Feasibility and Potential Effects of Multidomain Interventions to Improve the Cognitive and Functional Well-Being of Elderly Individuals in Residential Structures: The I-COUNT Pilot Study Protocol
Abstract
1. Introduction
2. Materials and Methods
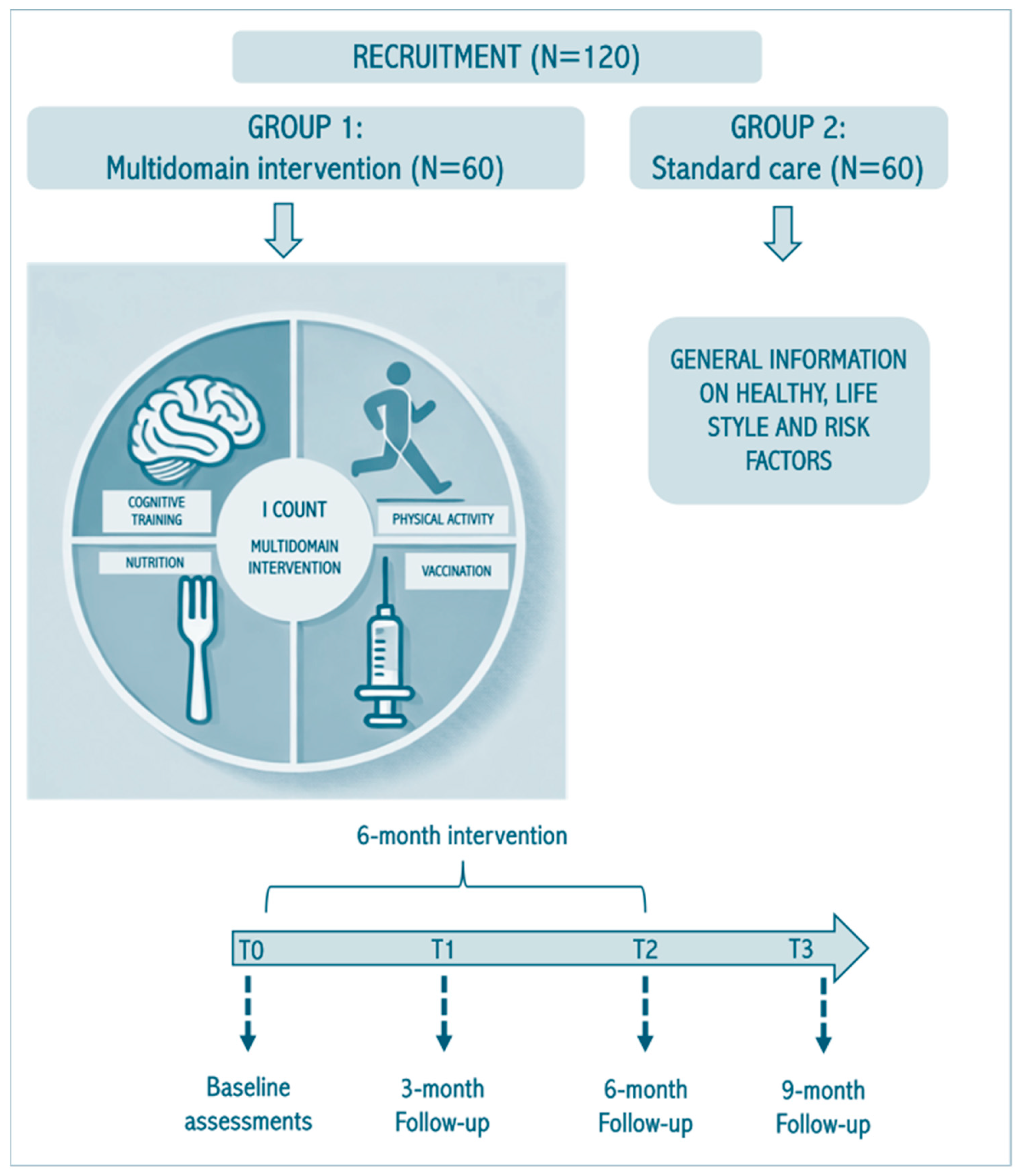
2.1. Study Design
2.2. Recruitment Process
2.2.1. Sample Size
2.2.2. Randomization and Blinding
2.3. Baseline Assessments
2.3.1. Dietary Assessment
2.3.2. Body Composition and Physical Evaluation
2.3.3. Neuropsychological and Psychological Assessments
2.3.4. Biomarkers
2.4. Multidomain Intervention
2.4.1. Nutritional Intervention
2.4.2. Physical Intervention
- Endurance exercises with muscle strengthening of the lower and upper limbs, such as getting up from a chair or bed, front and side step-ups, lifting the lower limbs, lifting water bottles, etc. The physiotherapists or exercise science experts will adapt the type of exercises to the participant’s abilities;
- Static and dynamic balance exercises of increasing difficulty, tailored to the patient’s initial performance level and subsequent improvements (e.g., exercises to maintain a standing position on a flat surface, load transfer, proprioceptive exercises on an unstable surface or balance board, maintaining balance on monopodal support, semi-tandem walking on a straight line, walking with small objects, etc.). If necessary, the physiotherapist or the exercise science expert will use tools to reduce the risk of falling during treatment;
- Exercises for aerobic activity will include walking for a number of minutes appropriate to the patient’s baseline functional performance level, following the patient’s subsequent progress during the sessions.
