Multidimensional Radiological Assessment of Delirium in the Emergency Department
Abstract
1. Introduction
2. Methods
2.1. Purpose of the Study
2.2. Inclusion Criteria
2.3. Study Design
2.4. Outcomes
2.5. Radiological Variables
- Cardiovascular calcifications;
- Body composition (visceral and abdominal fat);
- Psoas muscle volume (as an estimate of sarcopenia/cachexia).
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Etiological Subgroups
- Septic Delirium (n = 9): Mean age 84 ± 3 years; 11.1% female. Hypoactive in 44.4%, hyperactive in 55.6%. Mean SBP 128 ± 17 mmHg, DBP 75 ± 10 mmHg, HR 93 ± 10 bpm. Charlson Index: 8 ± 3. One-year mortality: 33.3% (n = 3).
- Neurological Delirium (n = 10): Mean age 86 ± 5 years; 50% female. Hypoactive 60%, hyperactive 40%. SBP 127 ± 25 mmHg, DBP 71 ± 13 mmHg, HR 83 ± 18 bpm. Charlson Index: 7 ± 3. One-year mortality: 70% (n = 7).
- Cardiac–hypoxic Delirium (n = 6): Mean age 83 ± 7 years; 50% female. Hypoactive and hyperactive equally distributed (50%). SBP 151 ± 39 mmHg, DBP 86 ± 18 mmHg, HR 89 ± 7 bpm. Charlson Index: 6 ± 1. One-year mortality: 50% (n = 3).
- Metabolic Delirium (n = 5): Mean age 87 ± 4 years; 80% female. Hypoactive 60%, hyperactive 40%. SBP 153 ± 21 mmHg, DBP 79 ± 12 mmHg, HR 83 ± 4 bpm. Charlson Index: 7 ± 2. One-year mortality: 40% (n = 2).
3.3. Clinical History and Outcomes
- 30-day: 7% (n = 2);
- 3-month: 13% (n = 4);
- 6-month: 40% (n = 12);
- 1-year: 50% (n = 15).
