Beyond the Growth: A Registry-Based Analysis of Global Imbalances in Artificial Intelligence Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Screening
2.3. Data Analysis
2.3.1. Geographic Distribution
2.3.2. Topic Modeling
2.3.3. Network Analysis
2.4. Statistical Analysis
3. Results
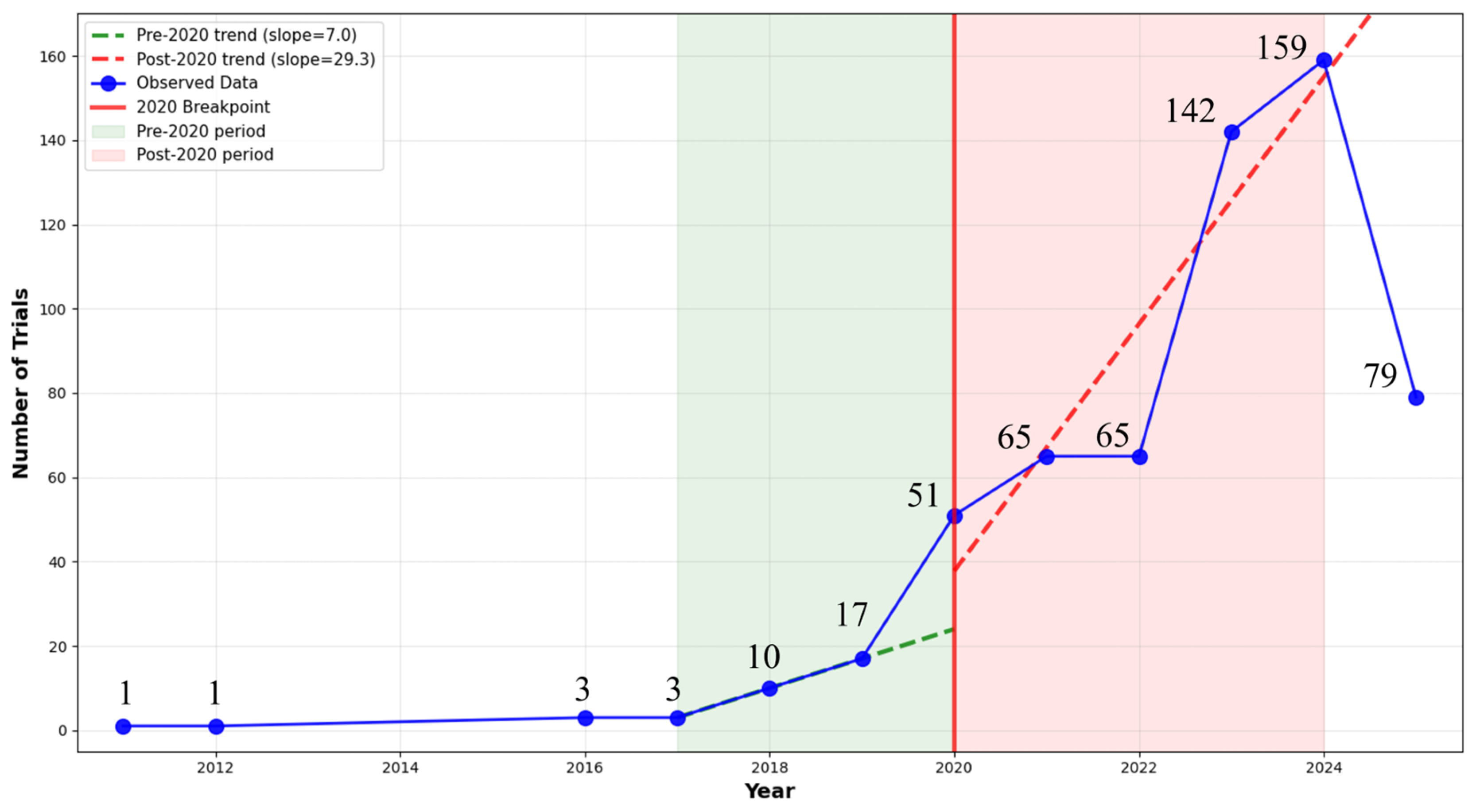
3.1. Study Selection and Characteristics
3.2. Disease and Technology Category Distribution
3.3. Geographic Distribution and Global Patterns
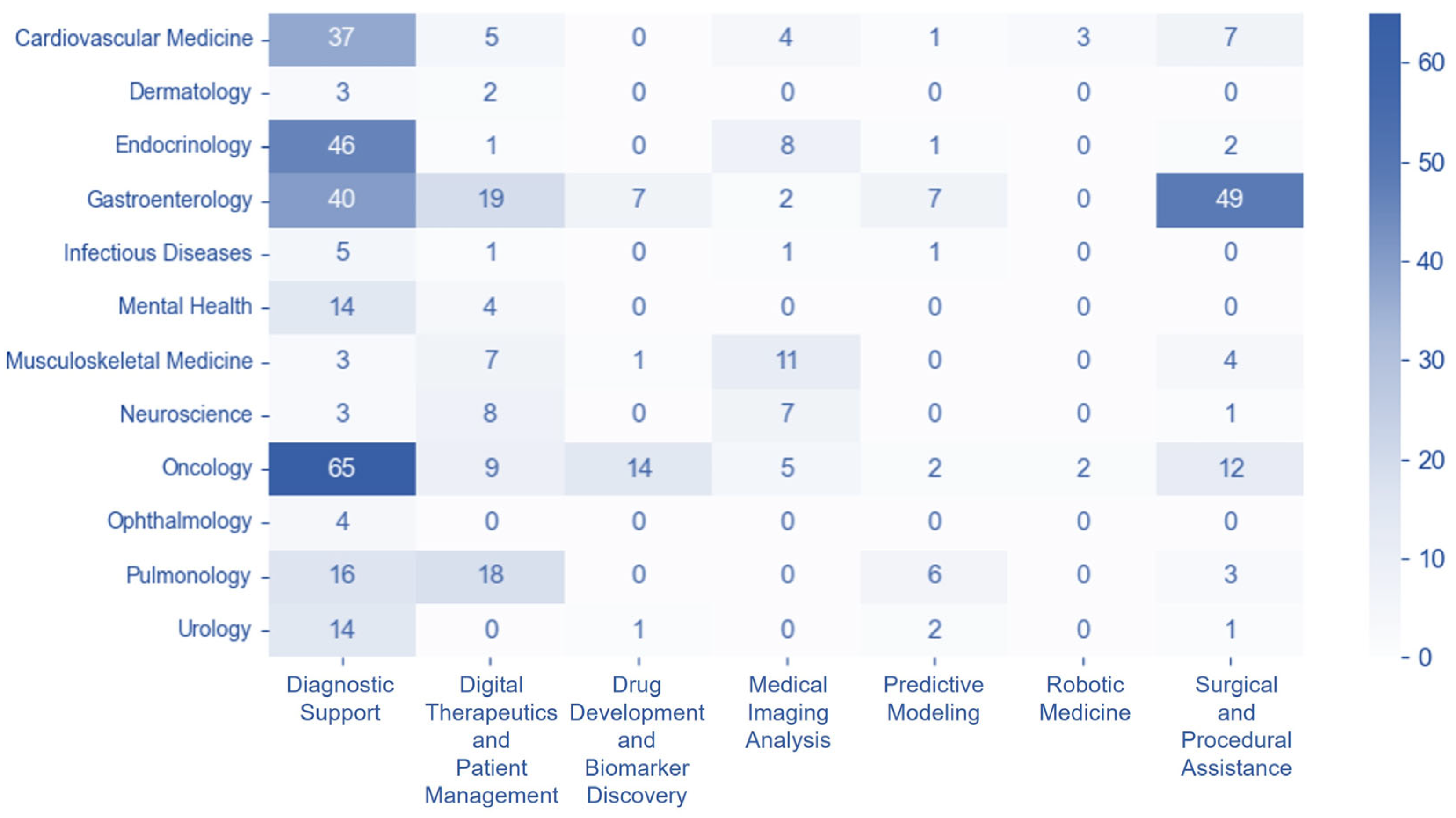
3.4. Disease-Technology Association Patterns
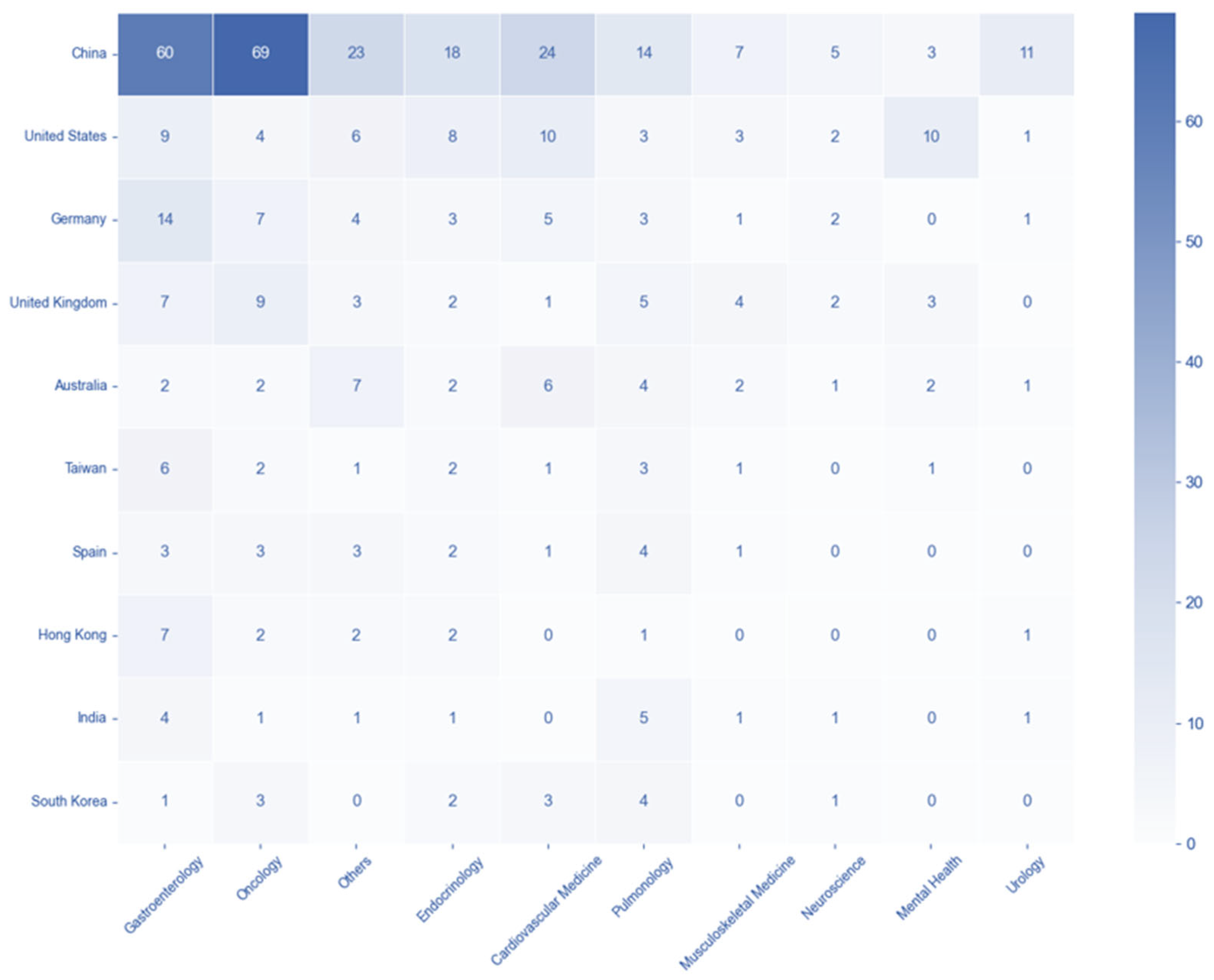
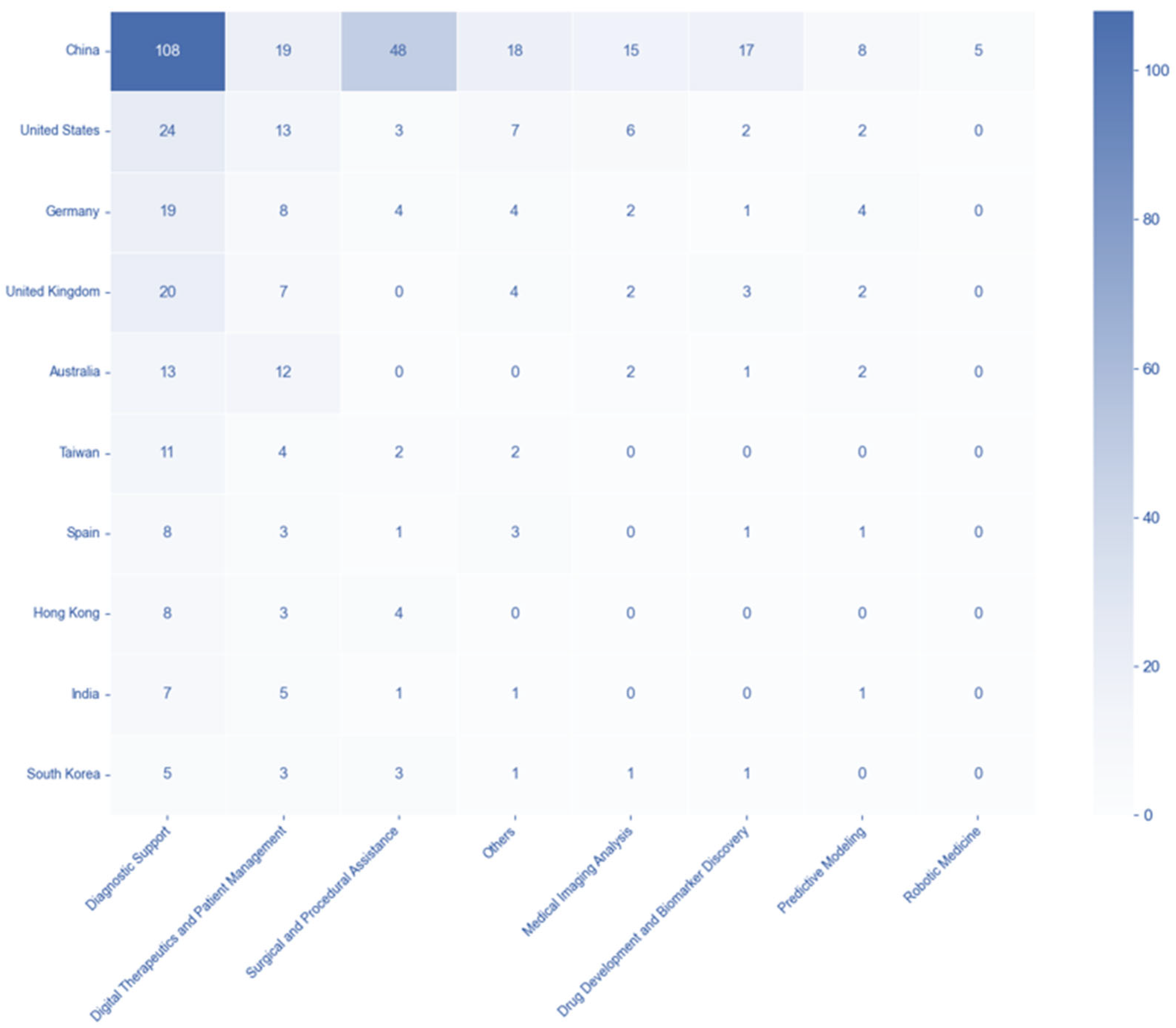
3.5. Country-Disease and -Technology Association Patterns
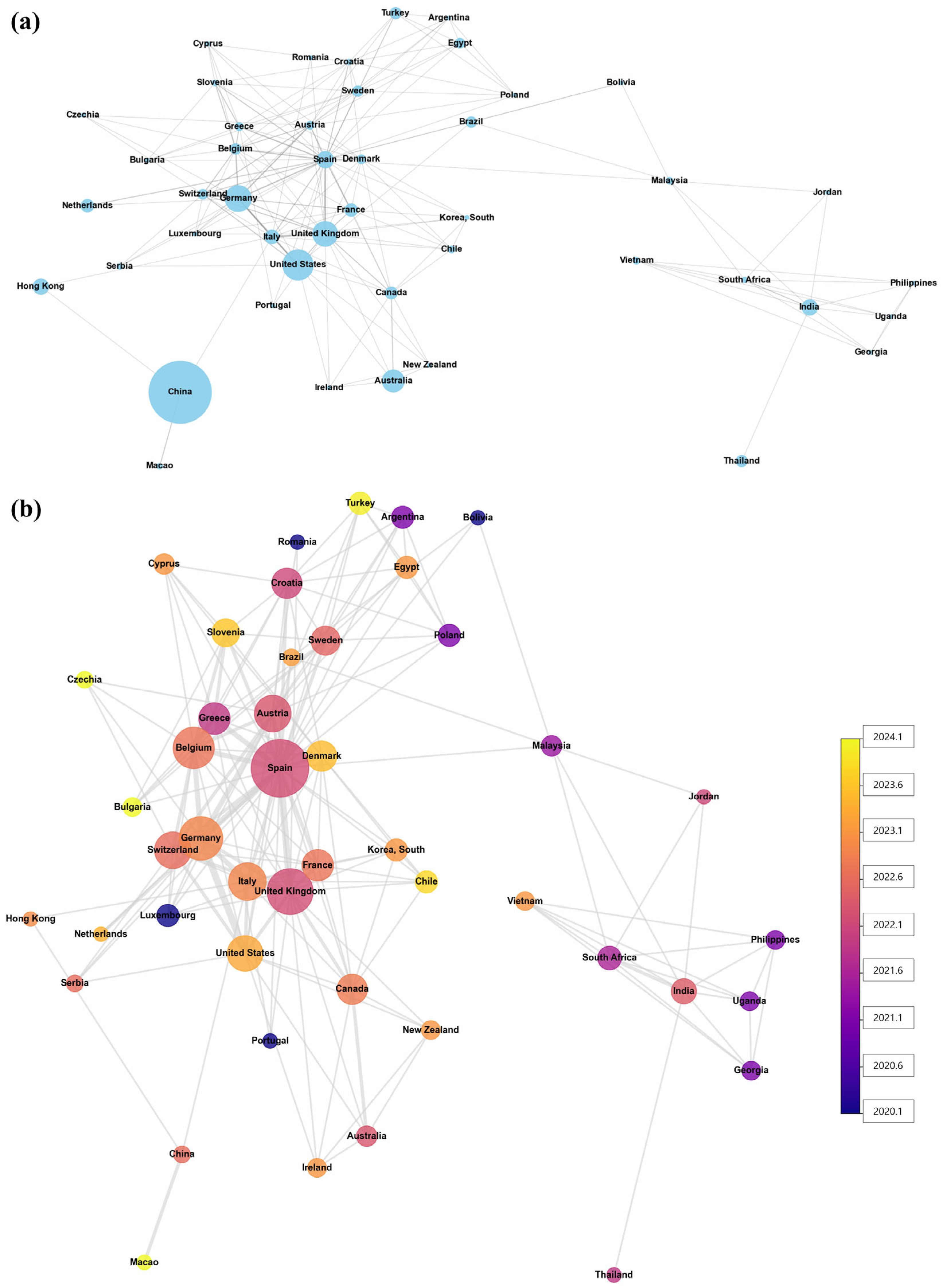
3.6. International Collaboration in AI Clinical Trials
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Academic Press: Cambridge, MA, USA, 2020; pp. 25–60. [Google Scholar] [CrossRef]
- Macheka, S.; Ng, P.Y.; Ginsburg, O.; Hope, A.; Sullivan, R.; Aggarwal, A. Prospective evaluation of artificial intelligence (AI) applications for use in cancer pathways following diagnosis: A systematic review. BMJ Oncol. 2024, 3, e000255. [Google Scholar] [CrossRef] [PubMed]