2.4.3. Cognitive Intervention
2.4.4. Vaccinations
2.5. Follow-Up Assessments
2.6. Retention
2.7. Analytical Approach
2.7.1. Outcomes
- Recruitment and retention rates over the study period;
- Adherence metrics (attendance at training sessions, completion of cognitive modules, dietary compliance);
- Technological acceptability, assessed via user feedback and actual usage data (wearable sensors, digital apps);
- Completeness and quality of data collected across domains;
- Operational challenges, including staffing, logistics, and data management within LTCFs.
- Gut microbiota composition and differences between the two groups at follow-up at 6 and 9 months (T2 and T3);
- Hemogram, electrolytes, cholesterol, triglycerides, blood glucose, vitamin D, and other parameters and differences between the two groups at 6- and 9-month follow-ups (T2 and T3);
- Additional biomarkers (including ESR, high-sensitivity CRP, albumin, transferrin, IL-6, BDNF, FT3/FT4, s-RAGE, irisin, IGF1, ghrelin) and differences between the two groups at 6- and 9- months follow-up (T2 and T3);
- Physical performance (SPPB) and differences between groups at 6- and 9-month follow-ups (T2 and T3);
- General and domain-specific cognitive functioning (mNTB, ACE-R) and differences between groups at 6- and 9-month follow-ups (T2 and T3);
- Psychological health (GDS, PSS, DASS), sleep quality (PSQI) and differences between groups at 6- and 9-month follow-ups (T2 and T3);
- Number of falls and number of hospital admissions and differences between groups at 6- and 9-month follow-ups (T2 and T3);
- Nutritional, anthropometric status (MNA-SF, BMI, waist circumference), and body composition, and differences between the groups at 6- and 9-month follow-ups (T2 and T3).
2.7.2. Adverse Events
2.7.3. Statistical Analyses
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-R | Addenbrooke’s Cognitive Examination Revised |
| ADL | Activities of Daily Living |
| ASMM | Appendicular Skeletal Muscle Mass |
| ASMMI | Appendicular Skeletal Muscle Mass Index |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| CIRS | Cumulative Illness Rating Scale |
| CRIq | Cognitive Reserve Index questionnaire |
| DASS | Depression Anxiety Stress Scale |
| ESS | Exton–Smith Scale |
| GDS | Geriatric Depression Scale |
| IADL | Instrumental Activities of Daily Living |
| I-COUNT | Multidomain Interventions to improve the COgnitive and fUNctional well-being of elderly individuals in residential sTructures |
| ITT | Intention-to-treat |
| LTCFs | Long-Term Care Facilities |
| MMSE | Mini-Mental State Examination |
| MNA-SF | Mini Nutritional Assessment-Short Form |
| MPI | Multidimensional Prognostic Index |
| MUAC | Mid-upper arm circumference |
| PNRR | National Recovery and Resilience Plan |
| PSQI | Pittsburgh Sleep Quality Index |
| PSS | Perceived Stress Scale |
| RECODE | Remote stimulation for Cognitive DEcline |
| SPMSQ | Short Portable Mental Status Questionnaire |
| SPPB | Short Physical Performance Battery |
| WHOQOL-BRIEF | World Health Organization Quality of Life scale |
References
- United Nations 2025. Available online: https://www.un.org/en/global-issues/ageing (accessed on 10 January 2025).
- Eurostat Database 2025. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing (accessed on 26 June 2025).