3.4. Hyperactive vs. Hypoactive Delirium
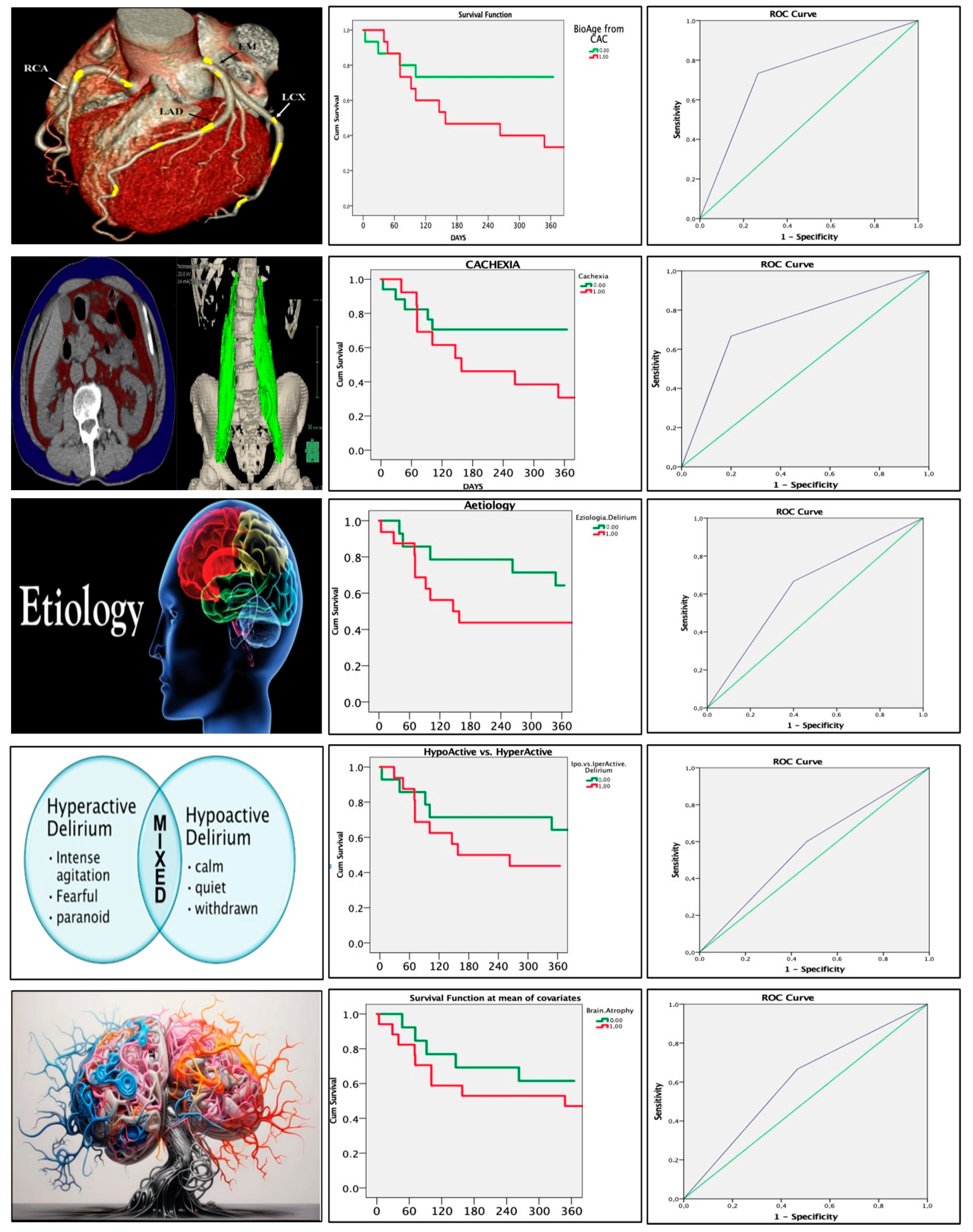
3.5. Radiological Parameters and Mortality
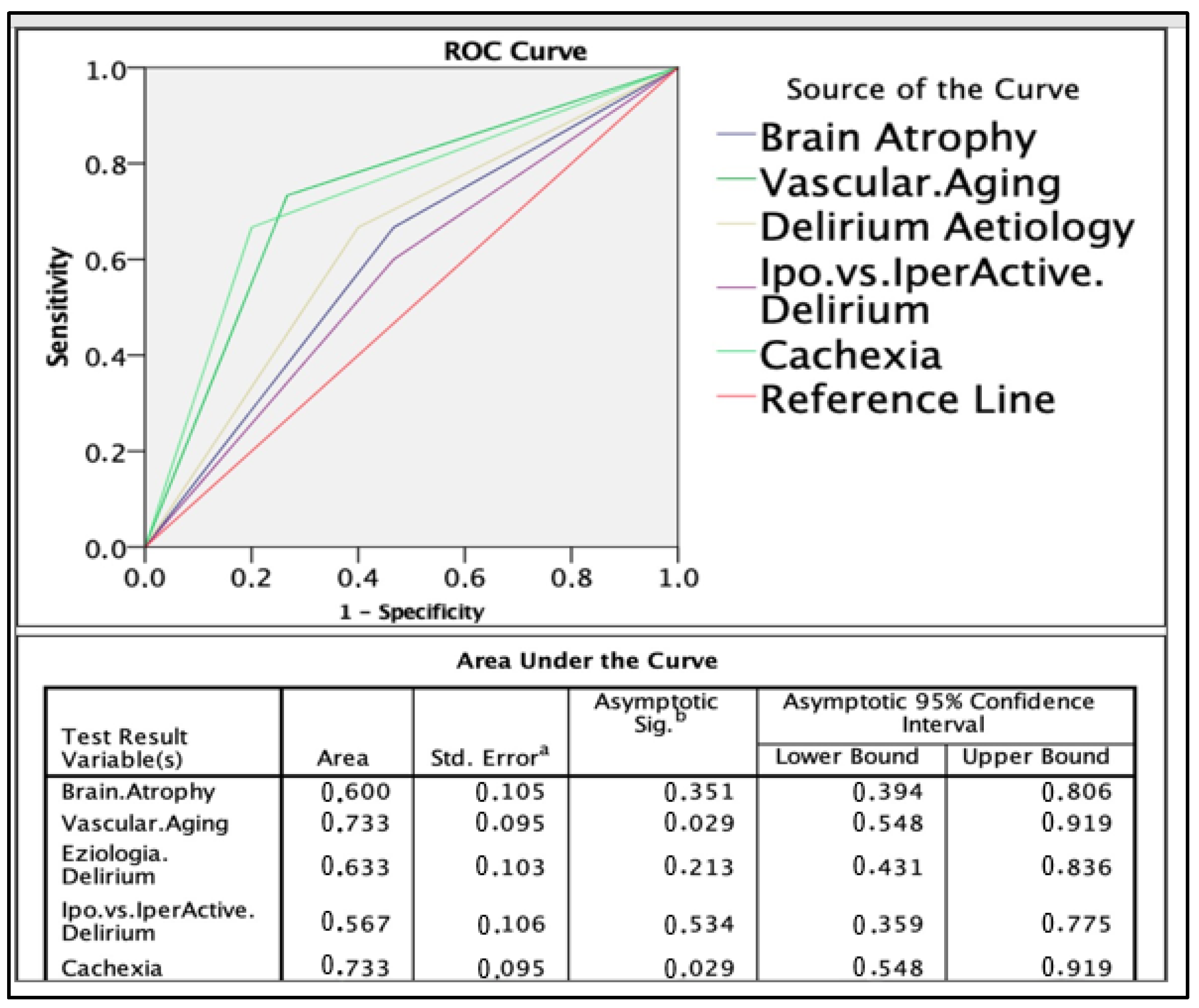
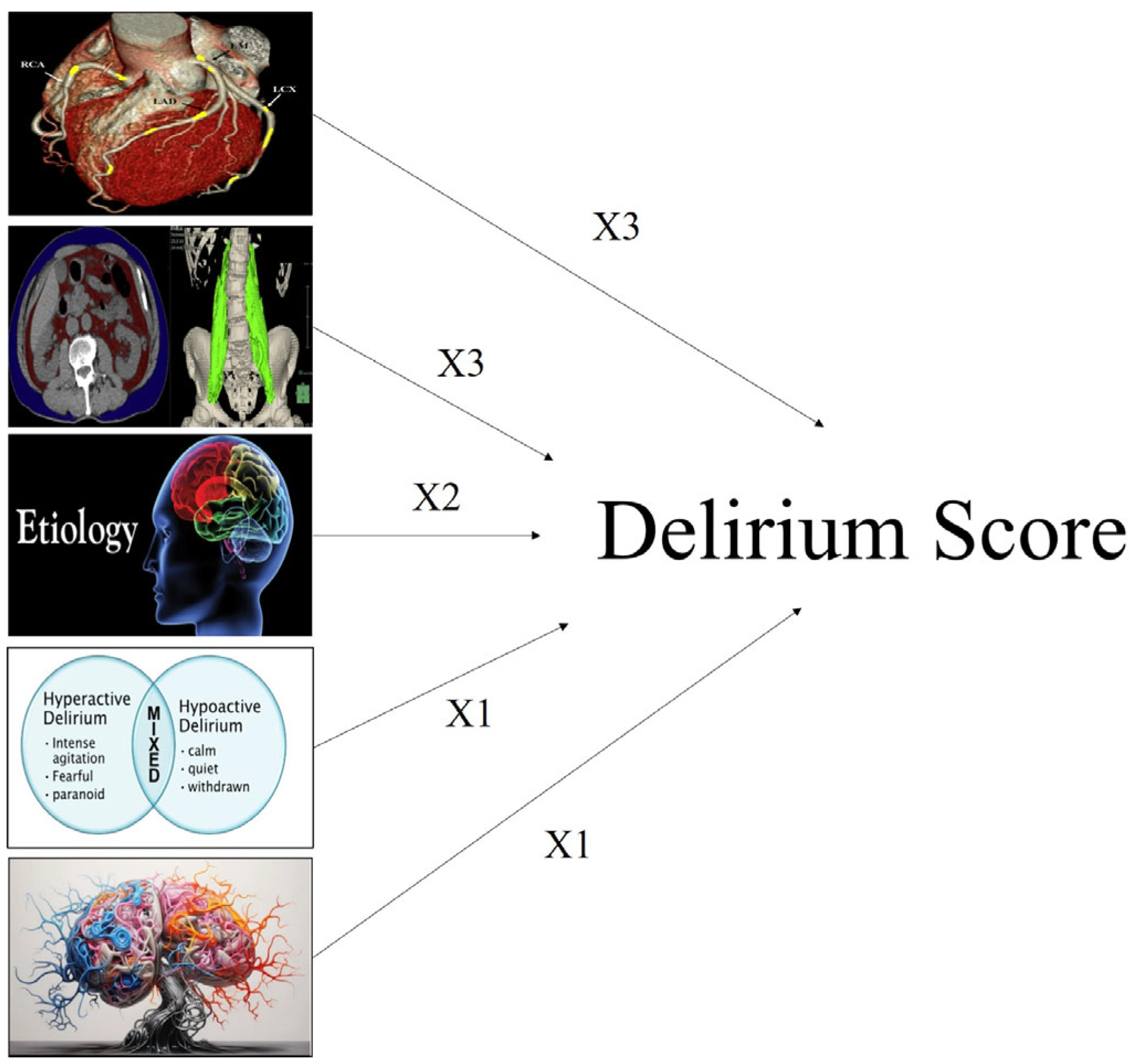
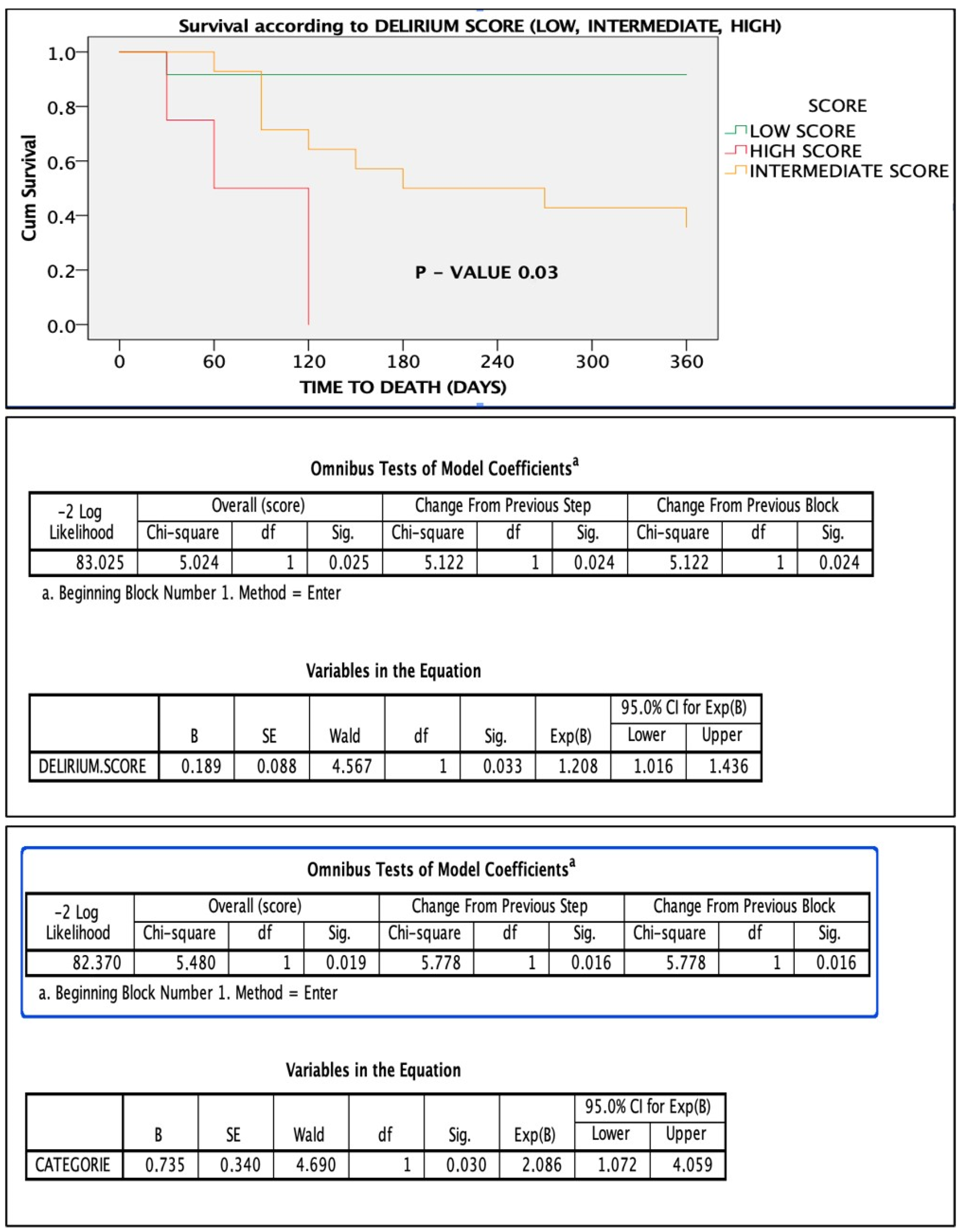
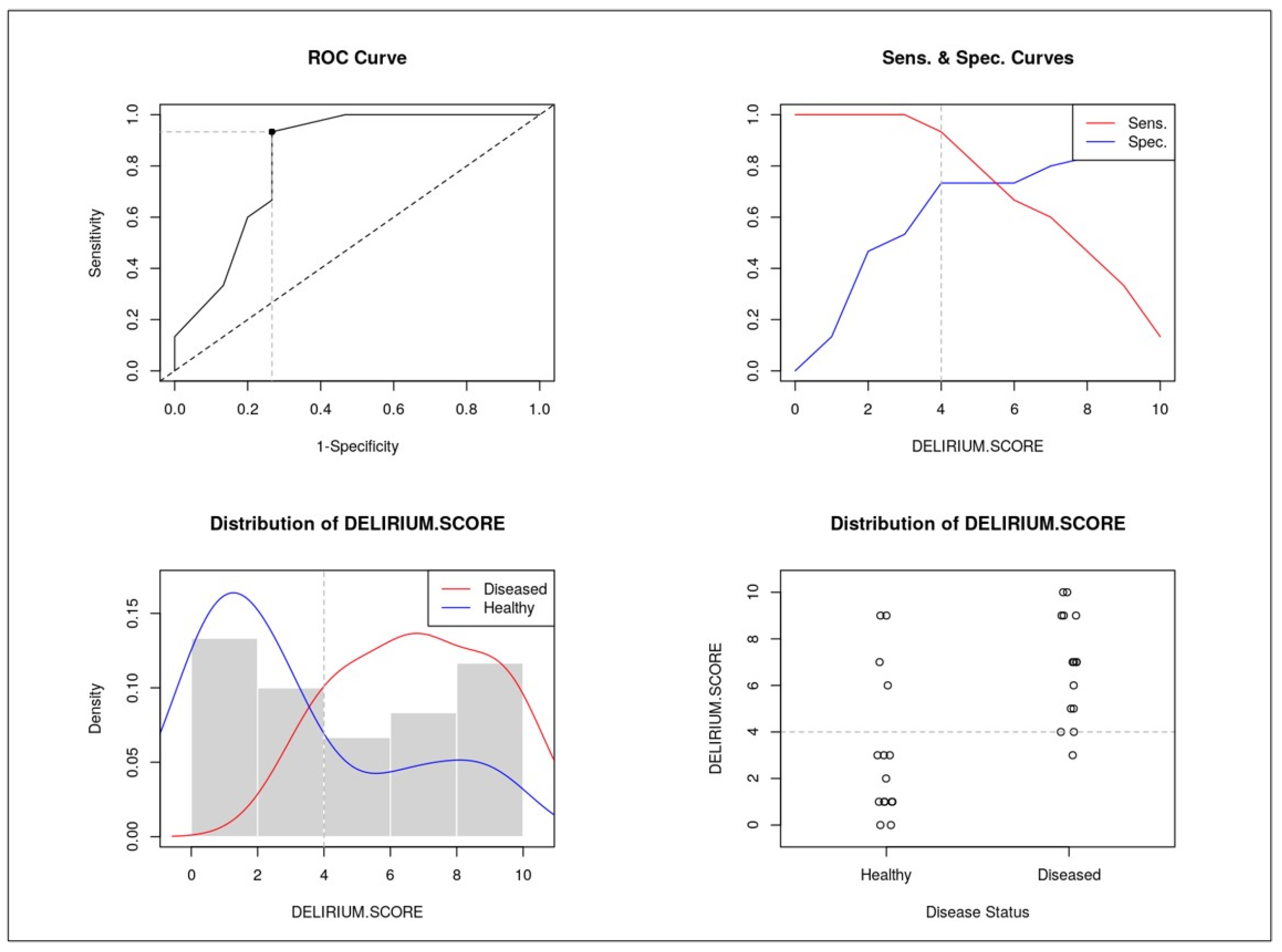
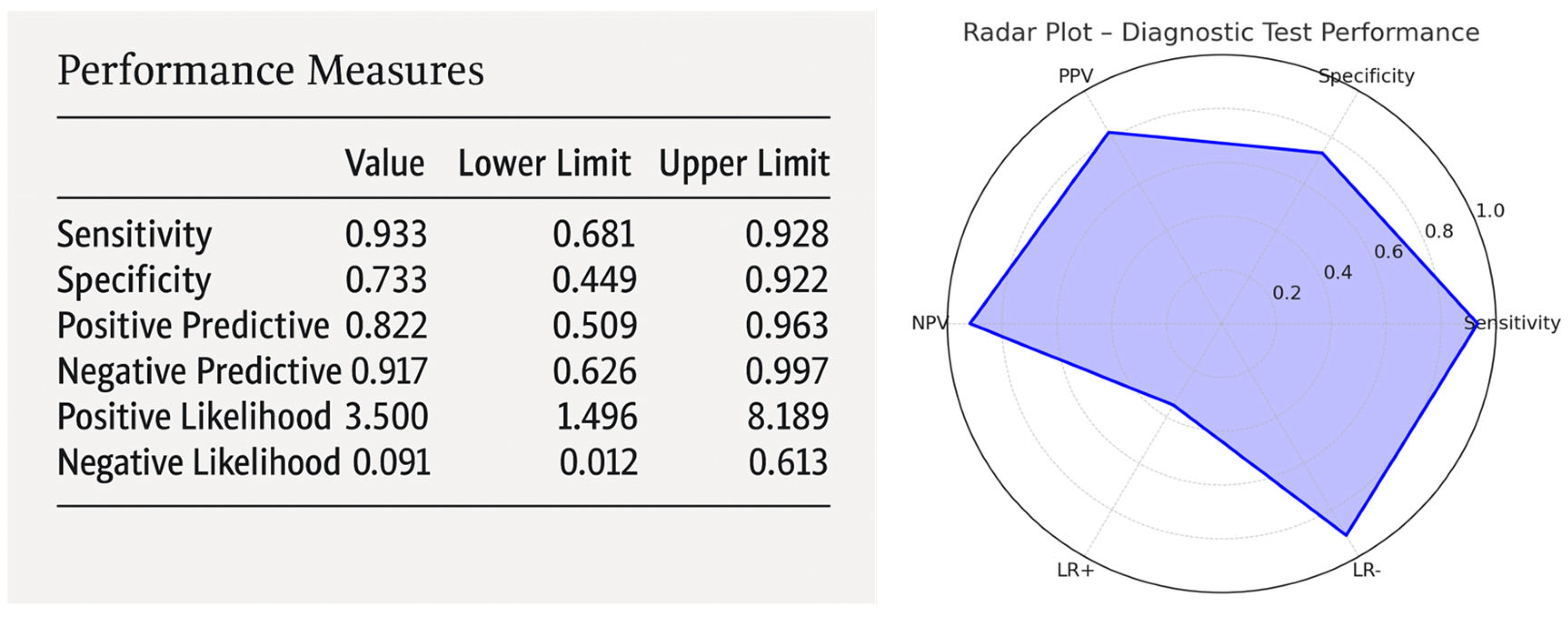
3.6. Delirium Score and ROC Analysis
- Cachexia: 3 points;
- Vascular aging: 3 points;
- Etiology of delirium: 2 points;
- Delirium type: 1 point;
- Brain atrophy: 1 point.
4. Discussion
4.1. Delirium Etiologies and Mortality
4.2. Hyperactive vs. Hypoactive Delirium
4.3. Radiological Features as Prognostic Indicators
4.4. The Delirium Score: Improving Mortality Prediction
4.5. Clinical Implications
4.6. Study Limitations
4.7. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattison, M.L.P. Delirium. Ann. Intern. Med. 2020, 173, ITC49–ITC64. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R. Delirium in hospitalized older adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Westendorp, R.G.J.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Howard, M.A., III; Han, J.H. Delirium and Delirium Prevention in the Emergency Department. Clin. Geriatr. Med. 2023, 39, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Inouye, S.K. The inter-relationship between delirium and dementia: The importance of delirium prevention. Nat. Rev. Neurol. 2022, 18, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.K.; Needham, E. Diagnosis of delirium: A practical approach. Pract. Neurol. 