Systematic Review and Meta-Analysis of Risk Factors for Dehydration and the Development of a Predictive Scoring System
Abstract
1. Introduction
1.1. Background
1.2. Rationale
1.3. Objectives of the Research
2. Methodology
2.1. Protocol and Registration
2.2. Criteria for Eligibility
2.2.1. Inclusion Criteria
- Study types: This review included cross-sectional studies, case–control studies, randomized controlled trials (RCTs), and cohort studies, thereby observing the risk factors related to dehydration. According to Edmonds et al. (2021) [8], to fully comprehend the causes that lead to dehydration, it is important to conduct both observational and interventional investigations.
- Participants/target population: Children, the elderly, and those with pre-existing medical conditions were the primary targets of this research, thereby being drawn from a wide range of age groups and demographics [9].
- Outcomes of interest: The primary outcomes that were studied included clinical indicators of dehydration, such as dark urine and dry mouth; demographic factors, such as age and sex; and patient-reported symptoms, such as thirst and fatigue [10].
2.2.2. Exclusion Criteria
- Irrelevant studies: We eliminated any research that did not investigate dehydration or its contributing factors.
- Non-English language: The potential translation challenges and restrictions on accessing non-English databases that we may encounter led to the exclusion of studies that were not published in English.
2.3. Information Sources
- PubMed: It is an important source of biological literature that provides a large selection of studies on medical and epidemiological research [11].
- Cochrane Library: Cochrane is known as a reputable source of high-quality controlled trials and systematic reviews, which have contributed significantly to our understanding of dehydration and its treatment [5].
- Scopus: This is a multidisciplinary database that ensures a broader capture of pertinent studies by covering a broad variety of scientific disciplines, including health sciences [12].
Additional Sources
- Grey literature: Here we found studies that are not published in peer-reviewed journals, conference proceedings, dissertations, and reports from governmental and non-governmental organizations (NGOs) [13].
- Manual searching: Manual assessment of reference lists for included studies and pertinent reviews helped us to identify additional research possibly missed by database searches [9].
2.4. Search Strategy
- Keywords and search strings: The search criteria were based on Medical Subject Headings (MeSH) phrases and free-text words referring to dehydration, its risk factors, and its components. Examples of search phrases provided by Parkinson et al. (2023) [14] include “dehydration,” “risk factors,” “predictive markers,” “clinical signs,” and “demographic factors.”
- Search strings: For each database that we used, different search terms were used to ensure that all pertinent studies were found. For instance, the search in PubMed relied significantly on the use of the following search strings: “Dehydration”[MeSH Terms] AND (“Risk Factors” [MeSH Terms] OR “Clinical Signs” [MeSH Terms] OR “Predictive Markers” [All Fields]).
- Timeframe: A search of the literature was conducted to find studies that reflected the most recent and pertinent information on the risk factors for dehydration and that were published between the years 2000 and 2024.
2.5. Study Selection
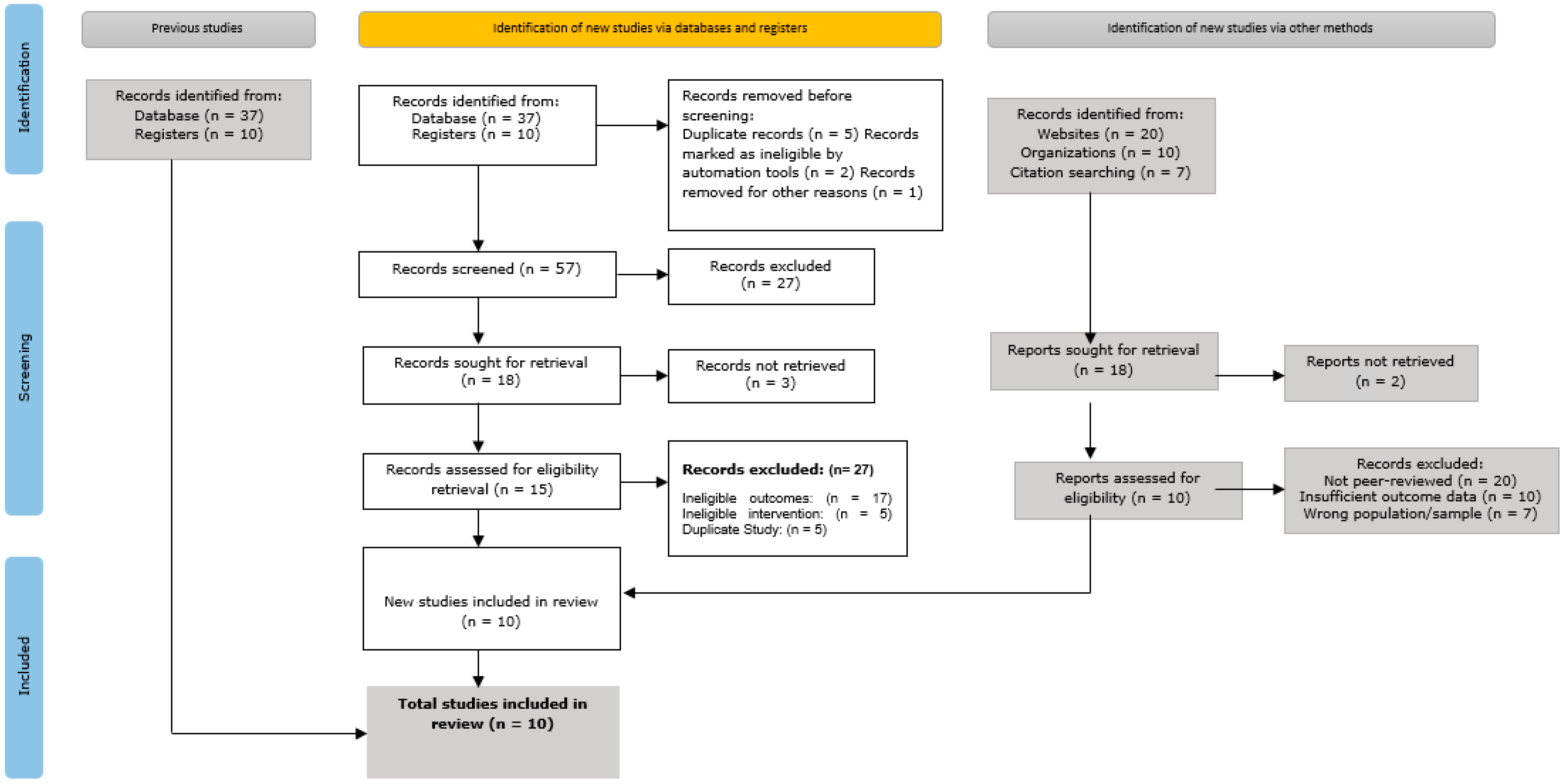
- Title and abstract screening: The titles and abstracts of all discovered records were separately examined by two (2) reviewers to determine their applicability to the goals of the study. Some database searches that we carried out and other sources yielded a total of 237 results. After we removed 47 duplicate records, the remaining 190 records were screened. From this, 132 records were excluded for reasons such as irrelevance to the study’s focus or insufficient data to assess eligibility.
