Beyond the Pill: Mapping Process-Oriented Decision Support Models in Pharmaceutical Policy
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Data Extraction and Quality Appraisal
3. Results
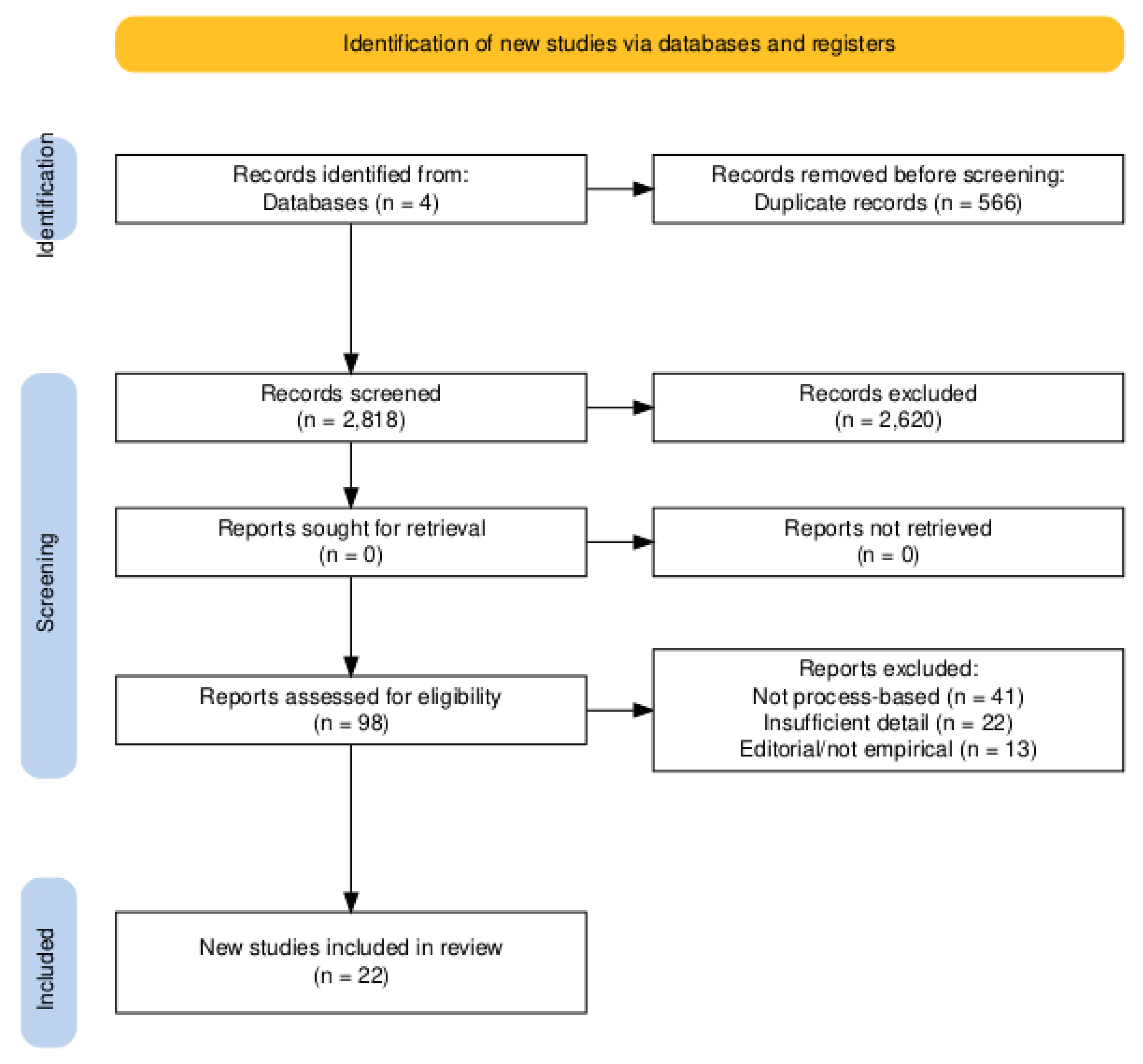
3.1. Study Selection
3.2. Characteristics of Identified DSMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Area | Evaluation Criteria (Score: 0–2) |
|---|---|
| 1. Structure | Are the stages of decision-making clearly defined and structured in the model? |
| 2. Transparency | Is the logic, structure, and data use of the DSM clearly documented and reproducible? |
| 3. Impact | Does the study include stakeholder input or address a relevant policy question? |
| 4. Evaluation | Is there evidence that the DSM was piloted, validated, or tested in practice? |
References
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Marsh, K.; Ijzerman, M.; Thokala, P.; Baltussen, R.; Boysen, M.; Kaló, Z.; Lönngren, T.; Mussen, F.; Peacock, S.; Watkins, J.; et al. ISPOR Task Force. Multiple Criteria Decision Analysis for Health Care Decision Making—Emerging Good Practices: Report 2 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health 2016, 19, 125–137. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Mauskopf, J.A.; Augustovski, F.; Caro, J.J.; Lee, K.M.; Minchin, M.; Orlewska, E.; Penna, P.; Barrios, J.-M.R.; Shau, W.-Y. Budget impact analysis—Principles of good practice: Report of the ISPOR 2012 Task Force. Value Health 2014, 17, 5–14. [Google Scholar] [CrossRef]
- Annemans, L.; Redekop, W.K.; Payne, K. Current methodological issues in the economic assessment of personalized medicine. Value Health 2013, 16, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Eddy, D.M.; Hollingworth, W.; Caro, J.J.; Tsevat, J.; McDonald, K.M.; Wong, J.B. Model transparency and validation: A report of the ISPOR–SMDM Modeling Good Research Practices Task Force. Med. Decis. Making 2012, 32, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Kwakkenbos, L.; Jewett, L.R.; Baron, M.; Bartlett, S.J.; Furst, D.; Gottesman, K.; Khanna, D.; Malcarne, V.L.; Mayes, M.D.; Mouthon, L.; et al. The scleroderma Patient-centered Intervention Network–Coaching for Health trial: Design, feasibility, and baseline data. Clin. Rheumatol. 2021, 40, 3205–3215. [Google Scholar]
- Thokala, P.; Devlin, N.; Marsh, K.; Baltussen, R.; Boysen, M.; Kalo, Z.; Longrenn, T.; Mussen, F.; Peacock, S.; Watkins, J.; et al. Multiple criteria decision analysis for health care decision making–an introduction: Report 1 of the ISPOR MCDA Emerging Good Practices Task Force. Value Health 2016, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bujar, M.; McAuslane, N.; Walker, S.; Salek, M.-S.S. Evaluating Quality of Decision-Making Processes in Medicines’ Development, Regulatory Review, and Health Technology Assessment: A Systematic Review of the Literature. Front. Pharmacol. 2017, 10, 189. [Google Scholar] [CrossRef]
- Donelan, R.; Walker, S.; Salek, S. The development and validation of a generic instrument, QoDoS, for assessing the quality of decision making. Front. Pharmacol. 2016, 7, 180. [Google Scholar] [CrossRef]