- Khan, H.T.A.; Addo, K.M.; Findlay, H. Public Health Challenges and Responses to the Growing Ageing Populations. Public Health Chall. 2024, 3, e213. [Google Scholar] [CrossRef]
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Marzetti, E.; Canevelli, M.; Guaraldi, G. Geriatric syndromes: How to treat. Virulence 2017, 8, 577–585. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Noutsias, M.; Hauptmann, M.; Völler, H. Pointing a FINGER at the contribution of lifestyle to cardiovascular events and dementia. Eur. Heart J. 2022, 43, 2062–2064. [Google Scholar] [CrossRef]
- Rosenberg, A.; Mangialasche, F.; Ngandu, T.; Solomon, A.; Kivipelto, M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. J. Prev. Alzheimer’s Dis. 2020, 7, 29–36. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimer’s Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Patel, H.; Wijk, H.; Ekman, I.; Olaya-Contreras, P. A systematic review on implementation of person-centered care interventions for older people in out-of-hospital settings. Geriatr. Nurs. 2021, 42, 213–224. [Google Scholar] [CrossRef]
- Ceolin, C.; Papa, M.V.; Scagnellato, L.; Doria, A.; Sergi, G.; Ramonda, R. Is sarcopenia a real concern in ankylosing spondylitis? A systematic literature review. Eur. Geriatr. Med. 2024, 15, 903–912. [Google Scholar] [CrossRef]
- Scisciola, L.; Fontanella, R.A.; Surina; Cataldo, V.; Paolisso, G.; Barbieri, M. Sarcopenia and cognitive function: Role of myokines in muscle brain cross-talk. Life 2021, 11, 173. [Google Scholar] [CrossRef]
- Gutiérrez-Reguero, H.; Buendía-Romero, Á.; Franco-López, F.; Martínez-Cava, A.; Hernández-Belmonte, A.; Courel-Ibáñez, J.; Ara, I.; Alcazar, J.; Pallarés, J.G. Effects of multicomponent training and HMB supplementation on disability, cognitive and physical function in institutionalized older adults aged over 70 years: A cluster-randomized controlled trial. J. Nutr. Health Aging 2024, 28, 100208. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mehdi, M.M. Functional foods, bioactives, and cognitive impairments during aging. In Plant Bioactives as Natural Panacea Against Age-Induced Diseases: Nutraceuticals and Functional Lead Compounds for Drug Development, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 271–286. [Google Scholar] [CrossRef]
- Aguirre, E.; Woods, R.T.; Spector, A.; Orrell, M. Cognitive stimulation for dementia: A systematic review of the evidence of effectiveness from randomised controlled trials. Ageing Res. Rev. 2013, 12, 253–262. [Google Scholar] [CrossRef]
- Ceolin, C.; De Rui, M.; Simonato, C.; Vergadoro, M.; Cazzavillan, S.; Acunto, V.; Papa, M.V.; Trapella, G.S.; Zanforlini, B.M.; Curreri, C.; et al. Sarcopenic patients “get even”: The impact of COVID-19 vaccination on mortality. Exp. Gerontol. 2024, 187, 112382. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Triolo, F.; Maggi, S.; Malley, R.; Jackson, T.A.; Poscia, A.; Bernabei, R.; Ferrucci, L.; Fratiglioni, L. Fostering healthy aging: The interdependency of infections, immunity and frailty. Ageing Res. Rev. 2021, 69, 101351. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, S.; Zhou, C.; Zhang, A.; Zhang, S.; Wang, Y. Effects of exercise interventions on cognition, physical function and quality of life among older adults with cognitive frailty: A systematic review and meta-analysis. Geriatr. Nurs. 2025, 62 Pt A, 96–107. [Google Scholar] [CrossRef]
- Rossi, P.G.; Carnavale, B.F.; Farche, A.C.S.; Ansai, J.H.; de Andrade, L.P.; Takahashi, A.C.M. Effects of physical exercise on the cognition of older adults with frailty syndrome: A systematic review and meta-analysis of randomized trials. Arch. Gerontol. Geriatr. 2021, 93, 104322. [Google Scholar] [CrossRef]
- Langlois, F.; Vu, T.T.; Chassé, K.; Dupuis, G.; Kergoat, M.J.; Bherer, L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2013, 68, 400–404. [Google Scholar] [CrossRef]
- Silva, A.F.; Silva, R.M.; Murawska-Ciałowicz, E.; Zurek, G.; Danek, N.; Cialowicz, M.; Carvalho, J.; Clemente, F.M. Cognitive Training with Older Adults Using Smartphone and Web-Based Applications: A Scoping Review. J. Prev. Alzheimers Dis. 2024, 11, 693–700. [Google Scholar] [CrossRef]