2023, 23, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Hshieh, T.T.; Inouye, S.K.; Oh, E.S. Delirium in the Elderly. Clin. Geriatr. Med. 2020, 36, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Ormseth, C.H.; LaHue, S.C.; Oldham, M.A.; Josephson, S.A.; Whitaker, E.; Douglas, V.C. Predisposing and Precipitating Factors Associated with Delirium: A Systematic Review. JAMA Netw. Open 2023, 6, e2249950. [Google Scholar] [CrossRef] [PubMed]
- Shenvi, C.; Kennedy, M.; Austin, C.A.; Wilson, M.P.; Gerardi, M.; Schneider, S. Managing Delirium and Agitation in the Older Emergency Department Patient: The ADEPT Tool. Ann. Emerg. Med. 2020, 75, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Giannini, F.; Toselli, M.; Palmisano, A.; Cereda, A.; Vignale, D.; Leone, R.; Nicoletti, V.; Gnasso, C.; Monello, A.; Manfrini, M.; et al. Coronary and total thoracic calcium scores predict mortality and provides pathophysiologic insights in COVID-19 patients. J. Cardiovasc. Comput. Tomogr. 2021, 15, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Miranda, F.; Gonzalez, F.; Plana, M.N.; Zamora, J.; Quinn, T.J.; Seron, P. Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) for the diagnosis of delirium in adults in critical care settings. Cochrane Database Syst. Rev. 2023, 11, CD013126. [Google Scholar] [CrossRef] [PubMed]
