Physical Training and Pulmonary Rehabilitation in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Eligibility Criteria
- Randomized controlled trials (RCTs), regardless of their specific design (e.g., crossover, parallel).
- Studies published in any language.
- Studies published between January 1990 and January 2025 were included to ensure a broad and representative evidence base from the period following the identification of the CFTR gene, as well as the evolution of exercise interventions in line with contemporary standards of care in cystic fibrosis.
- Pediatric and adult patients with a diagnosis of cystic fibrosis, enrolled in pulmonary rehabilitation or physical training programs of any modality (in-person or remote), regardless of intensity and setting (hospital, community center, or home).
- Studies reporting at least one of the following outcomes: pulmonary function (FEV1, FVC, FEV1/FVC, RV/TLC), exercise capacity (6MWT, VO2 max, Wmax), pulmonary exacerbations, hospitalizations, health-related quality of life, and adverse effects.
- Article preprints and letters to the editor.
- Studies published as conference abstracts.
- Studies not available in accessible formats.
- Patients with cardiac, orthopedic, or traumatic complications that prevent exercise performance or adequate participation in the rehabilitation program.
- Studies reporting on the same patient cohort as previous publications of similar research.
2.3. Data Sources and Search Strategy
2.4. Study Selection and Data Extraction
2.5. Risk of Bias Assessment
2.6. Assessment of Evidence Quality
2.7. Statistical Analysis
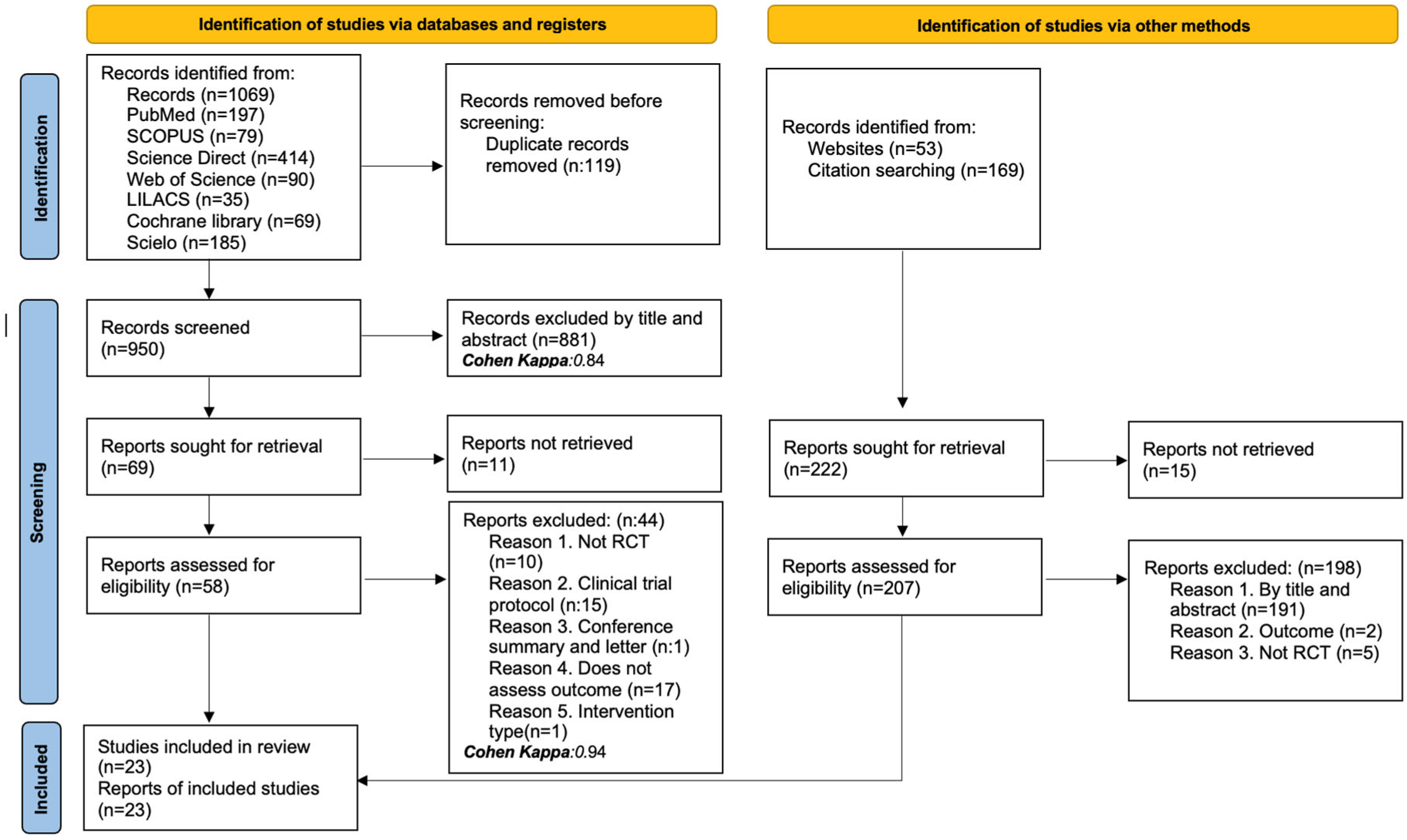
3. Results
3.1. Studies Identified for the Review
3.2. Characteristics of the Studies Included in the Review
3.3. Characteristics of the Population and the Applied Intervention
3.4. Results of the Risk of Bias Assessment
3.4.1. Random Sequence Generation
3.4.2. Allocation Concealment
3.4.3. Blinding of Participants and Personnel
3.4.4. Blinding of Outcome Assessment
3.4.5. Incomplete Outcome Data
3.4.6. Selective Reporting
3.4.7. Summary of Risk of Bias
3.5. Qualitative Synthesis of the Scientific Evidence
3.5.1. Exacerbations
3.5.2. Hospitalization
3.5.3. Health-Related Quality of Life
3.5.4. Adverse Events
3.6. Meta-Analysis
3.6.1. Results of the Evidence Quality Assessment
3.6.2. Pulmonary Function
Forced Expiratory Volume in the First Second (FEV1)
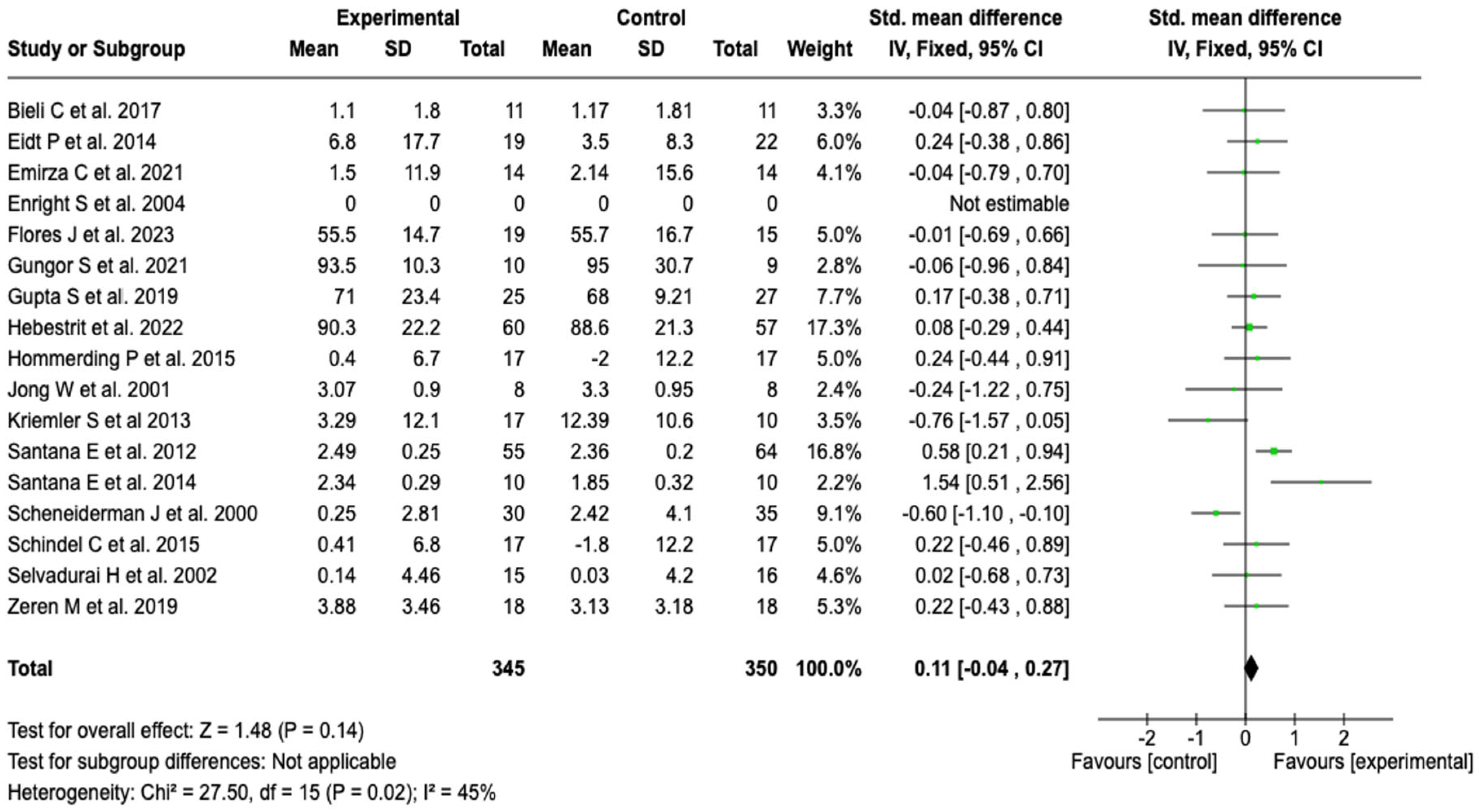
Forced Vital Capacity (FVC)
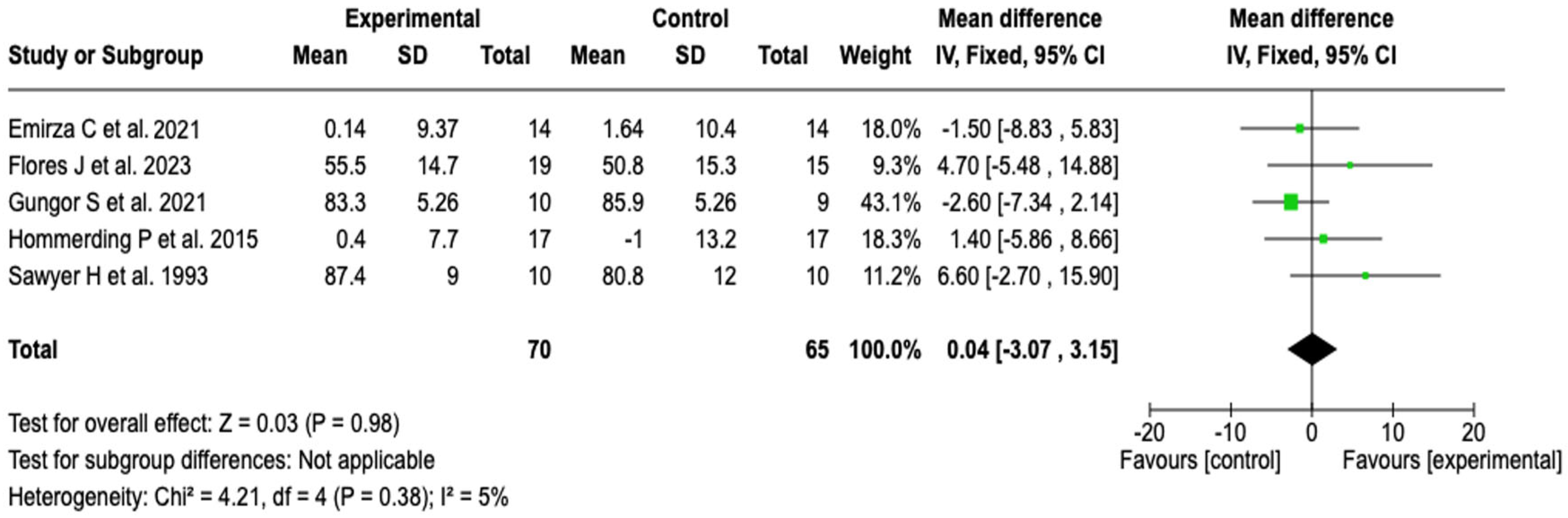
FEV1/FVC Ratio
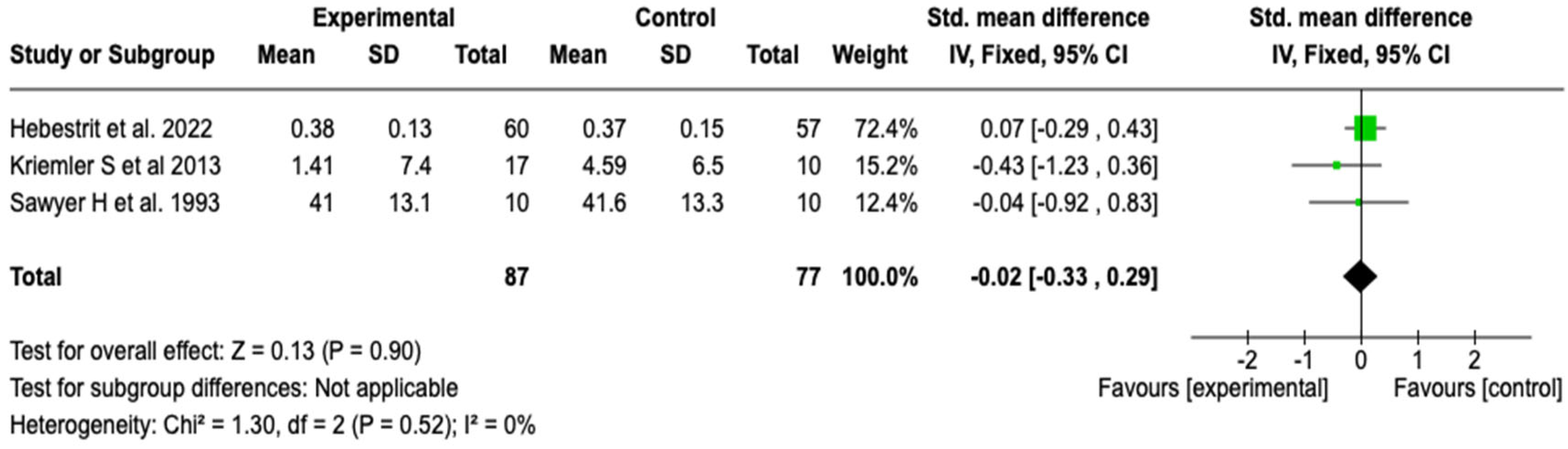
Residual Volume to Total Lung Capacity Ratio (RV/TLC)
3.6.3. Exercise Capacity
6-Minute Walk Distance (6MWD)
Oxygen Consumption (VO2 Max)
Wmax
3.6.4. Publication Bias
3.6.5. Results of the GRADE Certainty of Evidence Assessment
4. Discussion
4.1. Main Findings of the Review
4.2. Comparison with Previous Studies
4.3. Limitations of the Included Studies
4.4. Limitations of the Review
4.5. Strengths of the Review
4.6. Clinical Implications
4.7. Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| VEF1 | Forced Expiratory Volume in 1 s |
| CVF | Forced Vital Capacity |
| VEF1/CVF | Ratio of Forced Expiratory Volume in 1 s to Forced Vital Capacity |
| RV/TLC | Residual Volume to Total Lung Capacity ratio |
| VO2 max | Maximum Oxygen Consumption |
| 6MWT | 6-Minute Walk Test |
| CF | Cystic Fibrosis |
References
- Bierlaagh, M.C.; Muilwijk, D.; Beekman, J.M.; van der Ent, C.K. A New Era for People with Cystic Fibrosis. Eur. J. Pediatr. 2021, 180, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Valerio, G.; Brodlie, M.; Donini, L.M.; Siervo, M. Cystic Fibrosis, Body Composition, and Health Outcomes: A Systematic Review. Nutrition 2018, 55–56, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic Fibrosis Genetics: From Molecular Understanding to Clinical Application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The Future of Cystic Fibrosis Care: A Global Perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Varkki, S.D.; Aaron, R.; Chapla, A.; Danda, S.; Medhi, P.; Jansi Rani, N.; Paul, G.R. CFTR Mutations and Phenotypic Correlations in People with Cystic Fibrosis: A Retrospective Study from a Single Centre in South India. Lancet Reg. Health—Southeast Asia 2024, 27, 100434. [Google Scholar] [CrossRef]