- World Health Organization. WHO-INTEGRATE Evidence to Decision Framework; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Nair, M.; Andersson, J.; Nygren, J.M.; Lundgren, L.E. Barriers and Enablers for Implementation of an Artificial Intelligence-Based Decision Support Tool to Reduce the Risk of Readmission of Patients With Heart Failure: Stakeholder Interviews. JMIR Form. Res. 2023, 23, e47335. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Fasseeh, A.; Karam, R.; Jameleddine, M.; George, M.; Kristensen, F.B.; Al-Rabayah, A.A.; Alsaggabi, A.H.; El Rabbat, M.; Alowayesh, M.S.; Chamova, J. Implementation of Health Technology Assessment in the Middle East and North Africa: Comparison Between the Current and Preferred Status. Front Pharmacol. 2020, 21, 15. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Holtorf, A.P.; Al-Hussaini, M.; Lemay, J.; Alowayesh, M.; Kaló, Z. Stakeholder driven development of a multi-criteria decision analysis tool for purchasing off-patent pharmaceuticals in Kuwait. J. Pharm. Policy Pract. 2019, 12, 9. [Google Scholar] [CrossRef]
- Al-Badriyeh, D.; Alabbadi, I.; Fahey, M.; Al-Khal, A.; Zaidan, M. Multi-indication Pharmacotherapeutic Multicriteria Decision Analytic Model for the Comparative Formulary Inclusion of Proton Pump Inhibitors in Qatar. Clin Ther. 2016, 38, 1158–1173. [Google Scholar] [CrossRef]
- Marsh, K.; Lanitis, T.; Neasham, D.; Orfanos, P.; Caro, J.J. Assessing the value of healthcare interventions using multi-criteria decision analysis: A review of the literature. Pharmacoeconomics 2014, 32, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Yfantopoulos, J.N.; Chantzaras, A. Drug Policy in Greece. Value Health Reg. Issues 2018, 16, 66–73. [Google Scholar] [CrossRef]
- Kaló, Z.; Petykó, Z.I.; Fricke, F.U.; Maniadakis, N.; Tesař, T.; Podrazilová, K.; Espin, J.; Inotai, A. Development of a core evaluation framework of value-added medicines: Report 2 on pharmaceutical policy perspectives. Cost. Eff. Resour. Alloc. 2021, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Visintin, E.; Tinelli, M.; Kanavos, P. Value assessment of disease-modifying therapies for Relapsing-Remitting Multiple Sclerosis: HTA evidence from seven OECD countries. Health Policy 2019, 123, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Girotti, S.; Pauro, F.; Leufkens, H.G.M.; Cipolli, M. The impact of FDA and EMA regulatory decision-making process on the access to CFTR modulators for the treatment of cystic fibrosis. Orphanet J. Rare Dis. 2022, 17, 188. [Google Scholar] [CrossRef]
- Laba, T.L.; Jiwani, B.; Crossland, R.; Mitton, C. Can multi-criteria decision analysis (MCDA) be implemented into real-world drug decision-making processes? A Canadian provincial experience. Int. J. Technol. Assess. Health Care 2020, 36, 434–439. [Google Scholar] [CrossRef]
- Moosivand, A.; Rangchian, M.; Zarei, L.; Peiravian, F.; Mehralian, G.; Sharifnia, H. An application of multi-criteria decision-making approach to sustainable drug shortages management: Evidence from a developing country. J. Pharm. Health Care Sci. 2021, 7, 14. [Google Scholar] [CrossRef]