- Duong, F.; Gadermayr, M.; Merhof, D.; Kuhl, C.; Bruners, P.; Loosen, S.H.; Roderburg, C.; Truhn, D.; Schulze-Hagen, M.F. Automated major psoas muscle volumetry in computed tomography using machine learning algorithms. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 355–361. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.J.; Naazie, I.N.; Cainzos-Achirica, M.; Dardari, Z.A.; DeFilippis, A.P.; McClelland, R.L.; Mirbolouk, M.; Orimoloye, O.A.; Dzaye, O.; Nasir, K.; et al. Derivation of a Coronary Age Calculator Using Traditional Risk Factors and Coronary Artery Calcium: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2021, 10, e019351. [Google Scholar] [CrossRef] [PubMed]
- Stollings, J.L.; Kotfis, K.; Chanques, G.; Pun, B.T.; Pandharipande, P.P.; Ely, E.W. Delirium in critical illness: Clinical manifestations, outcomes, and management. Intensive Care Med. 2021, 47, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Nisgaard, M.G.; Boesen, S.V.; Jensen, J. Assessing Delirium in Patients with Neurological Diseases. J. Neurosci. Nurs. 2024, 56, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C.J.; Marra, A.; Han, J.H.M.; Patel, M.B.; Brummel, N.E.M.; Thompson, J.L.; Jackson, J.C.P.; Chandrasekhar, R.; Ely, E.W.; Pandharipande, P.P.M.; et al. Association of Hypoactive and Hyperactive Delirium with Cognitive Function After Critical Illness. Crit. Care Med. 2020, 48, e480–e488. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, A.; Manzoni, F.; Morandi, A.; Di Santo, S.; Rossi, E.; Valsecchi, M.G.; Inzitari, M.; Cherubini, A.; Bo, M.; Mossello, E.; et al. The association between low skeletal muscle mass and delirium: Results from the nationwide multi-centre Italian Delirium Day 2017. Aging Clin. Exp. Res. 2022, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Mart, M.F.; Roberson, S.W.; Salas, B.; Pandharipande, P.P.; Ely, E.W. Prevention and Management of Delirium in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2021, 42, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Hosker, C.; Ward, D. Hypoactive delirium. BMJ 2017, 357, j2047. [Google Scholar] [CrossRef] [PubMed]
| Number of patients, N | 30 |
| Average age, years ± SD | 85 ± 5 |
| Female sex, n/N (%) | 13 (43.3) |
| Delirium etiology | |
| Cardiac/hypoxic, n/N (%) | 6 (20%) |
| Septic, n/N (%) | 9 (30%) |
| Neurological, n/N (%) | 10 (33.3%) |
| Metabolic, n/N (%) | 5 (16.7%) |
| Delirium type | |
| Hypoactive, n/N (%) | 16 (53.3%) |
| Hyperactive, n/N (%) | 14 (46.7%) |
| Systolic BP, mmHg ± SD | 136 ± 27 |
| Diastolic BP, mmHg ± SD | 77 ± 14 |
| Heart rate, bpm ± SD | 88 ± 13 |
| Charlson Comorbidity Index, N ± SD | 7 ± 2 |
| Cognitive decline history, n/N (%) | 12 (40%) |
| Disability, n/N (%) | 16 (53.3%) |
| Anemia history, n/N (%) | 10 (33.3%) |
| Electrolyte abnormalities, n/N (%) | 8 (26.7%) |
| Length of stay, days ± SD | 18 ± 15 |
| Mortality | |
| 30-day mortality, n/N (%) | 2 (7%) |
| 3-month mortality, n/N (%) | 7 (13%) |