- Polgreen, P.M.; Comellas, A.P. Clinical Phenotypes of Cystic Fibrosis Carriers. Annu. Rev. Med. 2022, 73, 563–574. [Google Scholar] [CrossRef]
- Farinha, C.M.; Callebaut, I. Molecular Mechanisms of Cystic Fibrosis—How Mutations Lead to Misfunction and Guide Therapy. Biosci. Rep. 2022, 42, BSR20212006. [Google Scholar] [CrossRef]
- Fanen, P.; Wohlhuter-Haddad, A.; Hinzpeter, A. Genetics of Cystic Fibrosis: CFTR Mutation Classifications toward Genotype-Based CF Therapies. Int. J. Biochem. Cell Biol. 2014, 52, 94–102. [Google Scholar] [CrossRef]
- Lewis, B.W.; Patial, S.; Saini, Y. Immunopathology of Airway Surface Liquid Dehydration Disease. J. Immunol. Res. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Ramananda, Y.; Naren, A.P.; Arora, K. Functional Consequences of CFTR Interactions in Cystic Fibrosis. Int. J. Mol. Sci. 2024, 25, 3384. [Google Scholar] [CrossRef]
- Fonseca, C.; Bicker, J.; Alves, G.; Falcão, A.; Fortuna, A. Cystic Fibrosis: Physiopathology and the Latest Pharmacological Treatments. Pharmacol. Res. 2020, 162, 105267. [Google Scholar] [CrossRef]
- Fuhrer, M.; Zampoli, M.; Abriel, H. Diagnosing Cystic Fibrosis in Low- and Middle-Income Countries: Challenges and Strategies. Orphanet J. Rare Dis. 2024, 19, 482. [Google Scholar] [CrossRef] [PubMed]
- Spoonhower, K.A.; Davis, P.B. Epidemiology of Cystic Fibrosis. Clin. Chest Med. 2016, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Audrézet, M.P.; Munck, A.; Scotet, V.; Claustres, M.; Roussey, M.; Delmas, D.; Férec, C.; Desgeorges, M. Comprehensive CFTR Gene Analysis of the French Cystic Fibrosis Screened Newborn Cohort: Implications for Diagnosis, Genetic Counseling, and Mutation-Specific Therapy. Genet. Med. 2015, 17, 108–116. [Google Scholar] [CrossRef]
- Skov, M.; Bækvad-Hansen, M.; Hougaard, D.M.; Skogstrand, K.; Lund, A.M.; Pressler, T.; Olesen, H.V.; Duno, M. Cystic Fibrosis Newborn Screening in Denmark: Experience from the First 2 Years. Pediatr. Pulmonol. 2020, 55, 549–555. [Google Scholar] [CrossRef]
- Lilley, M.; Christian, S.; Hume, S.; Scott, P.; Montgomery, M.; Semple, L.; Zuberbuhler, P.; Tabak, J.; Bamforth, F.; Somerville, M.J. Newborn Screening for Cystic Fibrosis in Alberta: Two Years of Experience. Paediatr. Child Health 2010, 15, 590–594. [Google Scholar] [CrossRef]
- Kosorok, M.R.; Wei, W.-H.; Farrell, P.M. THE INCIDENCE OF CYSTIC FIBROSIS. Statist. Med. 1996, 15, 449–462. [Google Scholar] [CrossRef]
- Silva Filho, L.V.R.F.; Castaños, C.; Ruíz, H.H. Cystic Fibrosis in Latin America—Improving the Awareness. J. Cyst. Fibros. 2016, 15, 791–793. [Google Scholar] [CrossRef]
- Fajac, I.; Burgel, P.-R. Croissance démographique et thérapeutiques ciblées: Le nouveau visage de la mucoviscidose. Rev. Des Mal. Respir. 2016, 33, 645–647. [Google Scholar] [CrossRef]
- Corriveau, S.; Sykes, J.; Stephenson, A.L. Cystic Fibrosis Survival: The Changing Epidemiology. Curr. Opin. Pulm. Med. 2018, 24, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.L.; Stanojevic, S.; Sykes, J.; Burgel, P.-R. The Changing Epidemiology and Demography of Cystic Fibrosis. La Presse Médicale 2017, 46, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Flume, P.A. Pulmonary Complications of Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2019, 40, 804–809. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Mall, M.A. The Future of Cystic Fibrosis Treatment: From Disease Mechanisms to Novel Therapeutic Approaches. Lancet 2023, 402, 1185–1198. [Google Scholar] [CrossRef]
- Connett, G. Lumacaftor-Ivacaftor in the Treatment of Cystic Fibrosis: Design, Development and Place in Therapy. Drug Des. Dev. Ther. 2019, 13, 2405–2412. [Google Scholar] [CrossRef]
- Braga, S.F.F.; Almgren, M.M. Complementary Therapies in Cystic Fibrosis: Nutritional Supplements and Herbal Products. J. Pharm. Pract. 2013, 26, 14–17. [Google Scholar] [CrossRef]
- Lonabaugh, K.P.; O’Neal, K.S.; McIntosh, H.; Condren, M. Cystic Fibrosis-Related Education: Are We Meeting Patient and Caregiver Expectations? Patient Educ. Couns. 2018, 101, 1865–1870. [Google Scholar] [CrossRef]