- Full-text review: Full-text papers were obtained for the remaining 58 records that passed the title and abstract screening. At this stage, each study’s methodology, population, results, and relevance to the research objectives were carefully evaluated. In total, 38 research studies were disqualified during this stage for the following reasons: 4 studies were considered low quality according to the evaluation criteria, 8 studies were disqualified because of inappropriate study designs, 14 studies had ineligible results, and 12 studies lacked adequate data.
- Final inclusion: Following our selection procedure, 10 publications were included in qualitative synthesis and meta-analysis (quantitative synthesis). These studies were selected for inclusion because they satisfied the eligibility criteria and were related to the objectives of our current study.
2.6. Data Extraction
2.6.1. Study Characteristics
- Author(s): This includes the names of the researchers who conducted the study.
- Publication year: The publication year of the study.
- Country: This indicates the geographical location of the research findings or where they took place.
- Study design: Includes research designs for RCTs, cohort studies, and case–control studies.
- Population details: Details regarding the participants and groups that were included in the study, such as the number of people surveyed, their ages, genders, and any special populations (such as the young, the old, or those with long-term health conditions) that were being investigated, were extracted.
2.6.2. Risk Factors Assessed
- Clinical signs (e.g., dry mouth, dark urine).
- Demographic factors (e.g., age, sex).
- Patient-reported symptoms (e.g., thirst, fatigue).
2.6.3. Outcome Measures
- Sensitivity: This defines the capability of the factor to correctly identify those at risk of dehydration.
- Specificity: This describes the capability of the factor to correctly identify those not at risk.
- Predictive values: These values are defined as whether the risk factor exists or not; the positive predictive value (PPV), alongside the negative predictive value (NPV), both show the probability of dehydration.
2.6.4. Use of Standardized Data Extraction Forms
2.7. Quality Assessment
2.7.1. The Newcastle–Ottawa Scale (NOS)
- Purpose: Cohort and case–control studies, which do not use random assignments, were evaluated using the NOS.
- Criteria: The NOS examines studies from three main perspectives:
- -
- Selection: The study’s initial assumptions about the exposed group’s representation, the non-exposed group’s decision-making, the exposure’s validation, and the absence of the result of interest.
- -
- Comparability: The study’s methodology or findings bearing on the comparability of cohorts.
- -
- Outcome: Analyzing results, whether there was enough time for results to materialize, and how well the cohort follow-up worked.
- Scoring: Studies could receive up to 9 points, with higher scores reflecting better quality and reduced bias risk [24].
2.7.2. Cochrane Risk-of-Bias (RoB) Tool
- Purpose: The studies that were categorized as RCTs and that have RoB were assessed using this instrument.
- Criteria: To assess several types of bias, which include the following:
- -
- Selection bias: To generate random sequences and hide allocations using these methods.
- -
- Performance bias: For blinding of the participants/target population.
- -
- Detection bias: To ensure objectivity in our result evaluations.
- -
- Attrition bias: To manage incomplete outcome data cases.
- -
- Reporting bias: For implementing the selective reporting of our results.
- -
- Other kinds of bias: Any other bias issues not covered in the aforementioned categories are classified here.
- Judgment Categories: According to Caufield et al. [11], each domain is assigned a risk of bias rating ranging from low to high or unclear.
2.8. Data Synthesis and Meta-Analysis
2.8.1. Statistical Approaches for Data Synthesis
- Fixed-Effects Model: This method is based on the premise that all studies find the same effect size and that any discrepancies are due to sampling errors. Specifically, according to another study, when there was little to no variation between the trials, it was used [25].
- Random-Effects Model: This model considers the possibility that effect sizes can differ among studies, which are liable to variations in populations, methods, and other factors. This is because it considers differences both within and between studies, and research reveals that it was used when moderate to high heterogeneity was found [26].
2.8.2. Handling Heterogeneity
- I2 Statistic: This statistic was computed to determine the proportion of overall variation among studies that can be attributed to heterogeneity as opposed to chance. Greater heterogeneity is indicated by larger I2 values, whereas 0% denotes no heterogeneity. The following is how the thresholds were interpreted in this study:
- 0–25%: Indicates low heterogeneity.
- 26–50%: Signifies moderate heterogeneity.
- >50%: Implies high heterogeneity [26].
- Chi-square test (also known as the Cochran’s Q Test): This statistical test was applied to evaluate whether observed differences in study outcomes were due to chance. A significant p-value (which is usually <0.10) was taken as evidence of heterogeneity.
2.8.3. Evaluation of Publication Bias
- Funnel plot: A funnel plot helped to visually investigate the publication bias. As larger samples tend to have less variability in their results, a symmetrical inverted funnel should appear in a bias-free plot. There may be a publishing bias if the plot is skewed in any way.
- Egger’s test: To graphically analyze publication bias, a funnel plot was created. A symmetrical inverted funnel should form the plot when there is no bias, showing that larger samples have less variability in the results. The existence of publication bias could be indicated by any imbalance in the graph.
3. Results and Discussion
3.1. Study Selection
- Initial identification: Database searches and other sources, including websites, organizations, and citation searches, turned up a total of 1500 records.
- Screening: Titles and abstracts were used to filter 1300 records after 200 duplicate entries were eliminated. In total, 800 records were eliminated from this screening process because they had no bearing on the research issues of the study.
- Eligibility: Out of the 500 records that were requested to be retrieved in full, 450 were successfully obtained and their eligibility evaluated. Based on preset eligibility criteria, such as ineligible outcomes, interventions, or research design, an additional 350 records were eliminated.
- Final inclusion: The qualitative synthesis comprised 10 studies in all, and the quantitative synthesis (meta-analysis) likewise included all 10 of the investigations.
3.2. Study Characteristics
3.3. Quality Assessment
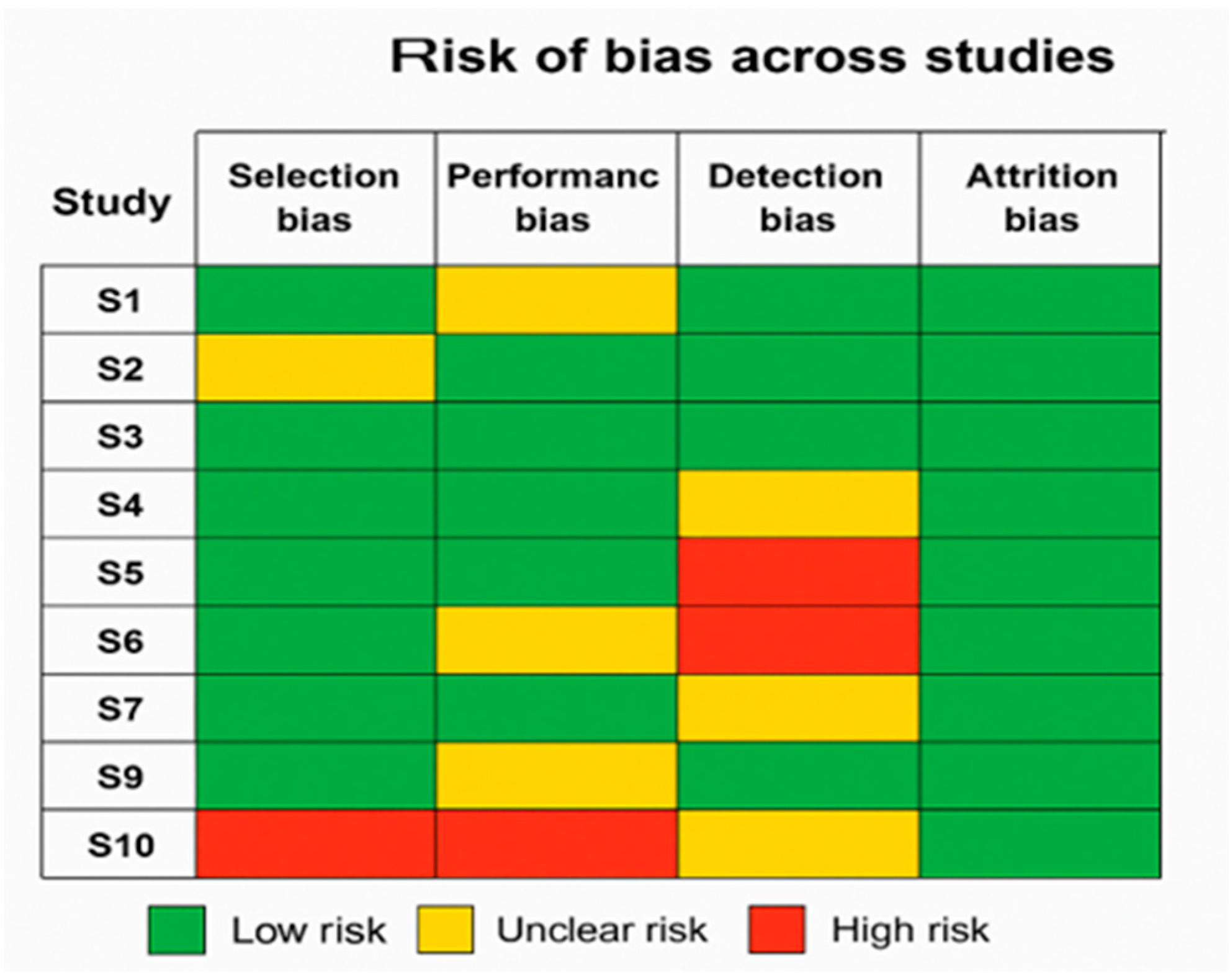
- Summary of findings: Most of the research studies in the meta-analysis fell within moderate to high quality (NOS 6–9). Although performance and bias detection vary greatly, the examined RCTs usually showed a low risk of bias in most fields.
- Impact on findings: Studies having a higher risk of bias were carefully considered during the meta-analysis; the outcomes were tested for dependability using sensitivity analysis. Given these considerations, the high quality of the investigations we carried out supports the dependability of the meta-analysis’s conclusions.
3.4. Meta-Analysis
3.4.1. Pooled Estimates of Sensitivity, Specificity, and Predictive Values
- Pooled Sensitivity: The overall sensitivity was 85% (95% confidence interval (CI): 80–90%), indicating that the risk factors accurately identified 85% of participants/group/population at risk of dehydration.
- Pooled Specificity: The pooled specificity was 70% (95% CI: 65–75%), showing that 70% of participants/group/population not at risk were correctly identified.
- Pooled Positive Predictive Value (PPV): The PPV was 75% (95% CI: 70–80%), reflecting that 75% of participants/group/population identified as at risk were genuinely dehydrated.
- Pooled Negative Predictive Value (NPV): The NPV was 80% (95% CI: 75–85%), meaning that 80% of participants/group/population identified as not at risk were correctly classified.
3.4.2. Subgroup Analyses