| 6-month mortality, n/N (%) | 12 (40%) |
| 1-year mortality, n/N (%) | 15 (50%) |
| Septic (1) | Neurological (2) | Cardiac–Hypoxic (3) | Metabolic (4) | |
|---|---|---|---|---|
| Number of patients, N | 9 | 10 | 6 | 5 |
| Average age, years ± SD | 84 ± 3 | 86 ± 5 | 83 ± 7 | 87 ± 4 |
| Female sex, n/N | 1 | 5 | 3 | 4 |
| Hypoactive, n/N | 4 | 6 | 3 | 3 |
| Hyperactive, n/N | 5 | 4 | 3 | 2 |
| Systolic BP, mmHg ± SD | 128 ± 17 | 127 ± 25 | 151 ± 39 | 153 ± 21 |
| Diastolic BP, mmHg ± SD | 75 ± 10 | 71 ± 13 | 86 ± 18 | 79 ± 12 |
| Heart rate, bpm ± SD | 93 ± 10 | 83 ± 18 | 89 ± 7 | 83 ± 4 |
| Charlson Comorbidity Index, N ± SD | 8 ± 3 | 7 ± 3 | 6 ± 1 | 7 ± 2 |
| Cognitive decline history, n/N | 3 | 4 | 4 | 1 |
| Disability, n/N | 6 | 5 | 3 | 2 |
| Anemia, n/N | 3 | 6 | 0 | 1 |
| Electrolyte abnormalities, n/N | 0 | 2 | 2 | 4 |
| Length of stay, days ± SD | 23 ± 13 | 20 ± 18 | 16 ± 14 | 7 ± 3 |
| 1-year mortality, n/N (%) | 3 (33.3%) | 7 (70%) | 3 (50%) | 2 (40%) |
| Hyperactive | Hypoactive | p-Value | |
|---|---|---|---|
| Average age, years ± SD | 86 ± 3 | 84 ± 6 | ns |
| Female sex, n/N | 6 | 7 | ns |
| Septic, n/N | 5 | 4 | ns |
| Neurological, n/N | 4 | 6 | ns |
| Cardiac hypoxic, n/N | 3 | 3 | ns |
| Metabolic, n/N | 2 | 3 | ns |
| Systolic BP, mmHg ± SD | 139 ± 31 | 134 ± 24 | ns |
| Diastolic BP, mmHg ± SD | 79 ± 15 | 75 ± 13 | ns |
| Charlson Comorbidity Index, N ± SD | 6 ± 2 | 8 ± 2 | 0.07 |