- Takita, H.; Kabata, D.; Walston, S.L.; Tatekawa, H.; Saito, K.; Tsujimoto, Y.; Miki, Y.; Ueda, D. A systematic review and meta-analysis of diagnostic performance comparison between generative AI and physicians. NPJ Digit. Med. 2025, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Zeltzer, D.; Herzog, L.; Pickman, Y.; Steuerman, Y.; Ber, R.I.; Kugler, Z.; Shaul, R.; Ebbert, J.O. Diagnostic Accuracy of Artificial Intelligence in Virtual Primary Care. Mayo Clin. Proc. Digit. Health 2023, 1, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.W.; Poliak, A.; Dredze, M.; Leas, E.C.; Zhu, Z.; Kelley, J.B.; Faix, D.J.; Goodman, A.M.; Longhurst, C.A.; Hogarth, M.; et al. Comparing Physician and Artificial Intelligence Chatbot Responses to Patient Questions Posted to a Public Social Media Forum. JAMA Intern. Med. 2023, 183, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, T.; Wang, H.; Zhang, R.; Ni, Z.; Liu, N.; Zhai, H.; Zhao, J.; Meng, F.; Zhou, Z.; et al. Multiple large language models versus experienced physicians in diagnosing challenging cases with gastrointestinal symptoms. NPJ Digit. Med. 2025, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Acosta, J.N.; Shakeri, Z.; Ioannidis, J.P.A.; Topol, E.J.; Rajpurkar, P. Randomised controlled trials evaluating artificial intelligence in clinical practice: A scoping review. Lancet Digit. Health 2024, 6, e367–e373. [Google Scholar] [CrossRef] [PubMed]
- Schmallenbach, L.; Bärnighausen, T.W.; Lerchenmueller, M.J. The global geography of artificial intelligence in life science research. Nat. Commun. 2024, 15, 7527. [Google Scholar] [CrossRef] [PubMed]
- Maslej, N.; Fattorini, L.; Perrault, R.; Gil, Y.; Parli, V.; Kariuki, N.; Capstick, E.; Reuel, A.; Brynjolfsson, E.; Etchemendy, J.; et al. Artificial intelligence index report 2025. arXiv 2025, arXiv:2504.07139. [Google Scholar] [CrossRef]
- Celi, L.A.; Cellini, J.; Charpignon, M.L.; Dee, E.C.; Dernoncourt, F.; Eber, R.; Mitchell, W.G.; Moukheiber, L.; Schirmer, J.; Situ, J.; et al. Sources of bias in artificial intelligence that perpetuate healthcare disparities-A global review. PLOS Digit. Health 2022, 1, e0000022. [Google Scholar] [CrossRef] [PubMed]