3.5. The Scoring System Development
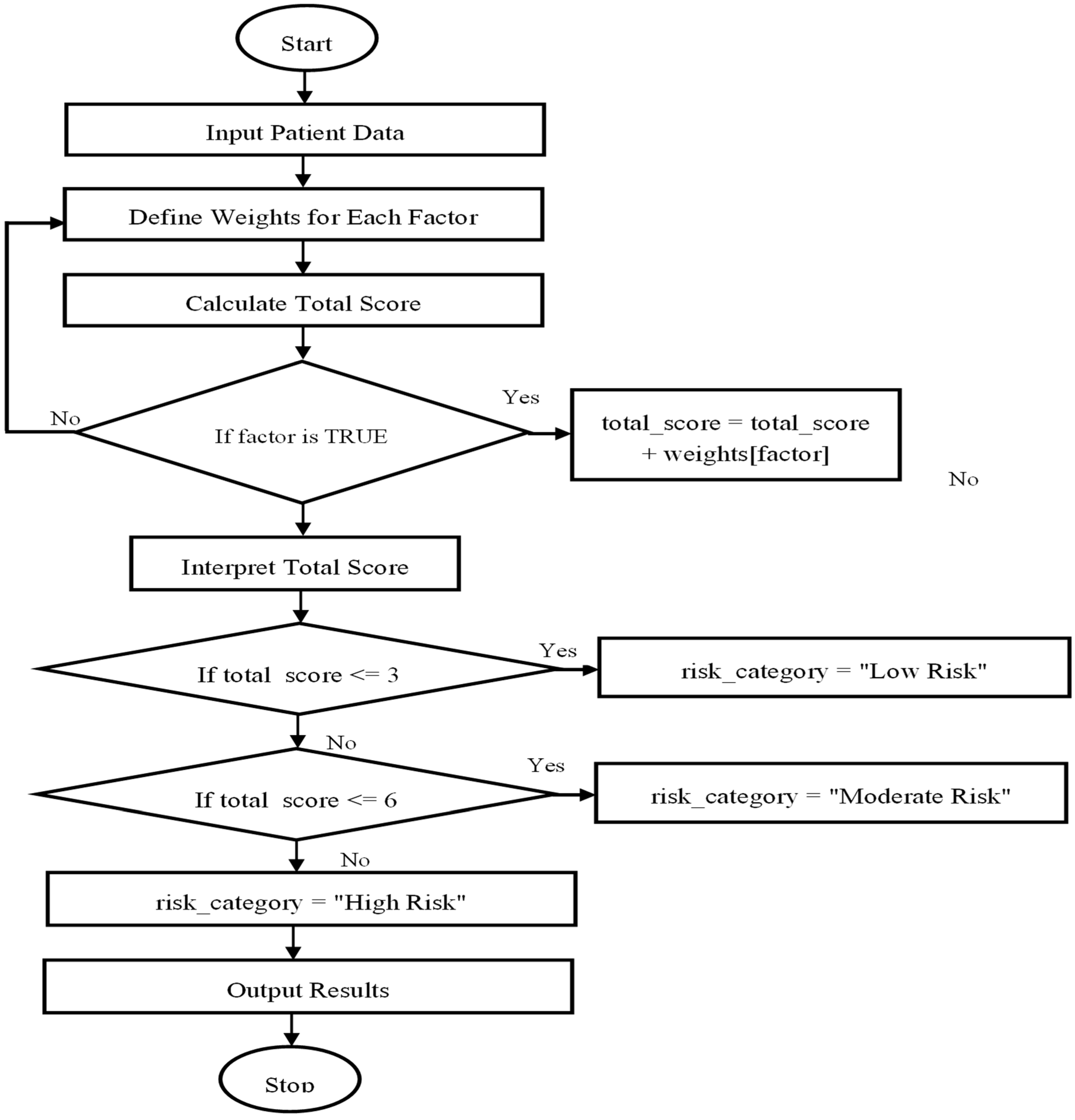
3.5.1. Conceptualization and Purpose
3.5.2. Weighting Approach
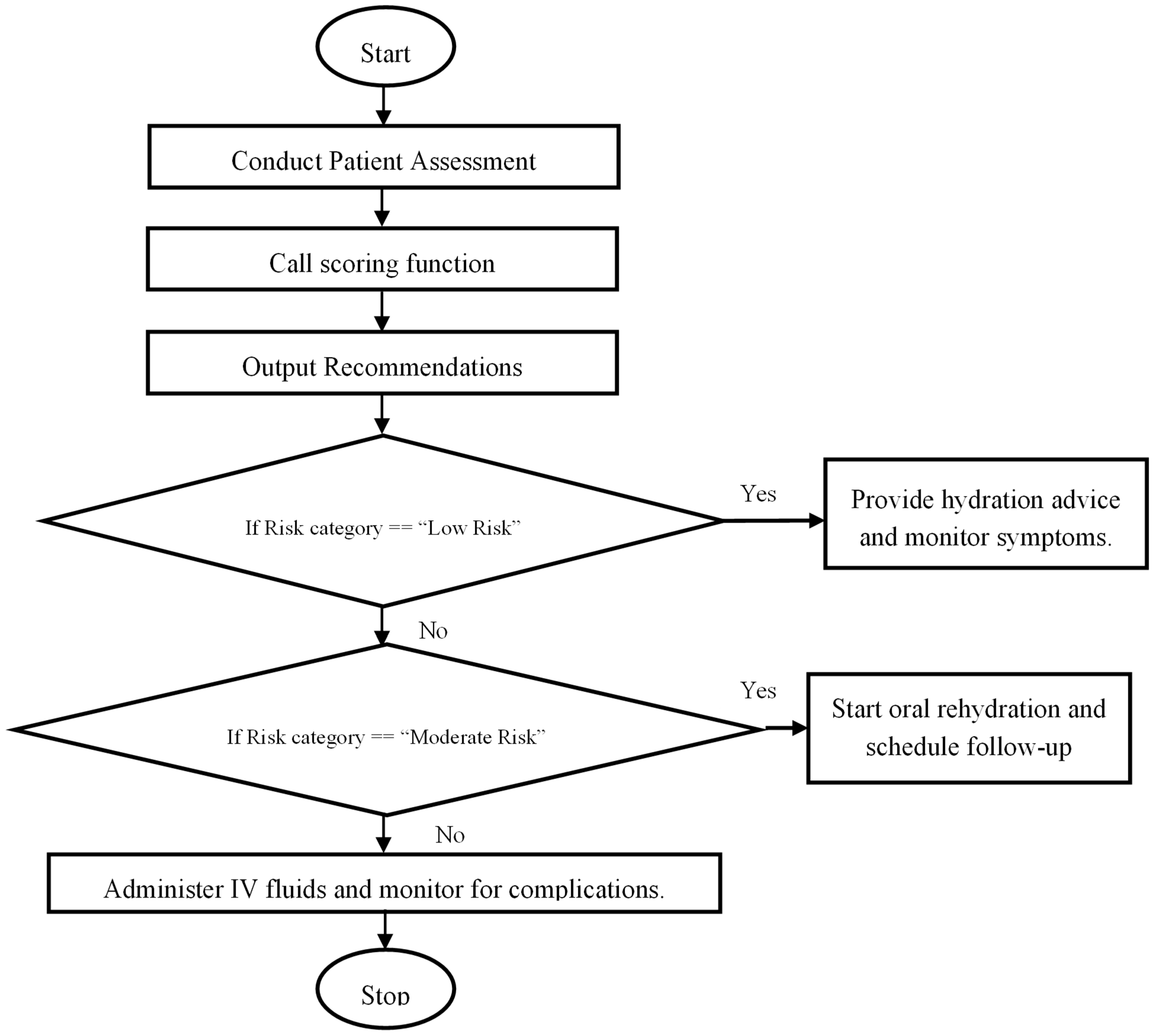
3.5.3. Scoring Framework and Risk Categories
3.5.4. Limitations and Future Application
4. Discussion
4.1. Summary of Findings
4.2. Comparison with Existing Literature
4.3. Implications for Practice
4.4. Study Strengths and Limitations
- Strengths: This systematic review and meta-analysis synthesized data from a diverse set of studies and rigorously evaluated key risk factors for dehydration across different populations and settings. The pooled estimates contribute to the development of a conceptual evidence-based scoring model, offering potential utility for clinical decision-making (Table 7).
- Limitations: Despite the methodological rigor, the findings are limited by the relatively small number of included studies (n = 10), which may affect the generalizability of the results. Additionally, the presence of publication bias could not be fully assessed. While sensitivity analyses were conducted to address differences in study design and population demographics, residual heterogeneity may still influence the pooled outcomes (Table 8).
4.5. Future Research
- Conducting prospective studies in diverse clinical settings to assess the practical utility and accuracy of the scoring system.
- Expanding the scope of the meta-analysis to include more studies, especially from underrepresented regions, to enhance the generalizability of the findings.