- Cruz Mosquera, F.E.; Perlaza, C.L.; Naranjo Rojas, A.; Murillo Rios, S.; Carrero Gallego, A.; Fischersworring, S.I.; Rodríguez, J.S.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina 2025, 61, 489. [Google Scholar] [CrossRef]
- Kalamara, E.I.; Ballas, E.T.; Pitsiou, G.; Petrova, G. Pulmonary Rehabilitation for Cystic Fibrosis: A Narrative Review of Current Literature. Monaldi Arch. Chest Dis. 2021, 91, 1501. [Google Scholar] [CrossRef]
- Ward, N.; Stiller, K.; Holland, A.E. Exercise as a Therapeutic Intervention for People with Cystic Fibrosis. Expert Rev. Respir. Med. 2019, 13, 449–458. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Jacobsen, U.; Bregnballe, V.; Olesen, H.V.; Ingemann-Hansen, T.; Thastum, M.; Oluf Schiøtz, P. Exercise and Quality of Life in Patients with Cystic Fibrosis: A 12-Week Intervention Study. Physiother. Theory Pract. 2011, 27, 548–556. [Google Scholar] [CrossRef]
- Hulzebos, H.J.; Snieder, H.; Van Der Et, J.; Helders, P.J.; Takken, T. High-Intensity Interval Training in an Adolescent with Cystic Fibrosis: A Physiological Perspective. Physiother. Theory Pract. 2011, 27, 231–237. [Google Scholar] [CrossRef]
- Gagulic, S.; Bártolo, A.; Marques, A. Effects of a Tailored Home-Based Exercise Program, “KidMove”, on Children with Cystic Fibrosis: A Quasi-Experimental Study. Healthcare 2024, 13, 4. [Google Scholar] [CrossRef]
- García-Pérez-de-Sevilla, G.; Yvert, T.; Blanco, Á.; Sosa Pedreschi, A.I.; Thuissard, I.J.; Pérez-Ruiz, M. Effectiveness of Physical Exercise Interventions on Pulmonary Function and Physical Fitness in Children and Adults with Cystic Fibrosis: A Systematic Review with Meta-Analysis. Healthcare 2022, 10, 2205. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Yoon, U.; Vogt, P.M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and Publication Bias. J. Cranio-Maxillofac. Surg. 2011, 39, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Flores, J.; Ziegler, B.; Silvello, D.; Dalcin, P.T.R. Effects of an Early Rehabilitation Program for Adult Cystic Fibrosis Patients during Hospitalization: A Randomized Clinical Trial. Braz. J. Med. Biol. Res. 2023, 56, e12752. [Google Scholar] [CrossRef]
- Hebestreit, H.; Kriemler, S.; Schindler, C.; Stein, L.; Karila, C.; Urquhart, D.S.; Orenstein, D.M.; Lands, L.C.; Schaeff, J.; Eber, E.; et al. Effects of a Partially Supervised Conditioning Program in Cystic Fibrosis: An International Multicenter, Randomized Controlled Trial (ACTIVATE-CF). Am. J. Respir. Crit. Care Med. 2022, 205, 330–339. [Google Scholar] [CrossRef]
- Kaltsakas, G.; Chynkiamis, N.; Anastasopoulos, N.; Zeliou, P.; Karapatoucha, V.; Kotsifas, K.; Diamantea, F.; Inglezos, I.; Koulouris, N.G.; Vogiatzis, I. Interval versus Constant-Load Exercise Training in Adults with Cystic Fibrosis. Respir. Physiol. Neurobiol. 2021, 288, 103643. [Google Scholar] [CrossRef]
- Güngör, S. The Clinical Effects of Combining Postural Exercises with Chest Physiotherapy in Cystic Fibrosis: A Single-Blind, Randomized-Controlled Trial. Turk. J. Phys. Med. Rehabil. 2021, 67, 91–98. [Google Scholar] [CrossRef]
- Emirza, C.; Aslan, G.K.; Kilinc, A.A.; Cokugras, H. Effect of Expiratory Muscle Training on Peak Cough Flow in Children and Adolescents with Cystic Fibrosis: A Randomized Controlled Trial. Pediatr. Pulmonol. 2021, 56, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mukherjee, A.; Lodha, R.; Kabra, M.; Deepak, K.K.; Khadgawat, R.; Talwar, A.; Kabra, S.K. Effects of Exercise Intervention Program on Bone Mineral Accretion in Children and Adolescents with Cystic Fibrosis: A Randomized Controlled Trial. Indian J. Pediatr. 2019, 86, 987–994. [Google Scholar] [CrossRef]
- Zeren, M.; Cakir, E.; Gurses, H.N. Effects of Inspiratory Muscle Training on Postural Stability, Pulmonary Function and Functional Capacity in Children with Cystic Fibrosis: A Randomised Controlled Trial. Respir. Med. 2019, 148, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Bieli, C.; Summermatter, S.; Boutellier, U.; Moeller, A. Respiratory Muscle Training Improves Respiratory Muscle Endurance but Not Exercise Tolerance in Children with Cystic Fibrosis. Pediatr. Pulmonol. 2017, 52, 331–336. [Google Scholar] [CrossRef]