- McEwin, E.J.; Hooimeyer, A.; Mintzes, B.J. Post-Market Evidence for Cancer Medicines in Regulatory and Clinical Decision-Making: A Scoping Review. Pharmacoepidemiol. Drug Saf. 2025, 34, e70093. [Google Scholar] [CrossRef] [PubMed]
- Grundy, Q.; Parker, L.; Wong, A.; Fusire, T.; Dimancesco, D.; Tisocki, K.; Walkowiak, H.; Vian, T.; Kohler, J. Disclosure, transparency, and accountability: A qualitative survey of public sector pharmaceutical committee conflict of interest policies in the World Health Organization South-East Asia Region. Glob. Health 2022, 18, 33. [Google Scholar] [CrossRef]
- Angelis, A.; Lange, A.; Kanavos, P. Using health technology assessment to assess the value of new medicines: Results of a systematic review and expert consultation across eight European countries. Eur. J. Health Econ. 2018, 19, 123–152. [Google Scholar] [CrossRef]
- Pisana, A.; Wettermark, B.; Kurdi, A.; Tubic, B.; Pontes, C.; Zara, C.; Van Ganse, E.; Petrova, G.; Mardare, I.; Fürst, J.; et al. Challenges and Opportunities with Routinely Collected Data on the Utilization of Cancer Medicines. Perspectives From Health Authority Personnel Across 18 European Countries. Front. Pharmacol. 2022, 13, 873556. [Google Scholar] [CrossRef]
- Almomani, E.; Hammad, E.; AlQutob, R.; Abu Hammour, K.; Al-Sharu, E.; Abu-Shaer, M.; Alabbadi, I.; Kaló, Z. Capacity Building for Health Technology Assessment in Jordan: Institutionalization and Its Use in Pricing and Reimbursement Decisions. Value Health Reg. Issues 2022, 32, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, M.A.; Møllebæk, M.; Vreman, R.A.; Lu, T.-A.; Wang, J.; De Bruin, M.L.; Leufkens, H.G.M.; Mantel-Teeuwisse, A.; Goettsch, W. Perspectives on how to build bridges between regulation, health technology assessment and clinical guideline development: A qualitative focus group study with European experts. BMJ Open 2023, 13, e072309. [Google Scholar] [CrossRef]
- Eskola, S.M.; Leufkens, H.G.; Bate, A.; De Bruin, M.L.; Gardarsdottir, H. Use of Real-World Data and Evidence in Drug Development of Medicinal Products Centrally Authorized in Europe in 2018–2019. Clin. Pharmacol. Ther. 2022, 111, 310–320. [Google Scholar] [CrossRef]
- Vitry, A.; Nguyen, T.; Entwistle, V.; Roughead, E. Regulatory withdrawal of medicines marketed with uncertain benefits: The bevacizumab case study. J. Pharm. Policy Pract. 2015, 8, 25. [Google Scholar] [CrossRef]
- Sehdev, S.; Chambers, A. Is It Time to Commit to a Process to Re-Evaluate Oncology Drugs? A Descriptive Analysis of Systemic Therapies for Solid Tumour Indications Reviewed in Canada from 2017 to 2021. Curr. Oncol. 2022, 29, 1919–1931. [Google Scholar] [CrossRef]
- Phelps, C.E.; Lakdawalla, D.N.; Basu, A.; Drummond, M.F.; Towse, A.; Danzon, P.M. Approaches to Aggregation and Decision Making—A Health Economics Approach: An ISPOR Special Task Force Report [5]. Value Health. 2018, 21, 146–154. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Process-Focused DSM | Country/Context | Policy Domain | Reported Outcomes |