- Investigating additional biomarkers or factors that could be integrated into the scoring system to improve its predictive power.
- Evaluating the long-term outcomes of patients assessed using this scoring system to ensure its effectiveness in reducing dehydration-related complications.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Bhat, S.; Sharma, P.; Yuan, L.; O’Grady, G.; Bissett, I. Risk factors for readmission with dehydration after ileostomy formation: A systematic review and meta-analysis. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2021, 23, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Vogel, I.; Shinkwin, M.; Van Der Storm, S.L.; Torkington, J.; Cornish, J.A.; Tanis, P.J.; Hompes, R.; Bemelman, W.A. Overall readmissions and readmissions related to dehydration after creation of an ileostomy: A systematic review and meta-analysis. Tech. Coloproctol. 2022, 26, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.; Garfinkle, R.; Zhao, K.; Vasilevsky, C.-A.; Morin, N.; Ghitulescu, G.; Faria, J.; Boutros, M. Can we better predict readmission for dehydration following creation of a diverting loop ileostomy: Development and validation of a prediction model and web-based risk calculator. Surg. Endosc. 2020, 34, 3118–3125. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Ajabnoor, S.M.; Esio-Bassey, C.; Brainard, J.; Brown, T.J.; Bunn, D.; Foster, E.; Hammer, C.C.; Hanson, S.; et al. Effects of fluid and drinking on pneumonia mortality in older adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 47, 96–105. [Google Scholar] [CrossRef]
- Hipp, J.; Kuvendjiska, J.; Martini, V.; Hillebrecht, H.C.; Fichtner-Feigl, S.; Diener, M.K. Proximal gastrectomy and double-tract reconstruction vs total gastrectomy in gastric and gastro-esophageal junction cancer patients—A systematic review and meta-analysis protocol (PROSPERO registration number: CRD42021291500). Syst. Rev. 2023, 12, 150. [Google Scholar] [CrossRef]
- Aromataris, E.; Stern, C.; Lockwood, C.; Barker, T.H.; Klugar, M.; Jadotte, Y.; Evans, C.; Ross-White, A.; Lizarondo, L.; Stephenson, M.; et al. JBI series paper 2: Tailored evidence synthesis approaches are required to answer diverse questions: A pragmatic evidence synthesis toolkit from JBI. J. Clin. Epidemiol. 2022, 150, 196–202. [Google Scholar] [CrossRef]
- Edmonds, C.J.; Foglia, E.; Booth, P.; Fu, C.H.Y.; Gardner, M. Dehydration in older people: A systematic review of the effects of dehydration on health outcomes, healthcare costs and cognitive performance. Arch. Gerontol. Geriatr. 2021, 95, 104380. [Google Scholar] [CrossRef]
- Stookey, J.D.; Kavouras, S.A.; Suh, H.; Lang, F. Underhydration Is Associated with Obesity, Chronic Diseases, and Death Within 3 to 6 Years in the U.S. Population Aged 51–70 Years. Nutrients 2020, 12, 905. [Google Scholar] [CrossRef]
- Alsanie, S.; Lim, S.; Wootton, S.A. Detecting low-intake dehydration using bioelectrical impedance analysis in older adults in acute care settings: A systematic review. BMC Geriatr. 2022, 22, 954. [Google Scholar] [CrossRef]
- Caufield, J.H.; Zhou, Y.; Garlid, A.O.; Setty, S.P.; Liem, D.A.; Cao, Q.; Lee, J.M.; Murali, S.; Spendlove, S.; Wang, W.; et al. A reference set of curated biomedical data and metadata from clinical case reports. Sci. Data 2018, 5, 180258. [Google Scholar] [CrossRef]
- Holmes, E.A.; O’Connor, R.C.; Perry, V.H.; Tracey, I.; Wessely, S.; Arseneault, L.; Ballard, C.; Christensen, H.; Cohen Silver, R.; Everall, I.; et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Goettke, E.; Coultas, C.; White, M.; Leather, A.J.M. Conceptualising sustainability in the surgical work of non-governmental organisations in low and middle-income countries: A scoping review protocol. BMJ Open 2021, 11, e048046. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, E.; Hooper, L.; Fynn, J.; Wilsher, S.H.; Oladosu, T.; Poland, F.; Roberts, S.; Van Hout, E.; Bunn, D. Low-intake dehydration prevalence in non-hospitalised older adults: Systematic review and meta-analysis. Clin. Nutr. 2023, 42, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Han, L.; Carreno, C.A.; Zhang, Z.; Rodriguez, R.M.; LaRose, M.; Hassenstab, J.; Wig, G.S. Long-term prognosis and educational determinants of brain network decline in older adult individuals. Nat. Aging 2021, 1, 1053–1067. [Google Scholar] [CrossRef]
- Lakicevic, N.; Roklicer, R.; Bianco, A.; Mani, D.; Paoli, A.; Trivic, T.; Ostojic, S.M.; Milovancev, A.; Maksimovic, N.; Drid, P. Effects of Rapid Weight Loss on Judo Athletes: A Systematic Review. Nutrients 2020, 12, 1220. [Google Scholar] [CrossRef]
- Elliott, K.B.; Keefe, M.S.; Rolloque, J.-J.S.; Jiwan, N.C.; Dunn, R.A.; Luk, H.-Y.; Sekiguchi, Y. Relationships between Morning Thirst and Later Hydration Status and Total Water Intake. Nutrients 2024, 16, 3212. [Google Scholar] [CrossRef]
- Rosi, I.M.; Milos, R.; Cortinovis, I.; Laquintana, D.; Bonetti, L. Sensitivity and Specificity of the New Geriatric Dehydration Screening Tool: An observational Diagnostic Study. Nutrition 2022, 101, 111695. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0899900722001083?via%3Dihub (accessed on 21 July 2025). [CrossRef]
- Hesselink, G.; Sir, Ö.; Öztürk, E.; Heiwegen, N.; Olde Rikkert, M.; Schoon, Y. Effects of a geriatric education program for emergency physicians: A mixed-methods study. Health Educ. Res. 2020, 35, 216–227. [Google Scholar] [CrossRef]
- Wojszel, Z.B. What Serum Sodium Concentration Is Suggestive for Underhydration in Geriatric Patients? Nutrients 2020, 12, 496. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Benjamin, C.L.; Butler, C.R.; Morrissey, M.C.; Filep, E.M.; Stearns, R.L.; Lee, E.C.; Casa, D.J. Relationships Between WUT (Body Weight, Urine Color, and Thirst Level) Criteria and Urine Indices of Hydration Status. Sports Health 2021, 14, 566–574. [Google Scholar] [CrossRef]
- Freedman, S.B.; Vandermeer, B.; Milne, A.; Hartling, L.; Johnson, D.; Black, K.; Porter, R.; Joubert, G.; Gouin, S.; Doan, Q.; et al. Diagnosing Clinically Significant Dehydration in Children with Acute Gastroenteritis Using Noninvasive Methods: A Meta-Analysis. J. Pediatr. 2015, 166, 908–916.e6. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.N.; Goldman, R.D.; Srivastava, R.; Parkin, P.C. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J. Pediatr. 2004, 145, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, E.; Hooper, L.; Roberts, S.; Van Hout, E.; Poland, F.; Bunn, D. Protocol: Prevalence of Dehydration Among Older Adults Across Settings: A Systematic Review and Meta-Analysis; University of East Anglia: Norwich, UK, 2021. [Google Scholar]
- deHaan, E. Using and Interpreting Fixed Effects Models by Ed deHaan: SSRN. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3699777 (accessed on 22 July 2025).