- Del Corral, T.; Cebrià I Iranzo, M.À.; López-de-Uralde-Villanueva, I.; Martínez-Alejos, R.; Blanco, I.; Vilaró, J. Effectiveness of a Home-Based Active Video Game Programme in Young Cystic Fibrosis Patients. Respiration 2018, 95, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Schindel, C.S.; Hommerding, P.X.; Melo, D.A.S.; Baptista, R.R.; Marostica, P.J.C.; Donadio, M.V.F. Physical Exercise Recommendations Improve Postural Changes Found in Children and Adolescents with Cystic Fibrosis: A Randomized Controlled Trial. J. Pediatr. 2015, 166, 710–716.e2. [Google Scholar] [CrossRef]
- Hommerding, P.X.; Baptista, R.R.; Makarewicz, G.T.; Schindel, C.S.; Donadio, M.V.; Pinto, L.A.; Marostica, P.J. Effects of an Educational Intervention of Physical Activity for Children and Adolescents With Cystic Fibrosis: A Randomized Controlled Trial. Respir. Care 2015, 60, 81–87. [Google Scholar] [CrossRef]
- Eidt, P.M.; Flores, J.; Ziegler, B.; Casarotto, F.; Jaques, P.; Barreto, S.S.M.; Dalcin, P.D.T.R. Exercise Programme in Patients with Cystic Fibrosis: A Randomized Controlled Trial. Respir. Med. 2014, 108, 1134–1140. [Google Scholar] [CrossRef]
- Kriemler, S.; Kieser, S.; Junge, S.; Ballmann, M.; Hebestreit, A.; Schindler, C.; Stüssi, C.; Hebestreit, H. Effect of Supervised Training on FEV1 in Cystic Fibrosis: A Randomised Controlled Trial. J. Cyst. Fibros. 2013, 12, 714–720. [Google Scholar] [CrossRef]
- Santana, E.; Gonzalez-Saiz, L.; Groeneveld, I.F.; Villa-Asensi, J.R.; Barrio Gómez De Aguero, M.I.; Fleck, S.J.; López-Mojares, L.M.; Pérez, M.; Lucia, A. Benefits of Combining Inspiratory Muscle with ‘Whole Muscle’ Training in Children with Cystic Fibrosis: A Randomised Controlled Trial. Br. J. Sports Med. 2014, 48, 1513–1517. [Google Scholar] [CrossRef]
- Santana, E.; Groeneveld, I.F.; Gonzalez-Saiz, L.; López-Mojares, L.M.; Villa-Asensi, J.R.; Gonzalez, M.I.B.; Fleck, S.J.; Pérez, M.; Lucia, A. Intrahospital Weight and Aerobic Training in Children with Cystic Fibrosis: A Randomized Controlled Trial. Med. Sci. Sports Exerc. 2012, 44, 2–11. [Google Scholar] [CrossRef]
- Sandsund, C.A.; Roughton, M.; Hodson, M.E.; Pryor, J.A. Musculoskeletal Techniques for Clinically Stable Adults with Cystic Fibrosis: A Preliminary Randomised Controlled Trial. Physiotherapy 2011, 97, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Klijn, P.H.C.; Oudshoorn, A.; Van Der Ent, C.K.; Van Der Net, J.; Kimpen, J.L.; Helders, P.J.M. Effects of Anaerobic Training in Children With Cystic Fibrosis. Chest 2004, 125, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Enright, S.; Chatham, K.; Ionescu, A.A.; Unnithan, V.B.; Shale, D.J. Inspiratory Muscle Training Improves Lung Function and Exercise Capacity in Adults With Cystic Fibrosis. Chest 2004, 126, 405–411. [Google Scholar] [CrossRef]
- Moorcroft, A.J. Individualised Unsupervised Exercise Training in Adults with Cystic Fibrosis: A 1 Year Randomised Controlled Trial. Thorax 2004, 59, 1074–1080. [Google Scholar] [CrossRef]
- Selvadurai, H.C.; Blimkie, C.J.; Meyers, N.; Mellis, C.M.; Cooper, P.J.; Van Asperen, P.P. Randomized Controlled Study of Inhospital Exercise Training Programs in Children with Cystic Fibrosis. Pediatr. Pulmonol. 2002, 33, 194–200. [Google Scholar] [CrossRef]
- De Jong, W.; Van Aalderen, W.M.C.; Kraan, J.; Koëter, G.H.; Van Der Schans, C.P. Inspiratory Muscle Training in Patients with Cystic Fibrosis. Respir. Med. 2001, 95, 31–36. [Google Scholar] [CrossRef]
- Schneiderman, J.; Pollock, S.L.; Corey, M.; Wilkes, D.D.; Canny, G.J.; Pedder, L.; Reisman, J.J. A Randomized Controlled Trial of a 3-Year Home Exercise Program in Cystic Fibrosis. J. Pediatr. 2000, 136, 304–310. [Google Scholar] [CrossRef]
- Sawyer, E.H.; Clanton, T.L. Improved Pulmonary Function and Exercise Tolerance With Inspiratory Muscle Conditioning in Children With Cystic Fibrosis. Chest 1993, 104, 1490–1497. [Google Scholar] [CrossRef]