- Chen, C.; Huang, N.; Hu, B.; Zhang, M.; Yuan, J.; Guo, J. The effectiveness of digital technology interventions for cognitive func-tion in older adults: A systematic review and meta-analysis of randomized controlled trials. Geroscience 2025, 47, 653–683. [Google Scholar] [CrossRef]
- Ristori, S.; Bertoni, G.; Bientinesi, E.; Monti, D. The Role of Nutraceuticals and Functional Foods in Mitigating Cellular Senescence and Its Related Aspects: A Key Strategy for Delaying or Preventing Aging and Neurodegenerative Disorders. Nutrients 2025, 17, 1837. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
- Mijan, M.A.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673–2685. [Google Scholar] [CrossRef]
- Resciniti, N.; Kaplan, D.; Sellner, J.; Lohman, M. Vaccine access shrinks disparities between long-term care and community rates of COVID-19 mortality. Innov. Aging 2021, 5 (Suppl. S1), 1019–1020. [Google Scholar] [CrossRef]
- Frangos, E.; Barratt, J.; Michel, J.P.; Ecarnot, F. Vaccines in Long-Term Care Settings: A Narrative Review. Gerontology. 2024, 70, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.E.; Brown, C.S.; Nguyen-Van-Tam, J.S. Influenza in long-term care facilities. Influ. Other Respir. Viruses 2017, 11, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Lewis, M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ferrucci, L.; Franceschi, M.; D’Ambrosio, L.P.; Scarcelli, C.; Cascavilla, L.; Paris, F.; Placentino, G.; Seripa, D.; Dallapiccola, B.; et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008, 11, 151–161. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2013, 17, 2769–2782. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Roche, A.F.; Steinbaugh, M.L. Estimating Stature from Knee Height for Persons 60 to 90 Years of Age. J. Am. Geriatr. Soc. 1985, 33, 116–120. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-mental state examination: A normative study in Italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.; Raimo, S.; Tufano, D.; Basile, G.; Grossi, D.; Santangelo, F.; Trojano, L.; Santangelo, G. The Addenbrooke’s Cognitive Examination Revised (ACE-R) and its sub-scores: Normative values in an Italian population sample. Neurol. Sci. 2016, 37, 385–392. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, G.; Rucci, P.; Scocco, P.; Becchi, A.; Coppa, F.; D’Addario, A.; Daru, E.; De Leo, D.; Galassi, L.; Mangelli, L. Quality of life assessment: Validation of the Italian version of the WHOQOL-Brief. Epidemiol. Psychiatr. Sci. 2000, 9, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2011, 24, 218–226. [Google Scholar] [CrossRef]
- Galaria, I.I.; Casten, R.J.; Rovner, B.W. Development of a shorter version of the geriatric depression scale for visually impaired older patients. Int. Psychogeriatr. 2000, 12, 435–443. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R. The short-form version of the Depression anxiety stress scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef]
- Cohen, S. Perceived stress in a probability sample of the United States. In The Social Psychology of Health: Claremont Symposium on Applied Social Psychology; Spacapan, S., Oskamp, S., Eds.; Sage: Thousand Oaks, CA, USA, 1988; pp. 31–67. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inf. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Da Ros, A.; Polo, A.; Giuseppe Rizzello, C.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, e0049421. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; di Palo, D.M.; Lorusso, M.P.; de Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough fermented breads are more digestible than those started with baker’s yeast alone: An in vivo challenge dissecting distinct gastrointestinal responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S.; Fki, I.; Kobayashi, I.; Khalid, N.; Neves, M.A.; Isoda, H.; Sayadi, S.; Nakajima, M. Emerging Technologies for Recovery of Value-Added Components from Olive Leaves and Their Applications in Food/Feed Industries. Food Bioprocess Technol. 2017, 10, 229–248. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Lonigro, S.L.; Visconti, A.; De Angelis, M.; Valerio, F.; Morelli, L. Table Olives Containing Probiotic Microorganisms. European Patent EP1843664, 8 July 2009. [Google Scholar]