- Alberto, I.R.I.; Alberto, N.R.I.; Altinel, Y.; Blacker, S.; Binotti, W.W.; Celi, L.A.; Chua, T.; Fiske, A.; Griffin, M.; Karaca, G.; et al. A scientometric analysis of fairness in health AI literature. PLOS Glob. Public Health 2024, 4, e0002513. [Google Scholar] [CrossRef] [PubMed]
- Cau, R.; Pisu, F.; Suri, J.S.; Saba, L. Addressing hidden risks: Systematic review of artificial intelligence biases across racial and ethnic groups in cardiovascular diseases. Eur. J. Radiol. 2025, 183, 111867. [Google Scholar] [CrossRef] [PubMed]
- Juneja, A.; Gupta, J.; Yadav, N.; Sharma, S.; Panchal, Y.; Adhikari, T.; Rao, M.V.V. An overview of primary registries of WHO’s international clinical trial registry platform. Ayu 2019, 40, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, L. Registering transparency: The making of the international clinical trial registry platform by the world health organization (2004–2006). Glob. Health 2023, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, M. BERTopic: Neural topic modeling with a class-based TF-IDF procedure. arXiv 2022, arXiv:2203.05794. [Google Scholar]
- Lee, J.; Yoon, W.; Kim, S.; Kim, D.; Kim, S.; So, C.H.; Kang, J. BioBERT: A pre-trained biomedical language representation model for biomedical text mining. Bioinformatics 2020, 36, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, A.; Swart, P.; Chult, D. Exploring Network Structure, Dynamics, and Function Using NetworkX. In Proceedings of the 7th Python in Science Conference, Pasadena, CA, USA, 19–24 August 2008. [Google Scholar]
- de-Graft Aikins, A.; Unwin, N.; Agyemang, C.; Allotey, P.; Campbell, C.; Arhinful, D. Tackling Africa’s chronic disease burden: From the local to the global. Glob. Health 2010, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.; Gaziano, T.A.; Bouillon Buendia, C.P.; Guanais de Aguiar, F.C. Confronting the chronic disease burden in Latin America and the Caribbean. Health Aff. 2010, 29, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhai, Y.; Lu, G. Evolution of artificial intelligence in healthcare: A 30-year bibliometric study. Front. Med. 2024, 11, 1505692. [Google Scholar] [CrossRef] [PubMed]

| Algorithm Component | Parameter | Value |
|---|---|---|
| Embedding Model | Model | pritamdeka/S-BioBert-snli-multinli-stsb |
| UMAP | n_neighbors | 5 |
| n_components | 5 | |
| min_dist | 0.0 | |