- da Silva, F.C.; Arancibia, B.A.V.; Iop, R.d.R.; Filho, P.J.B.G.; Silva, R.d. Escalas y listas de evaluación de la calidad de estudios científicos. Rev. Cuba. De Inf. En Cienc. De La Salud (ACIMED) 2013, 24, 295–312. [Google Scholar]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE Approach in Systematic Reviews and Guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef]
- Cai, W.; Li, M.; Xu, Y.; Li, M.; Wang, J.; Zuo, Y.; Cao, J. The Effect of Respiratory Muscle Training on Children and Adolescents with Cystic Fibrosis: A Systematic Review and Meta-Analysis. BMC Pediatr. 2024, 24, 252. [Google Scholar] [CrossRef]
- Stanford, G.; Ryan, H.; Solis-Moya, A. Respiratory Muscle Training for Cystic Fibrosis. Cochrane Database Syst. Rev. 2020, 12, CD006112. [Google Scholar] [CrossRef]
- Radtke, T.; Nevitt, S.J.; Hebestreit, H.; Kriemler, S. Physical Exercise Training for Cystic Fibrosis. Cochrane Database Syst. Rev. 2017, 11, CD002768. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, N. Exercise Programs for Children with Cystic Fibrosis: A Systematic Review of Randomized Controlled Trials. Disabil. Rehabil. 2010, 32, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gruber, W.; Stehling, F.; Blosch, C.; Dillenhoefer, S.; Olivier, M.; Koerner-Rettberg, C.; Sutharsan, S.; Mellies, U.; Taube, C.; Welsner, M. Effects of a Long-Term Monitored Exercise Program on Aerobic Fitness in a Small Group of Children with Cystic Fibrosis. Int. J. Environ. Res. Public Health 2022, 19, 7923. [Google Scholar] [CrossRef] [PubMed]
- Rosolia Capasso, C.; Miniato, A.L.; Di Filippo, P.; Di Ludovico, A.; Di Pillo, S.; Chiarelli, F.; Sferrazza Papa, G.F.; Attanasi, M. The Impact of Physical Activity on Clinical Outcomes in Children with Cystic Fibrosis: A Narrative Review. Children 2025, 12, 831. [Google Scholar] [CrossRef]
- Hilliard, T.N.; Regamey, N.; Shute, J.K.; Nicholson, A.G.; Alton, E.W.F.W.; Bush, A.; Davies, J.C. Airway Remodelling in Children with Cystic Fibrosis. Thorax 2007, 62, 1074–1080. [Google Scholar] [CrossRef]
- Levine, H.; Cohen-Cymberknoh, M.; Klein, N.; Hoshen, M.; Mussaffi, H.; Stafler, P.; Breuer, O.; Kerem, E.; Blau, H. Reversible Airway Obstruction in Cystic Fibrosis: Common, but Not Associated with Characteristics of Asthma. J. Cyst. Fibros. 2016, 15, 652–659. [Google Scholar] [CrossRef]
- Pastré, J.; Prévotat, A.; Tardif, C.; Langlois, C.; Duhamel, A.; Wallaert, B. Determinants of Exercise Capacity in Cystic Fibrosis Patients with Mild-to-Moderate Lung Disease. BMC Pulm. Med. 2014, 14, 74. [Google Scholar] [CrossRef]
- Pianosi, P. Peak Oxygen Uptake and Mortality in Children with Cystic Fibrosis. Thorax 2005, 60, 50–54. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.; Grevink, R.G.; Roorda, R.J.; Kaptein, A.A.; Van Der Schans, C.P. Effect of a Home Exercise Training Program in Patients With Cystic Fibrosis. Chest 1994, 105, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Reuveny, R.; DiMenna, F.J.; Gunaratnam, C.; Arad, A.D.; McElvaney, G.N.; Susta, D.; Peled, M.; Moyna, N.M. High-Intensity Interval Training Accelerates Oxygen Uptake Kinetics and Improves Exercise Tolerance for Individuals with Cystic Fibrosis. BMC Sports Sci. Med. Rehabil. 2020, 12, 9. [Google Scholar] [CrossRef]
- Hackshaw, A. Small Studies: Strengths and Limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Cruz Mosquera, F.E.; Murillo, S.R.; Naranjo Rojas, A.; Perlaza, C.L.; Castro Osorio, D.; Liscano, Y. Effect of Exercise and Pulmonary Rehabilitation in Pre- and Post-Surgical Patients with Lung Cancer: Systematic Review and Meta-Analysis. Medicina 2024, 60, 1725. [Google Scholar] [CrossRef]
- Da Silva Filho, L.V.R.F.; Zampoli, M.; Cohen-Cymberknoh, M.; Kabra, S.K. Cystic Fibrosis in Low and Middle-Income Countries (LMIC): A View from Four Different Regions of the World. Paediatr. Respir. Rev. 2021, 38, 37–44. [Google Scholar] [CrossRef]



| Author, Year | Country | Patients | Sex (% Male) | Age (Years) | Program Duration (Weeks) | Outcomes Assessed |
|---|---|---|---|---|---|---|
| Flores J et al. [37], 2023 | Brazil | n: 34, I: 19, C: 15 | 62% | 28 | 2 | Pulmonary function, exercise capacity, health-related quality of life. |
