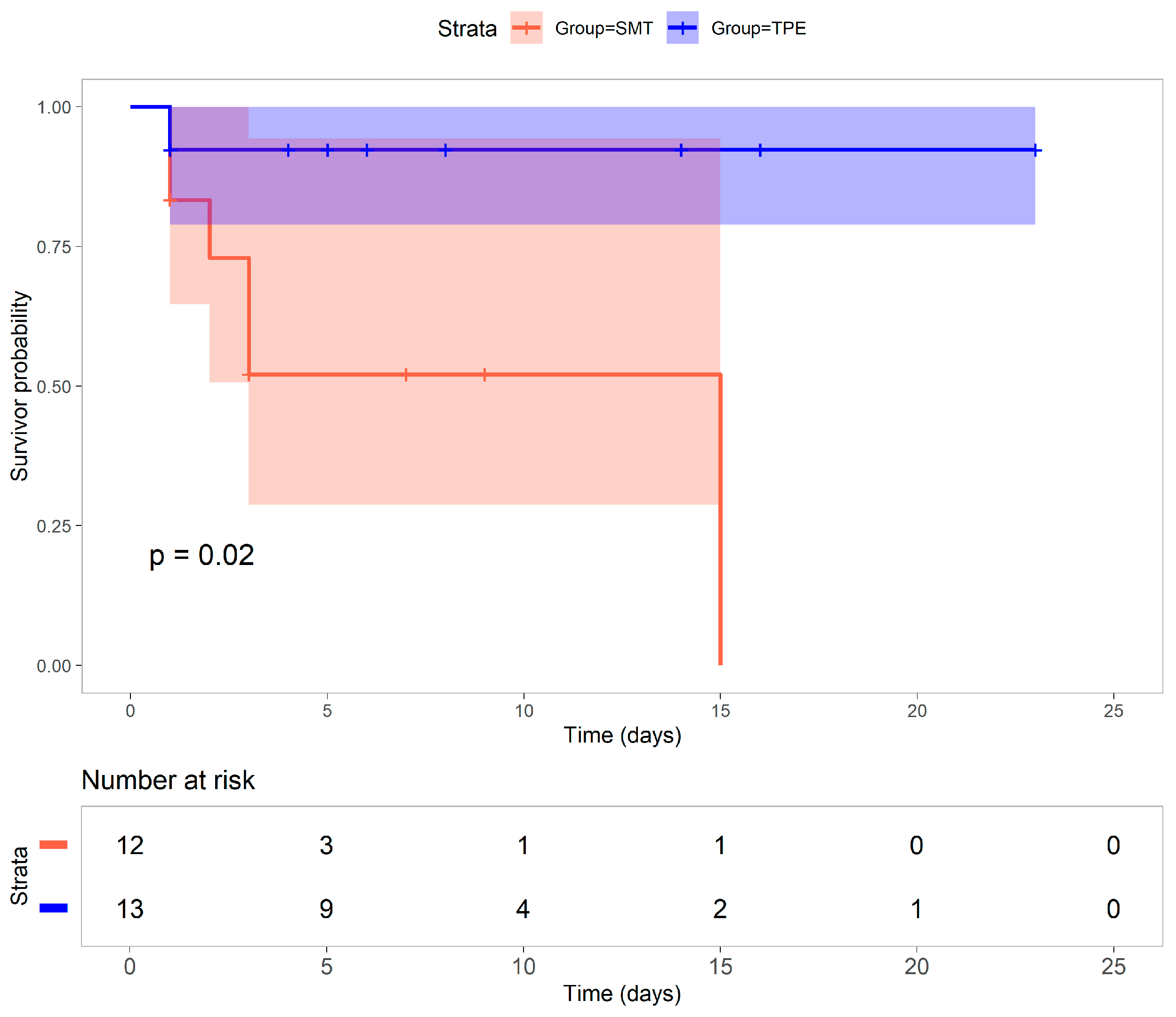
| SMT (n = 12) | TPE (n = 13) | p Value | |
|---|---|---|---|
| Gender/female (%) | 33 | 85 | 0.02 |
| Age | 28.3 ± 9.3 | 29.6 ± 7.8 | 0.51 |
| Body mass index | 27.9 ± 3.9 | 27.4 ± 3.8 | 0.71 |
| Etiology (%) | |||
| Hepatitis A | 33 | 69 | 0.16 |
| DILI | 25 | 8 | 0.32 |
| Mushroom poisoning | 17 | 8 | 0.59 |
| Ischemic hepatitis | 17 | 0 | 0.22 |
| Autoimmune hepatitis | 0 | 8 | 1.00 |
| ALL | 8 | 0 | 0.48 |
| AFLP | 0 | 8 | 1.00 |
| APACHE II | 16.6 ± 8.6 | 18.8 ± 7.4 | 0.49 |
| Kings College | 1.9 ± 1.2 | 1.8 ± 0.7 | 0.53 |
| SOFA | 7.8 ± 5.0 | 12.2 ± 5.4 | 0.11 |
| MELD | 34.0 ± 7.2 | 36.9 ± 7.6 | 0.35 |
| MELD (%) | 59.4 ± 13.9 | 61.6 ± 8.7 | 0.87 |
| Hepatic encephalopathy | 1.7 ± 1.5 | 3.4 ± 1.2 | 0.005 |
| Grade 0–1 | 5 | 1 | |
| Grade 2–3 | 5 | 3 | |
| Grade 4 | 2 | 9 | |
| Creatinine | 2.1 ± 1.3 | 3.1 ± 3.7 | 0.79 |
| Lactate | 6.7 ± 5.0 | 9.0 ± 6.1 | 0.35 |
| Bilirubin | 13.5 ± 10.5 | 15.1 ± 9.0 | 0.25 |
| INR | 10.3 ± 6.6 | 15.6 ± 7.3 | 0.07 |
| DHL | 1078.8 ± 1234.2 | 1721.8 ± 2092.1 | 0.51 |
| TGO | 2240.7 ± 2880.4 | 1786.4 ± 1309.9 | 0.70 |
| TGP | 2189.9 ± 2438.8 | 3678.6 ± 2233.9 | 0.04 |
| Sodium level | 139.0 ± 7.0 | 135.3 ± 7.0 | 0.20 |
| Estimates | Standard Error | p-Value | |
|---|---|---|---|
| (Intercept) | −1.40 | 1.12 | 0.21 |
| Group TPE | −3.18 | 1.43 | 0.03 |
| Encephalopathy (Grades 2–3) | 2.43 Survival | 1.46 | 0.10 |