| metric | cosine | |
| HDBSCAN | min_cluster_size | 5 |
| min_samples | 3 | |
| metric | Euclidean | |
| CountVectorizer | min_df | 2 |
| max_features | 1000 | |
| ngram_range | (1, 2) | |
| Topic Number | Determination | Automatic (data-driven) |
| Rank | Country/Region | Total Participation | Lead Studies | Multicountry Participation (%) | Single Country Studies | Total Partners | Avg. Partners per Study |
|---|---|---|---|---|---|---|---|
| 1 | China | 238 | 238 | 3 (1.3) | 235 | 4 | 1 |
| 2 | United States | 57 | 53 | 6 (10.5) | 51 | 18 | 1.3 |
| 3 | Germany | 42 | 36 | 9 (21.4) | 33 | 27 | 1.6 |
| 4 | UK | 38 | 31 | 7 (18.4) | 31 | 30 | 1.8 |
| 5 | Australia | 30 | 30 | 2 (6.7) | 28 | 6 | 1.2 |
| 6 | Taiwan | 19 | 19 | 0 (0) | 19 | 0 | 1 |
| 7 | Spain | 17 | 6 | 11 (64.7) | 6 | 47 | 3.8 |
| 8 | Hong Kong | 15 | 14 | 1 (6.7) | 14 | 2 | 1.1 |
| 9 | India | 15 | 14 | 3 (20) | 12 | 9 | 1.6 |
| 10 | South Korea | 14 | 14 | 0 (0) | 14 | 0 | 1 |
| 11 | Italy | 12 | 7 | 7 (58.3) | 5 | 20 | 2.7 |
| 12 | Japan | 11 | 11 | 0 (0) | 11 | 0 | 1 |
| 13 | Netherlands | 10 | 9 | 2 (20) | 8 | 3 | 1.3 |
| 14 | France | 10 | 8 | 2 (20) | 8 | 14 | 2.4 |