| Hebestrit H et al. [38], 2021 | International | n: 117, I: 60, C: 57 | 44% | 24 | 52 | Pulmonary function, exercise capacity, exacerbations, hospitalizations, health-related quality of life, and adverse effects. |
| Kaltsakas G et al. [39], 2021 | Greece and UK | n: 24, I: 12, C: 12 | 54% | 32 | 12 | Exercise capacity, health-related quality of life. |
| Gungor S et al. [40], 2022 | Turkey | n: 19, I: 10, C: 9 | 58% | 9 | 6 | Pulmonary function, health-related quality of life. |
| Emirza C et al. [41], 2021 | Turkey | n: 28, I: 14, C: 14 | 43% | 13 | 6 | Pulmonary function, exercise capacity, health-related quality of life, adverse effects. |
| Gupta S et al. [42], 2019 | India | n: 52, I: 25, C: 27 | 58% | 12.5 | 52 | Pulmonary function, exercise capacity, health-related quality of life. |
| Zeren M et al. [43], 2019 | Turkey | n: 36, I: 18, C: 18 | 47% | 13 | 8 | Pulmonary function, exercise capacity, and adverse events. |
| Bieli C et al. [44], 2017 | Switzerland | n: 22, I: 11, C: 11 | 45% | 14 | 8 | Pulmonary function, health-related quality of life. |
| Del corral T et al. [45], 2017 | Spain | n: 40, I: 20, C: 20 | 53% | 12 | 6 | Exercise capacity, health-related quality of life, and adverse events. |
| Schindel C et al. [46], 2015 | Brazil | n: 34, I: 17, C: 17 | 59% | 13 | 12 | Pulmonary function. |
| Hommerding P et al. [47], 2015 | Brazil | n: 34, I: 17, C: 17 | 59% | 13 | 12 | Pulmonary function, exercise capacity, and health-related quality of life. |
| Eidt P et al. [48], 2014 | Brazil | n: 41, I: 19, C: 22 | 34.15% | 24 | 12 | Pulmonary function, exercise capacity, and health-related quality of life. |
| Kriemler S et al. [49], 2013 | Switzerland | n: 39, I: 29, C: 10 | 58% | 21 | 24 | Pulmonary function, exercise capacity, health-related quality of life, and adverse effects. |
| Santana et al. [50], 2013 | Spain | n: 20, I: 10, C: 10 | 60% | 11 | 8 | Pulmonary function, exercise capacity, and adverse events. |
| Santana et al. [51], 2012 | Spain | n: 22, I: 11, C: 11 | 59% | 11 | 8 | Pulmonary function, exercise capacity, health-related quality of life, and adverse events. |
| Sandsund C et al. [52], 2011 | UK | n: 20, I: 10, C: 10 | 50% | 27 | 6 | Pulmonary function, health-related quality of life, and adverse events. |
| klijn P et al. [53], 2004 | Netherlands | n: 20, I: 11, C: 9 | NR | 14 | 12 | Exercise capacity, health-related quality of life, and adverse events. |
| Enright S et al. [54], 2004 | UK | n: 19, I: 9, C: 10 | 55% | 22 | 8 | Pulmonary function and health-related quality of life. |
| Moorcroft A et al. [55], 2004 | UK | n: 48, I: 30, C: 18 | NR | 23 | 52 | Exercise capacity. |
| Selvadurai et al. [56], 2002 | Australia | n: 31, I: 15, C: 16 | 61% | 11 | 8 | Pulmonary function, exercise capacity, and adverse events. |
| Jong W et al. [57], 2001 | Netherlands | n: 15, I: 7, C: 8 | 50% | 18 | 6 | Pulmonary function and exercise capacity. |
| Schneiderman J et al. [58], 2000 | Canada | n: 65, I: 30, C: 35 | 59% | 13 | 156 | Pulmonary function and exercise capacity. |
| Sawyer H et al. [59], 1993 | USA | n: 20, I: 10, C: 10 | 55% | 11 | 10 | Pulmonary function. |
| Author, Year | Setting | Program Activities | Number of Sessions | Conclusion |
|---|---|---|---|---|
| Flores J et al. [37], 2023 | Hospital | Early rehabilitation with aerobic exercise on a treadmill + strength. | 10 | The intervention improved muscle strength, fatigue, and quality of life. No significant differences were found in pulmonary function. |
| Hebestrit H et al. [38], 2021 | Hospital | Vigorous physical activity with motivational feedback. Included 30 min of strength exercises and 2 h of aerobic exercises. | 156 | The program increased vigorous physical activity and exercise capacity, but the control group showed superior improvement in FEV1 at 6 months. |
| Kaltsakas G et al. [39], 2021 | Hospital | Interval exercise. | 36 | The intervention improved respiratory muscle strength, with less desaturation and dyspnea during exercise, although it did not improve functional capacity. |
| Gungor S et al. [40], 2022 | Hospital | Respiratory physiotherapy, postural exercises, muscle strengthening, and core stability. | 6 | The intervention produced no significant changes in respiratory function, exercise tolerance, or postural stability. |