|---|---|---|---|---|
| Donelan et al., 2021 [9] | QoDoS | UK/Regulatory and HTA | Quality of decision-making | Improved consistency and transparency |
| WHO, 2019 [10] | WHO-INTEGRATE | Global/LMICs | HTA, UHC policy | Value alignment, contextual integration |
| Fasseeh et al., 2020 [13] | Stakeholder MCDA | Oman | Pricing, value assessment | Stakeholder alignment, Legitimacy |
| Bujar et al., 2017 [8] | QoDoS | Global | Industry-regulator interface | Evaluation framework Development |
| Al- Badriyeh et al., 2016 [15] | QoDoS, MCDA | Kuwait, Quatar | Regulatory process | Quality benchmarking, internal process improvement |
| Marsh et al., 2014 [16] | EVIDEM | International case comparisons | MCDA in HTA | Structured evaluation criteria, stakeholder integration |
| Yfantopoulos and Chantzaras, 2018 [17] | Policy analysis | Greece | Pricing reforms | Governance challenges, transparency deficits |
| Kaló et al., 2021 [18] | Value-Added Meds Eval | EU (pilot cases) | Reimbursement/value | Value optimization and HTA acceptability |
| Visintin et al., 2019 [19] | HTA framework comparison | 7 OECD countries | Reimbursement | Variability in appraisal criteria across HTA bodies |
| Costa et al., 2022 [20] | Regulatory assessment | EU/US | Access to orphan drugs | Delays in access, regulatory divergence |
| Laba et al., 2020 [21] | Consumer-engaged MCDA | Canada | Public drug plans | MCDA feasibility, transparency |
| Moosivand et al., 2021 [22] | AHP-TOPSIS MCDA | Iran | Drug shortages policy | Multi-criteria prioritization framework |
| McEwin et al., 2025 [23] | Post-market evidence scoping | Australia | Oncology decision-making | Gaps in real-world integration in decisions |
| Grundy et al., 2022 [24] | Governance and COI analysis | SE Asia Region | Regulatory governance | Conflict of interest management frameworks |
| Angelis et al., 2018 [25] | HTA practices comparison | 8 EU countries | Value assessment | Divergence in criteria and transparency |
| Pisana et al., 2022 [26] | Real-World Data evaluation | 18 European countries | Oncology utilization | RWD potential and limitations in routine decisions |
| Almomani et al., 2022 [27] | Local HTA capacity | Jordan | Pricing and reimbursement | Institutionalization progress |
| Hogervorst et al., 2023 [28] | Stakeholder consultation | EU | Regulatory-HTA convergence | Recommendations for Integration |
| Eskola et al., 2022 [29] | RWE mapping | EMA/EU | Marketing authorization | Frequency of RWD in Approvals |
| Vitry et al., 2015 [30] | Regulatory ethics review | EU | Adaptive licensing | Evaluation uncertainty, transparency gaps |
| Sehdev and Chambers, 2022 [31] | CADTH Re-evaluation framework | Canada | Reimbursement revisions | Justification and accountability mechanisms |
| Abdullah et al., 2019 [14] | Stakeholder MCDA | Kuwait | Procurement | Stakeholder alignment, MCDA |
| # | Study/(Author, Year) | DSM Used | Structure | Transparency | Impact | Evaluation | Total/8 |