| Estimated 10-year survival, % | 25 | 6 | 0.013 |
| Cognitive decline history, n/N (%) | 4 (33.3%) | 8 (66.7%) | ns |
| Disability, n/N | 7 | 9 | ns |
| Anemia, n/N | 6 | 4 | ns |
| Electrolyte abnormalities, n/N | 3 | 5 | ns |
| Length of stay, days ± SD | 17 ± 13 | 18 ± 16 | ns |
| 1-year mortality, n/N (%) | 6 (43%) | 8 (50%) | ns |
| Radiological Variables | Deceased | Living | All | p-Value |
|---|---|---|---|---|
| Number of patients, N | 15 | 15 | 30 | - |
| Average age, years ± SD | 85 ± 5 | 84 ± 4 | 85 ± 5 | ns |
| Common trunk calcifications, median (IQR) | 0 (0.02)) | 0 (0) | 0(0) | ns |
| Anterior descending calcifications, median (IQR) | 100 (259) | 79 (313) | 87 (290) | ns |
| Circumflex calcifications, median (IQR) | 2 (72) | 0 (18) | 0.75 (27) | ns |
| Right coronary calcifications, median (IQR) | 76 (418) | 0 (12) | 3.7 (215) | ns |
| Total calcium score, median (IQR) | 300 (711) | 100 (724) | 192 (838) | ns |
| Aortic valve calcifications, median (IQR) | 0 (71) | 0 (0) | 0 (35) | ns |
| Abdominal fat percentage, % median (IQR) | 30 (7) | 37 (15) | 33 (10) | 0.06 |
| Visceral abdominal fat, % median (IQR) | 30 (10) | 35 (18.5) | 32 (19) | ns |
| Psoas volume, mm3 ± SD, median (IQR) | 150 (40) | 200 (41.5) | 199 (62) | 0.02 |
| Vascular age, years ± SD | 83 ± 23 | 72 ± 16 | 78 ± 20 | ns |
| Cachexia, n/N (%) | 10 (66.6%) | 3 (20%) | 13 (76%) | 0.025 |
| Vascular elder, n/N (%) | 11 (73%) | 4 (26.6%) | 15 (50%) | 0.027 |