| Emirza C et al. [41], 2021 | Home | Expiratory muscle training with a PEP device. | 60 | The intervention was related to improvements in PCF, MEP, MIP, 6MWT, and Quality of Life. There were no adverse effects. |
| Gupta S et al. [42], 2019 | Home | Progressive resistance training and plyometric jumping exercises. | 144 | The intervention improved exercise capacity and quality of life. |
| Zeren M et al. [43], 2019 | Hospital and Home | Chest physiotherapy and inspiratory muscle training. | 56 | Inspiratory muscle training improved inspiratory strength, with no changes in pulmonary function, exercise tolerance, or quality of life. |
| Bieli C et al. [44], 2017 | Home | Respiratory resistance training with SpiroTiger®. | 40 | The intervention reduced respiratory muscle endurance; however, it did not improve exercise endurance or the other measured outcomes. |
| Del corral T et al. [45], 2017 | Home | Training through active video games (Nintendo Wii®). | 40 | The intervention improved respiratory endurance, with no changes in pulmonary function, tolerance, or quality of life. |
| Schindel C et al. [46], 2015 | Hospital | Aerobic activities, stretching exercises for upper and lower limbs. | 39 | The intervention did not generate significant changes in pulmonary function. |
| Hommerding P et al. [47], 2015 | Hospital | Verbal and written instructions to perform aerobic activities. | 24 | The intervention increased the frequency of physical exercise practice reported by participants, but did not generate improvements in pulmonary function, exercise capacity, or quality of life domains. |
| Eidt P et al. [48], 2014 | Home | Daily aerobic and strength exercise supervised by telephone. | 84 | The intervention improved muscle strength; however, there were no changes in functional capacity or quality of life. |
| Kriemler S et al. [49], 2013 | Home | Strength or endurance training. | 72 | The treatment improved FEV1 at 6 months. However, the benefits decreased at 24 months without continued training, indicating the importance of long-term regular physical activity. |
| Santana et al. [50], 2013 | Hospital and Home | Inspiratory muscle training, aerobic and strength training. | 24 | Improvement in exercise capacity and muscle strength. Some benefits were maintained after detraining. |
| Santana et al. [51], 2012 | Hospital | Combination of aerobic exercise on cycle ergometers and circuit weight training. | 24 | The combined in-hospital strength and aerobic training significantly improved cardiorespiratory capacity and muscle strength in children with cystic fibrosis. However, no improvements were observed in pulmonary function, body composition, or quality of life. |
| Sandsund C et al. [52], 2011 | Hospital | Musculoskeletal treatment, which included mobilizations of the rib cage and thoracic spine. | 6 | Although no significant short-term changes in pulmonary function were observed, improvements in the thoracic index and rib cage mobility were identified. |
| klijn P et al. [53], 2004 | Hospital | High-intensity anaerobic training. | 24 | The treatment improved anaerobic performance, aerobic performance, and quality of life. |
| Enright S et al. [54], 2004 | Hospital | Inspiratory muscle training using an electronic manometer. | 24 | High-intensity training improved strength, lung volumes, and psychosocial status. |
| Moorcroft A et al. [55], 2004 | Home | Individualized and unsupervised home exercise program: Aerobic activities and upper limb strength exercises. | 312 | The intervention achieved improvements in physical fitness and preserved pulmonary function in adults after 1 year, without the need for continuous supervision. |
| Selvadurai et al. [56], 2002 | Hospital | Aerobic or strength training. | 24 | The intervention improved exercise capacity and strength. Additionally, an improvement in cardiorespiratory capacity was evidenced. |
| Jong W et al. [57], 2001 | Hospital | Inspiratory muscle training using threshold load devices. | 30 | The intervention significantly improved inspiratory endurance in patients with cystic fibrosis, but there were no changes in pulmonary function, exercise capacity, dyspnea, or fatigue. |