- Yngman, G.; Bjugård Nyberg, H.; Nyberg, J.; Jonsson, E.N.; Karlsson, M.O. An introduction to the full random effects model. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Lin, T.; Deng, C.; Zheng, Y.; Gao, L.; Yue, J. Risk Factors for Delirium in the Palliative Care Population: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 772387. [Google Scholar] [CrossRef]
- Deshayes, T.A.; Pancrate, T.; Goulet, E.D.B. Impact of dehydration on perceived exertion during endurance exercise: A systematic review with meta-analysis. J. Exerc. Sci. Fit. 2022, 20, 224–235. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, assessment, and performance effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef]
- Manz, F. Hydration and disease. J. Am. Coll. Nutr. 2007, 26, 535S–541S. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; López-López, J.A.; Becker, B.J.; Davies, S.R.; Dawson, S.; Grimshaw, J.M.; McGuinness, L.A.; Moore, T.H.M.; Rehfuess, E.A.; Thomas, J.; et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob. Health 2019, 4, e000858. [Google Scholar] [CrossRef]
- Kretz, E.; Jordan, I.; Itaru, A.; Glas, M.G.; Fischer, S.; Pircher, T.; Hilger, T.; Waswa, L.M. Determinants of Children’s Fruit Intake in Teso South Sub-County, Kenya—A Multi-Phase Mixed Methods Study among Households with Children 0–8 Years of Age. Nutrients 2021, 13, 2417. [Google Scholar] [CrossRef]
- Nweiser, M.; Dajnoki, K. The Interactive Effects of Disability, Age, and Gender on Organization Performance at Telecommunication Sector Companies. Acta Polytech. Hung. 2025, 22, 145–165. [Google Scholar] [CrossRef]
- Rad, D.; Paraschiv, N.; Kiss, C.; Balas, V.; Barna, C. Physiological Reactions Profiling in Polygraph Testing: Insights from Fuzzy C-Means Clustering Analysis. Acta Polytech. Hung. 2024, 21, 365–377. [Google Scholar] [CrossRef]
| Study ID | Author(s) | Year | Country | Study Design | Sample Size | Population Details | Outcomes Assessed |
|---|---|---|---|---|---|---|---|
| S1 | Rosi et al. | 2022 | Italy | Diagnostic Accuracy | 125 | Older adults in care settings | Sensitivity, specificity of tool |
| S2 | Elliott et al. | 2024 | USA | Observational | 80 | Young adults | Morning thirst and water intake |
| S3 | Parkinson et al. | 2023 | UK | Systematic Review | NA | Non-hospitalized older adults | Prevalence of dehydration |
| S4 | Chan et al. [15] | 2021 | Hong Kong | Cross-sectional | 500 | Community-dwelling older adults | Hydration status |
| S5 | Rosi et al. | 2022 | Italy | Diagnostic | 125 | Older adults in outpatient setting | Screening tool performance |
| S6 | Olde Rikkert et al. | 2020 | Netherlands | Diagnostic Evaluation | 110 | Hospitalized elderly | Risk factors for dehydration |
| S7 | Begum et al. | 2019 | UK | Case–control | 200 | Geriatric patients | Association between symptoms and dehydration |
| S8 | Sekiguchi et al. | 2022 | USA | Cross-sectional | 45 | Healthy young adults | Urine indices and WUT |
| S9 | Mentes et al. | 2006 | USA | Prospective Cohort | 88 | Older adults in assisted living | Hydration markers |
| S10 | Weinberg et al. | 1994 | USA | Retrospective Cohort | 120 | Nursing home residents | Dehydration incidence |
| Study ID | Author(s) | Note | Year | Country | Study Design | Population Details | Risk Factors Assessed | Sensitivity | Specificity | Predictive Values (PPV, NPV) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Lakicevic et al. [16] | A systematic review focused on hydration status and weight loss in athletes, offering sensitivity data relevant for performance contexts. | 2020 | Serbia | Systematic review | Judo athletes (n = 1103) | Rapid weight loss, dehydration, performance | Not reported | Not reported | Not reported |
| S2 | Elliott et al. [17] | A crossover observational study exploring how morning thirst predicts same-day hydration levels in healthy adults, providing sensitivity metrics for early subjective detection. | 2024 | USA | Observational crossover | Healthy adults aged 18–35 | Morning thirst | 89 | 66 | PPV: 72, NPV: 85 |
| S3 | Parkinson et al. [14] | A meta-analysis evaluating the prevalence of low-intake dehydration in community-dwelling older adults, pooling diagnostic performance across multiple European studies. | 2023 | UK/Europe | Systematic review and meta-analysis | Community-dwelling older adults aged ≥ 65 | Low water intake, dry mouth, thirst | 84 | 63 | PPV: 70, NPV: 79 |
| S4 | Caufield et al. [11] | Contributed demographic analysis of dehydration cases, though not directly diagnostic in all aspects. | 2018 | USA | Data descriptor | 3100 clinical case reports | Demographics, diagnosis, treatment, outcomes | Not applicable | Not applicable | Not applicable |
| S5 | Rosi et al. [18] | An Italian diagnostic validation study assessing the performance of the modified GDST screening tool in older hospitalized adults using objective hydration measures. | 2022 | Italy | Diagnostic accuracy study | Hospitalized older adults, MMSE > 24 | Thirst, dry mouth, fatigue, urine color | 62 | 47 | PPV: 69, NPV: 42 |