|---|---|---|---|---|---|---|---|
| 1 | Donelan et al., 2016 [9] | QoDoS | 2 | 2 | 2 | 2 | 8 |
| 2 | WHO, 2019 [10] | WHO-INTEGRATE | 1 | 2 | 2 | 1 | 6 |
| 3 | Fasseeh et al., 2020 [13] | Stakeholder MCDA | 1 | 2 | 2 | 0 | 5 |
| 4 | Bujar et al., 2017 [8] | Multiple (incl. QoDoS) | 2 | 1 | 1 | 1 | 5 |
| 5 | Al- Badriyeh et al., 2016 [15] | Adapted QoDoS, MCDA | 1 | 2 | 1 | 1 | 5 |
| 6 | Marsh et al., 2014 [16] | EVIDEM | 1 | 2 | 2 | 1 | 6 |
| 7 | Yfantopoulos and Chantzaras, 2018 [17] | Policy Analysis | 1 | 1 | 1 | 0 | 3 |
| 8 | Kalo et al., 2021 [18] | Value-Added Meds Eval | 1 | 2 | 1 | 1 | 5 |
| 9 | Visintin et al., 2019 [19] | HTA Framework Analysis | 2 | 2 | 1 | 1 | 6 |
| 10 | Costa et al., 2022 [20] | Regulatory Pathway Eval | 1 | 1 | 1 | 1 | 6 |
| 11 | Laba et al., 2020 [21] | MCDA | 1 | 2 | 2 | 1 | 6 |
| 12 | Moosivand et al., 2021 [22] | MCDM/AHP-TOPSIS | 1 | 1 | 2 | 1 | 5 |
| 13 | McEwin et al., 2025 [23] | Scoping RWD | 1 | 2 | 1 | 1 | 5 |
| 14 | Grundy et al., 2022 [24] | Governance/Disclosure | 2 | 2 | 2 | 0 | 6 |
| 15 | Angelis et al., 2018 [25] | Comparative HTA Review | 2 | 2 | 1 | 1 | 6 |
| 16 | Pisana et al., 2022 [26] | RWD Evaluation | 1 | 2 | 1 | 1 | 5 |
| 17 | Almomani et al., 2022 [27] | Local HTA Implementation | 2 | 2 | 1 | 1 | 6 |
| 18 | Hogervorst et al., 2023 [28] | Qualitative Policy Eval | 1 | 2 | 2 | 0 | 5 |
| 19 | Eskola et al., 2022 [29] | RWE Mapping | 1 | 2 | 1 | 1 | 5 |
| 20 | Vitry et al., 2015 [30] | FDA/EMA Decision Review | 1 | 1 | 1 | 1 | 4 |
| 21 | Sehdev and Chambers, 2022 [31] | CADTH Re-Eval Framework | 1 | 1 | 1 | 1 | 4 |
| 22 | Abdullah et al., 2019 [14] | Stakeholder MCDA (Kuwait) | 2 | 2 | 2 | 1 | 7 |
| Tool | Transparency | Stakeholder Inclusion | Consistency | Equity Focus | Usability (Applied Use) |
|---|---|---|---|---|---|
| QoDoS | High | Moderate | High | Low | High (industry, regulators) |
| WHO-INTEGRATE | Moderate | High | Moderate | High | Moderate (LMIC policy use) |
| AGREE II | High | Low | High | Low | High (guideline contexts) |
| EVIDEM (MCDA) | Moderate | High | High | Moderate | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theiakou, F.; Kastanioti, C.; Zavras, D.; Rekkas, D. Beyond the Pill: Mapping Process-Oriented Decision Support Models in Pharmaceutical Policy. Healthcare 2025, 13, 1861. https://doi.org/10.3390/healthcare13151861
Theiakou F, Kastanioti C, Zavras D, Rekkas D. Beyond the Pill: Mapping Process-Oriented Decision Support Models in Pharmaceutical Policy. Healthcare. 2025; 13(15):1861. https://doi.org/10.3390/healthcare13151861
Chicago/Turabian StyleTheiakou, Foteini, Catherine Kastanioti, Dimitris Zavras, and Dimitrios Rekkas. 2025. "Beyond the Pill: Mapping Process-Oriented Decision Support Models in Pharmaceutical Policy" Healthcare 13, no. 15: 1861. https://doi.org/10.3390/healthcare13151861
APA StyleTheiakou, F., Kastanioti, C., Zavras, D., & Rekkas, D. (2025). Beyond the Pill: Mapping Process-Oriented Decision Support Models in Pharmaceutical Policy. Healthcare, 13(15), 1861. https://doi.org/10.3390/healthcare13151861