| Schneiderman J et al. [58], 2000 | Hospital | Aerobic exercise. | 468 | Regular home aerobic exercise reduced the decline in pulmonary function in patients with cystic fibrosis over 3 years and was feasible to maintain with high adherence. |
| Sawyer H et al. [59], 1993 | Hospital | Inspiratory muscle training using threshold load devices. | 70 | Inspiratory muscle training significantly improved inspiratory strength, lung capacity, and exercise tolerance. |
| Author | The Study Is Randomized | The Intervention Is Double-Blind | Study Withdrawals Are Accounted for and Described | The Randomization Procedure Is Adequate | Selection Criteria | Score |
|---|---|---|---|---|---|---|
| Flores J et al. [37], 2023 | 1 | 1 | 1 | 1 | 1 | 5 |
| Hebestrit H et al. [38], 2021 | 1 | 0 | 1 | 1 | 1 | 4 |
| Kaltsakas G et al. [39], 2021 | 1 | 0 | 1 | 1 | 1 | 4 |
| Gungor S et al. [40], 2022 | 1 | 0 | 1 | 1 | 1 | 4 |
| Emirza C et al. [41], 2021 | 1 | 1 | 1 | 1 | 1 | 5 |
| Gupta S et al. [42], 2019 | 1 | 0 | 1 | 1 | 1 | 4 |
| Zeren M et al. [43], 2019 | 1 | 0 | 1 | 1 | 1 | 4 |
| Bieli C et al. [44], 2017 | 1 | 0 | 1 | 1 | 1 | 4 |
| Del corral T et al. [45], 2017 | 1 | 0 | 1 | 1 | 1 | 4 |
| Schindel C et al. [46], 2015 | 1 | 0 | 1 | 1 | 1 | 4 |
| Hommerding P et al. [47], 2015 | 1 | 0 | 1 | 1 | 1 | 4 |
| Eidt P et al. [48], 2014 | 1 | 0 | 1 | 1 | 1 | 4 |
| Kriemler S et al. [49], 2013 | 1 | 0 | 1 | 1 | 1 | 4 |
| Santana et al. [50], 2013 | 1 | 0 | 1 | 1 | 1 | 4 |
| Santana et al. [51], 2012 | 1 | 0 | 1 | 1 | 1 | 4 |
| Sandsund C et al. [52], 2011 | 1 | 0 | 0 | 1 | 1 | 3 |
| klijn P et al. [53], 2004 | 1 | 0 | 1 | 1 | 1 | 4 |
| Enright S et al. [54], 2004 | 1 | 1 | 1 | 1 | 1 | 5 |
| Moorcroft A et al. [55], 2004 | 1 | 0 | 1 | 1 | 1 | 4 |
| Selvadurai et al. [56], 2002 | 1 | 0 | 1 | 1 | 1 | 4 |
| Jong W et al. [57], 2001 | 1 | 1 | 1 | 1 | 1 | 5 |
| Schneiderman J et al. [58], 2000 | 1 | 0 | 1 | 1 | 1 | 4 |
| Sawyer H et al. [59], 1993 | 1 | 1 | 1 | 0 | 1 | 4 |
| Outcome | Effect Size (MD or SMD) | GRADE Certainty |
|---|---|---|
| Pulmonary function (FEV1) | SMD: 0.05 (−0.09 to 0.20) | ⬤⬤◯◯ |
| Pulmonary function (FVC) | SMD: 0.11 (−0.04 to 0.27) | ⬤⬤◯◯ |
| Pulmonary function (FEV1/FVC) | MD: 0.04 (−3.07 to 3.15) | ⬤⬤◯◯ |
| Pulmonary function (RV/TLC) | SMD: −0.02 (−0.33 to 0.29) | ⬤⬤◯◯ |
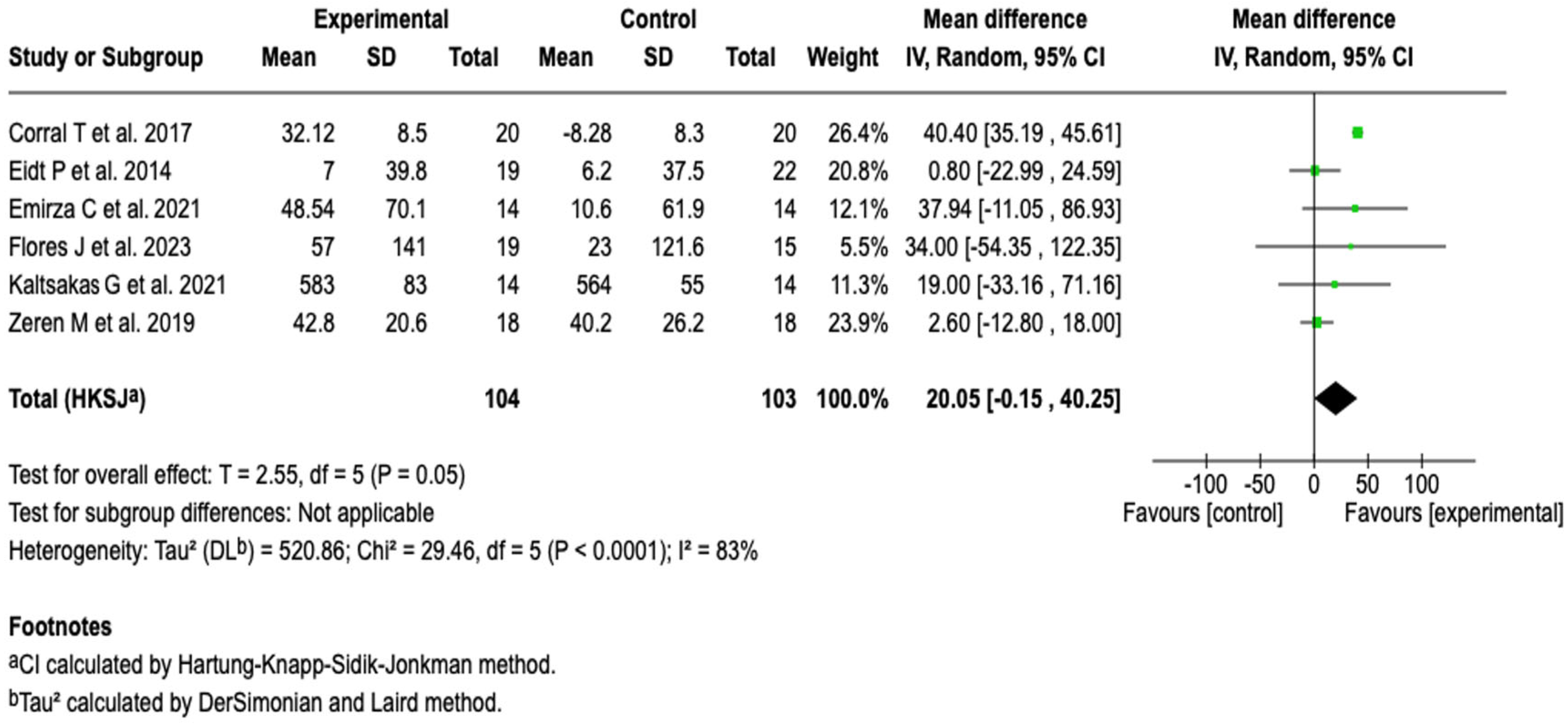
| Exercise capacity (6MWT) | MD: 20.05 (−0.15 to 40.25) | ⬤◯◯◯ |
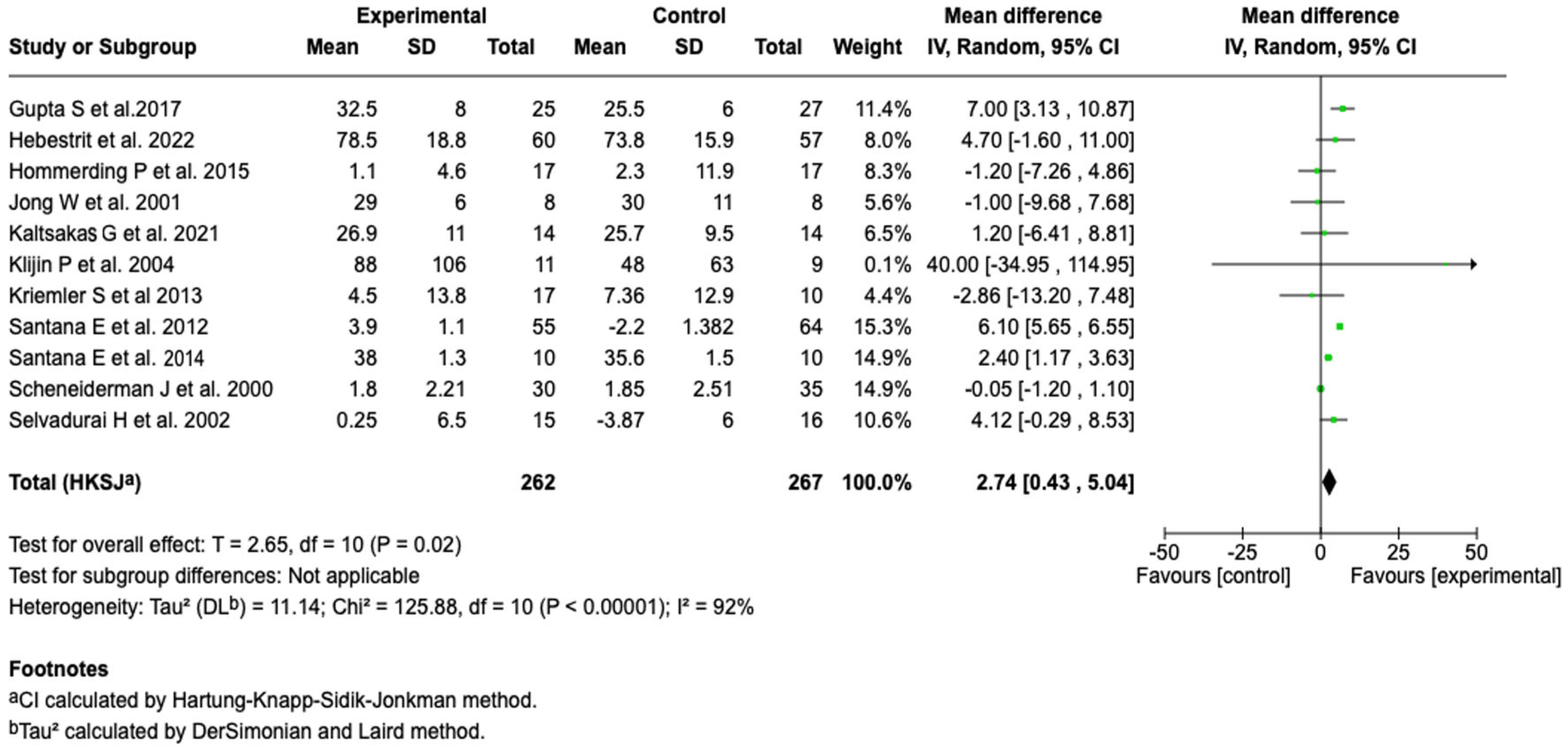
| Exercise capacity (VO2 max) | MD: 2.74 (0.43 to 5.04) | ⬤⬤◯◯ |
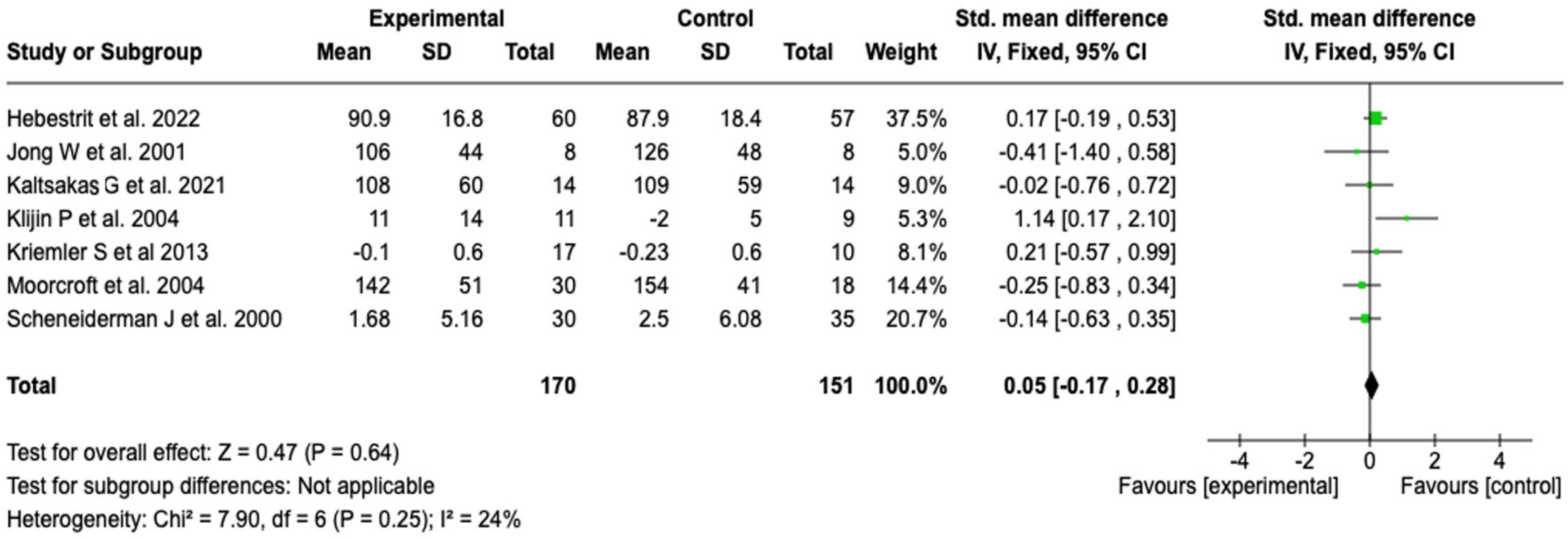
| Exercise capacity (Wmax) | SMD: 0.05 (−0.17 to 0.28) | ⬤⬤◯◯ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo, S.R.; Hinestroza Mancilla, A.M.; Pérez Ordoñez, L.M.; Ararat Carabalí, N.Y.; Cruz Mosquera, F.E.; Liscano, Y. Physical Training and Pulmonary Rehabilitation in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis of Clinical Trials. Healthcare 2025, 13, 2017. https://doi.org/10.3390/healthcare13162017
Murillo SR, Hinestroza Mancilla AM, Pérez Ordoñez LM, Ararat Carabalí NY, Cruz Mosquera FE, Liscano Y. Physical Training and Pulmonary Rehabilitation in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis of Clinical Trials. Healthcare. 2025; 13(16):2017. https://doi.org/10.3390/healthcare13162017
Chicago/Turabian StyleMurillo, Saray Ríos, Angie Melissa Hinestroza Mancilla, Lina Manuela Pérez Ordoñez, Naudy Yulisa Ararat Carabalí, Freiser Eceomo Cruz Mosquera, and Yamil Liscano. 2025. "Physical Training and Pulmonary Rehabilitation in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis of Clinical Trials" Healthcare 13, no. 16: 2017. https://doi.org/10.3390/healthcare13162017
APA StyleMurillo, S. R., Hinestroza Mancilla, A. M., Pérez Ordoñez, L. M., Ararat Carabalí, N. Y., Cruz Mosquera, F. E., & Liscano, Y. (2025). Physical Training and Pulmonary Rehabilitation in Patients with Cystic Fibrosis: A Systematic Review and Meta-Analysis of Clinical Trials. Healthcare, 13(16), 2017. https://doi.org/10.3390/healthcare13162017