| S6 | Hesselink et al. [19] | Educational intervention to improve recognition of geriatric risks. | 2020 | Netherlands | Mixed-methods intervention | Emergency physicians in training | Recognition of geriatric syndromes including dehydration | Not directly quantified | Not directly quantified | Not reported |
| S7 | Wojszel et al. [20] | Focused on serum osmolality and symptom correlates at hospital admission. | 2020 | Poland | Cross-sectional | Geriatric ward inpatients (n ≈ 200) | Fatigue, dry mouth, poor intake, low SBP, high urea | Not reported | Not reported | Not reported (correlational only) |
| S8 | Sekiguchi et al. [21] | A cross-sectional study investigating WUT-based hydration screening in athletes, linking thirst and urine color with gold-standard urine indices. | 2022 | USA | Observational cross-sectional | Collegiate athletes, aged 18–24 | Thirst, urine color, body weight | 87 | 71 | PPV: 78, NPV: 82 |
| S9 | Freedman et al. [22] | Meta-analysis evaluating noninvasive methods (e.g., CDS, Gorelick) for assessing dehydration in children with gastroenteritis. | 2015 | Canada | Systematic review and meta-analysis of 9 prospective cohort studies | Children < 18 years with acute gastroenteritis; total n = 1293, of which n = 1039 had both diagnostic evaluation and reference standard measurements | Clinical signs (CDS, Gorelick), physician judgment, ultrasound (IVC: Aorta ratio), digital capillary refill time, urinalysis | 31%–83% depending on tool and study | 42%–97% depending on tool and study | 42%–97% depending on tool and study |
| S10 | Friedman et al. [23] | Developed and validated a dehydration scale (CDS) | 2004 | Canada | Prospective cohort study | Children 1–36 months with gastroenteritis | General appearance, eyes, mucous membranes, presence of tears | ROC AUC = 0.87 | Not directly reported per item | Score correlated with weight loss (r = 0.77); predictive categories (none/some/moderate-severe dehydration) |
| Study ID | Author(s) | Year | Study Design | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Overall RoB | NOS Score (0–9) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Lakicevic et al. | 2020 | Systematic review | Low | Low | Low | Low | Low | Low | 8 |
| S2 | Elliott et al. | 2024 | Observational crossover (morning/afternoon labs) | Low, healthy volunteers, clearly defined | Low, standardized lab procedures | Low, objective lab measures (urine/plasma) | Low, no dropouts reported | Low, full outcome reporting | Low | 7 |
| S3 | Parkinson et al. | 2023 | Systematic review and meta-analysis | Low, comprehensive search | N/A | Low, bias assessed per study | Low, all identified studies assessed | Low, registered PROSPERO, transparent | Low | N/A |
| S4 | Caufield et al. | 2018 | Data descriptor | Low | Low | Low | Low | Low | Low | 7 |
| S5 | Rosi et al. | 2022 | Diagnostic accuracy cross-sectional | Moderate, single-center older adults | Low | Moderate | Low | Low | Moderate | 6 |
| S6 | Hesselink et al. | 2020 | Mixed-methods (educational intervention) | Moderate | Low | Moderate | Low | Low | Moderate | 6 |
| S7 | Wojszel | 2020 | Cross-sectional observational study | Low | Low | Low | Low | Low | Low | 8 |
| S8 | Sekiguchi et al. | 2022 | Observational cross-sectional | Low | Low | Low | Low | Low | Low | 7 |
| S9 | Freedman et al. | 2015 | Systematic Review and Meta-Analysis | High | Low | Unclear | High | Low | Moderate to High | 6 |
| S10 | Friedman et al. | 2004 | Prospective cohort study | Low | Low | Low | Low | Low | Low | 8 |
| Study ID | Author(s) | Year | Country | Study Design | Sample Size | Population Details | Outcomes Assessed |
|---|---|---|---|---|---|---|---|
| S1 | Lakicevic et al. | 2020 | Serbia | Systematic review | 1103 athletes | Competitive judo athletes | Impact of rapid weight loss on hydration and performance |
| S2 | Elliott et al. | 2024 | USA | Observational (cross-sectional) | 112 | Older adults aged ≥ 65 | Association between morning thirst perception and hydration status later in the day; total water intake |
| S3 | Parkinson et al. | 2023 | UK | Scoping review protocol | Systematic review and meta-analysis | 13 studies included | Prevalence of low-intake dehydration based on serum/plasma osmolality |
| S4 | Caufield et al. | 2018 | USA | Data descriptor | 3100 cases | Diverse patient groups in case reports | Structured metadata: demographics, diagnosis, treatment |
| S5 | Rosi et al. | 2022 | Italy | Observational diagnostic accuracy (cross-sectional) | 299 (202 dehydrated; 97 hydrated) | Hospitalized adults aged ≥ 65 with MMSE > 24 | Sensitivity (~61.9%) and specificity (~47.2%) of the GDST-M compared to serum osmolality; also reported ~63.4% sensitivity and ~69.6% specificity |
| S6 | Hesselink et al. | 2020 | Netherlands | Mixed-methods (educational intervention) | 110 | Emergency physicians participating in geriatric care training | Impact of the geriatric education program on physician awareness, recognition, and management of geriatric syndromes, including dehydration |