- Lavermicocca, P.; Lonigro, S.L.; Valerio, F.; Visconti, A.; Vanadia, S.; Calabrese, N.; Di Venere, D.; Morelli, L. Process for the Preparation of Vegetable Preserves Containing Probiotic Micro-Organisms. Italian Patent 001357149, 9 March 2009. [Google Scholar]
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; De Angelis, M.; Morelli, L.; Callegari, M.L.; Rizzello, C.G.; Visconti, A. Study of adhesion and survival of lactobacilli and bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl. Environ. Microbiol. 2005, 71, 4233–4240. [Google Scholar] [CrossRef]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Guerra, V.; Valerio, F.; Lavermicocca, P.; De Candia, S. Randomised clinical trial: Efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation-a double-blind, controlled, crossover study. Aliment. Pharmacol. Ther. 2005, 35, 441–450. [Google Scholar] [CrossRef]
- Valerio, F.; De Bellis, P.; Lonigro, S.L.; Morelli, L.; Visconti, A.; Lavermicocca, P. In vitro and in vivo survival and transit tolerance of potentially probiotic strains carried by artichokes in the gastrointestinal tract. Appl. Environ. Microbiol. 2006, 72, 3042–3045. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Parise, A.; Mena, P.; Meschi, T. The interaction between Mediterranean diet and intestinal microbiome: Relevance for preventive strategies against frailty in older individuals. Aging Clin. Exp. Res. 2024, 36, 58. [Google Scholar] [CrossRef]
- Fabbri, E.; Zoli, M.; Gonzalez-Freire, M.; Salive, M.E.; Studenski, S.A.; Ferrucci, L. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. J. Am. Med. Dir. Assoc. 2015, 16, 640–647. [Google Scholar] [CrossRef]
- Barbera, M.; Perera, D.; Matton, A.; Mangialasche, F.; Rosenberg, A.; Middleton, L.; Ngandu, T.; Solomon, A.; Kivipelto, M. Multimodal Precision Prevention—A New Direction in Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2023, 10, 718–728. [Google Scholar] [CrossRef]
- Dedeyne, L.; Deschodt, M.; Verschueren, S.; Tournoy, J.; Gielen, E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: A systematic review. Clin. Interv. Aging 2017, 12, 873–896. [Google Scholar] [CrossRef]
- Barnes, J.N. Exercise, cognitive function, and aging. Adv. Physiol. Educ. 2015, 39, 55–62. [Google Scholar] [CrossRef]
- Xu, L.; Gu, H.; Cai, X.; Zhang, Y.; Hou, X.; Yu, J.; Sun, T. The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2023, 20, 1088. [Google Scholar] [CrossRef]
- Doherty, T.M.; Connolly, M.P.; Del Giudice, G.; Flamaing, J.; Goronzy, J.J.; Grubeck-Loebenstein, B.; Lambert, P.H.; Maggi, S.; McElhaney, J.E.; Nagai, H.; et al. Vaccination programs for older adults in an era of demographic change. Eur. Geriatr. Med. 2018, 9, 289–300. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, Z.; Macchia, E.; Ceolin, C.; Devita, M.; Morandi, A.; Noale, M.; Maggi, S., on behalf of I-COUNT Study Group. Feasibility and Potential Effects of Multidomain Interventions to Improve the Cognitive and Functional Well-Being of Elderly Individuals in Residential Structures: The I-COUNT Pilot Study Protocol. Healthcare 2025, 13, 1999. https://doi.org/10.3390/healthcare13161999
Romeo Z, Macchia E, Ceolin C, Devita M, Morandi A, Noale M, Maggi S on behalf of I-COUNT Study Group. Feasibility and Potential Effects of Multidomain Interventions to Improve the Cognitive and Functional Well-Being of Elderly Individuals in Residential Structures: The I-COUNT Pilot Study Protocol. Healthcare. 2025; 13(16):1999. https://doi.org/10.3390/healthcare13161999
Chicago/Turabian StyleRomeo, Zaira, Eleonora Macchia, Chiara Ceolin, Maria Devita, Alessandro Morandi, Marianna Noale, and Stefania Maggi on behalf of I-COUNT Study Group. 2025. "Feasibility and Potential Effects of Multidomain Interventions to Improve the Cognitive and Functional Well-Being of Elderly Individuals in Residential Structures: The I-COUNT Pilot Study Protocol" Healthcare 13, no. 16: 1999. https://doi.org/10.3390/healthcare13161999
APA StyleRomeo, Z., Macchia, E., Ceolin, C., Devita, M., Morandi, A., Noale, M., & Maggi, S., on behalf of I-COUNT Study Group. (2025). Feasibility and Potential Effects of Multidomain Interventions to Improve the Cognitive and Functional Well-Being of Elderly Individuals in Residential Structures: The I-COUNT Pilot Study Protocol. Healthcare, 13(16), 1999. https://doi.org/10.3390/healthcare13161999