| Brain atrophy, n/N (%) | 10 (66.6%) | 7 (46%) | 17 (56.6%) | ns |
| Length of stay, days ± SD | 19 ± 17 | 11 ± 15 | 18 ± 15 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cereda, A.F.; Frangi, C.; Rocchetti, M.; Spangaro, A.; Tua, L.; Franchina, A.G.; Carlà, M.; Colavolpe, L.; Carelli, M.; Palmisano, A.; et al. Multidimensional Radiological Assessment of Delirium in the Emergency Department. Healthcare 2025, 13, 1871. https://doi.org/10.3390/healthcare13151871
Cereda AF, Frangi C, Rocchetti M, Spangaro A, Tua L, Franchina AG, Carlà M, Colavolpe L, Carelli M, Palmisano A, et al. Multidimensional Radiological Assessment of Delirium in the Emergency Department. Healthcare. 2025; 13(15):1871. https://doi.org/10.3390/healthcare13151871
Chicago/Turabian StyleCereda, Alberto Francesco, Claudia Frangi, Matteo Rocchetti, Andrea Spangaro, Lorenzo Tua, Antonio Gabriele Franchina, Matteo Carlà, Lucia Colavolpe, Matteo Carelli, Anna Palmisano, and et al. 2025. "Multidimensional Radiological Assessment of Delirium in the Emergency Department" Healthcare 13, no. 15: 1871. https://doi.org/10.3390/healthcare13151871
APA StyleCereda, A. F., Frangi, C., Rocchetti, M., Spangaro, A., Tua, L., Franchina, A. G., Carlà, M., Colavolpe, L., Carelli, M., Palmisano, A., Etteri, M., & Lucreziotti, S. (2025). Multidimensional Radiological Assessment of Delirium in the Emergency Department. Healthcare, 13(15), 1871. https://doi.org/10.3390/healthcare13151871