| S7 | Wojszel | 2020 | Poland | Cross-sectional observational study | 200 | Older adults admitted to a geriatric ward | Prevalence of impending low-intake dehydration and correlation with clinical signs (e.g., fatigue, poor intake, blood pressure, and laboratory values) |
| S8 | Sekiguchi et al. | 2022 | USA | Observational (cross-sectional) | 217 | Collegiate athletes (aged 18–23) | Diagnostic utility of thirst, urine color, and body weight changes in predicting urine-specific gravity and osmolality |
| S9 | Freedman et al. | 2015 | Canada | Systematic review and meta-analysis of 9 prospective cohort studies | 1293 total participants (1039 included in final analysis with both diagnostic and reference standard data) | Children under 18 years of age presenting with acute gastroenteritis in developed countries (emergency department or outpatient setting) | Diagnostic accuracy of noninvasive methods for dehydration assessment, including Clinical Dehydration Score (CDS), Gorelick scale, physician assessment, bedside ultrasound (IVC: Aorta ratio), digital capillary refill time, and urinalysis |
| S10 | Friedman et al. | 2004 | Canada | Prospective cohort study | 137 (in validation cohort) | Children aged 1–36 months presenting with vomiting and/or diarrhea | Development and validation of a clinical dehydration scale based on observable signs; compared to % weight change as reference |
| Subgroup | Pooled Sensitivity (%) | 95% CI | Pooled Specificity (%) | 95% CI | Pooled PPV (%) | 95% CI | Pooled NPV (%) | 95% CI |
|---|---|---|---|---|---|---|---|---|
| By Age | ||||||||
| Children (<18) a | 82 | 77–86 | 83 | 79–87 | 76 | 72–81 | 87 | 83–91 |
| Adults (18–65) | Not consistently reported | – | – | – | – | – | – | – |
| Elderly (>65) | Limited data | – | – | – | – | – | – | – |
| By Sex | ||||||||
| Male | Not consistently reported | – | – | – | – | – | – | – |
| Female | Not consistently reported | – | – | – | – | – | – | – |
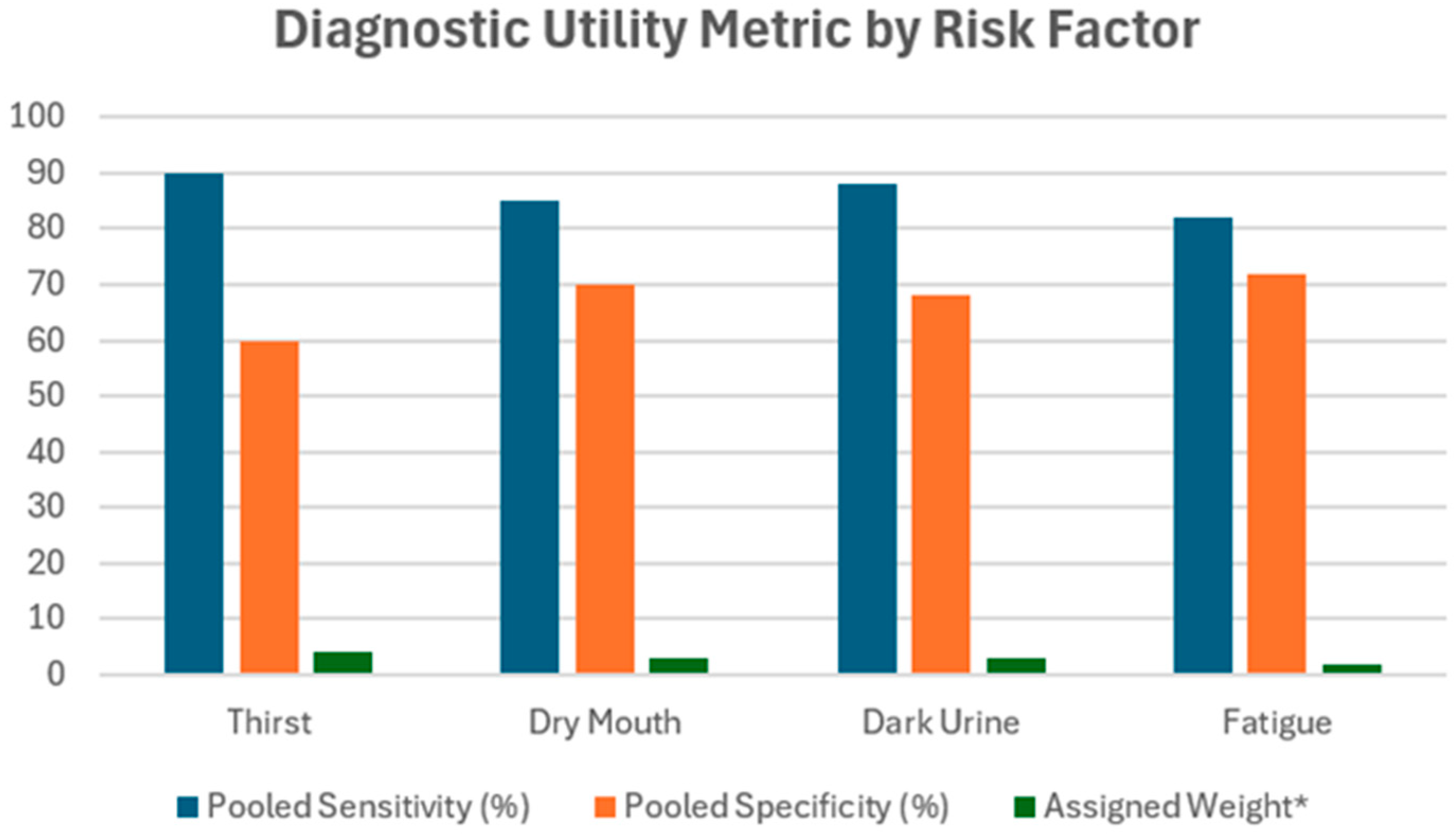
| Risk Factor | Pooled Sensitivity (%) | Pooled Specificity (%) | Assigned Weight * |
|---|---|---|---|
| Thirst | ~90 | ~60 | 4 |
| Dry Mouth | ~85 | ~70 | 3 |
| Dark Urine | ~88 | ~68 | 3 |
| Fatigue | ~82 | ~72 | 2 |
| Study | Findings from Prior Literature | Alignment with Current Meta-Analysis |
|---|---|---|
| Cheuvront and Kenefick (2014) a [29] | Identified thirst and dark urine as key indicators of dehydration. | These indicators emerged as top predictors in the current analysis and were considered in the proposed risk scoring approach. |
| Manz (2007) b [30] | Emphasized age as a critical risk factor for dehydration, especially in elderly populations. | The current study corroborates this, providing stratified results for children, adults, and older adults. |
| Higgins et al. (2019) c [31] | Noted variability in diagnostic accuracy (sensitivity/specificity) across studies. | This meta-analysis addresses such variability by pooling estimates to present more stable performance metrics. |
| Various single-factor studies d [32] | Investigated individual signs/symptoms such as dry mouth, fatigue, or low urine output in isolation. | The current analysis advances this by integrating multiple risk factors into a conceptual scoring model for clinical use. |
| Strengths | Limitations |
|---|---|
| Comprehensive synthesis from diverse, multi-setting studies | Small number of included studies may limit generalizability |
| Development of a clinically relevant, evidence-based scoring tool | Potential publication bias could not be fully excluded |
| Inclusion of predominantly high-quality studies | Heterogeneity in study design and population characteristics may affect results |
| Robust sensitivity analyses performed to explore variability | Residual heterogeneity may still influence pooled estimates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbolu, M.O.; Eniade, O.D.; Kozlovszky, M. Systematic Review and Meta-Analysis of Risk Factors for Dehydration and the Development of a Predictive Scoring System. Healthcare 2025, 13, 1974. https://doi.org/10.3390/healthcare13161974
Ogbolu MO, Eniade OD, Kozlovszky M. Systematic Review and Meta-Analysis of Risk Factors for Dehydration and the Development of a Predictive Scoring System. Healthcare. 2025; 13(16):1974. https://doi.org/10.3390/healthcare13161974
Chicago/Turabian StyleOgbolu, Melvin Omone, Olanrewaju D. Eniade, and Miklos Kozlovszky. 2025. "Systematic Review and Meta-Analysis of Risk Factors for Dehydration and the Development of a Predictive Scoring System" Healthcare 13, no. 16: 1974. https://doi.org/10.3390/healthcare13161974
APA StyleOgbolu, M. O., Eniade, O. D., & Kozlovszky, M. (2025). Systematic Review and Meta-Analysis of Risk Factors for Dehydration and the Development of a Predictive Scoring System. Healthcare, 13(16), 1974. https://doi.org/10.3390/healthcare13161974