| Encephalopathy (Grade 4) | 2.07 | 1.69 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasca-Aldama, J.C.; Castrejón-Sánchez, J.E.; Carrasco Flores, M.A.; Vásquez-Jiménez, E.; Carpinteyro-Espin, P.; Pérez-Escobar, J.; Gutierrez-Toledo, K.D.; Galindo, P.E.; Vidals-Sanchez, M.; Costa-Urrutia, P. Therapeutic Plasma Exchange in Acute Liver Failure: A Real-World Study in Mexico. Healthcare 2025, 13, 2059. https://doi.org/10.3390/healthcare13162059
Gasca-Aldama JC, Castrejón-Sánchez JE, Carrasco Flores MA, Vásquez-Jiménez E, Carpinteyro-Espin P, Pérez-Escobar J, Gutierrez-Toledo KD, Galindo PE, Vidals-Sanchez M, Costa-Urrutia P. Therapeutic Plasma Exchange in Acute Liver Failure: A Real-World Study in Mexico. Healthcare. 2025; 13(16):2059. https://doi.org/10.3390/healthcare13162059
Chicago/Turabian StyleGasca-Aldama, Jose Carlos, Jesús Enrique Castrejón-Sánchez, Mario A. Carrasco Flores, Enzo Vásquez-Jiménez, Paulina Carpinteyro-Espin, Juanita Pérez-Escobar, Karlos Dhamian Gutierrez-Toledo, Pablo E. Galindo, Marcos Vidals-Sanchez, and Paula Costa-Urrutia. 2025. "Therapeutic Plasma Exchange in Acute Liver Failure: A Real-World Study in Mexico" Healthcare 13, no. 16: 2059. https://doi.org/10.3390/healthcare13162059
APA StyleGasca-Aldama, J. C., Castrejón-Sánchez, J. E., Carrasco Flores, M. A., Vásquez-Jiménez, E., Carpinteyro-Espin, P., Pérez-Escobar, J., Gutierrez-Toledo, K. D., Galindo, P. E., Vidals-Sanchez, M., & Costa-Urrutia, P. (2025). Therapeutic Plasma Exchange in Acute Liver Failure: A Real-World Study in Mexico. Healthcare, 13(16), 2059. https://doi.org/10.3390/healthcare13162059