| 15 | Canada | 9 | 7 | 3 (33.3) | 6 | 13 | 2.4 |
| 16 | Thailand | 8 | 7 | 1 (12.5) | 7 | 1 | 1.1 |
| 17 | Turkey | 8 | 7 | 1 (12.5) | 7 | 7 | 1.9 |
| 18 | Brazil | 7 | 5 | 2 (28.6) | 5 | 4 | 1.6 |
| 19 | Belgium | 7 | 5 | 5 (71.4) | 2 | 24 | 4.4 |
| 20 | Singapore | 7 | 7 | 0 (0) | 7 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, C.-Y. Beyond the Growth: A Registry-Based Analysis of Global Imbalances in Artificial Intelligence Clinical Trials. Healthcare 2025, 13, 2018. https://doi.org/10.3390/healthcare13162018
Kwon C-Y. Beyond the Growth: A Registry-Based Analysis of Global Imbalances in Artificial Intelligence Clinical Trials. Healthcare. 2025; 13(16):2018. https://doi.org/10.3390/healthcare13162018
Chicago/Turabian StyleKwon, Chan-Young. 2025. "Beyond the Growth: A Registry-Based Analysis of Global Imbalances in Artificial Intelligence Clinical Trials" Healthcare 13, no. 16: 2018. https://doi.org/10.3390/healthcare13162018
APA StyleKwon, C.-Y. (2025). Beyond the Growth: A Registry-Based Analysis of Global Imbalances in Artificial Intelligence Clinical Trials. Healthcare, 13(16), 2018. https://doi.org/10.3390/healthcare13162018






