Continuous Radiofrequency for Morton’s Neuroma: Is There Complete Ablation? A Preliminary Report
Abstract
1. Introduction
2. Case Reports
2.1. Case 1
2.2. Case 2
2.3. Surgical Technique
2.4. Application of Radiofrequency
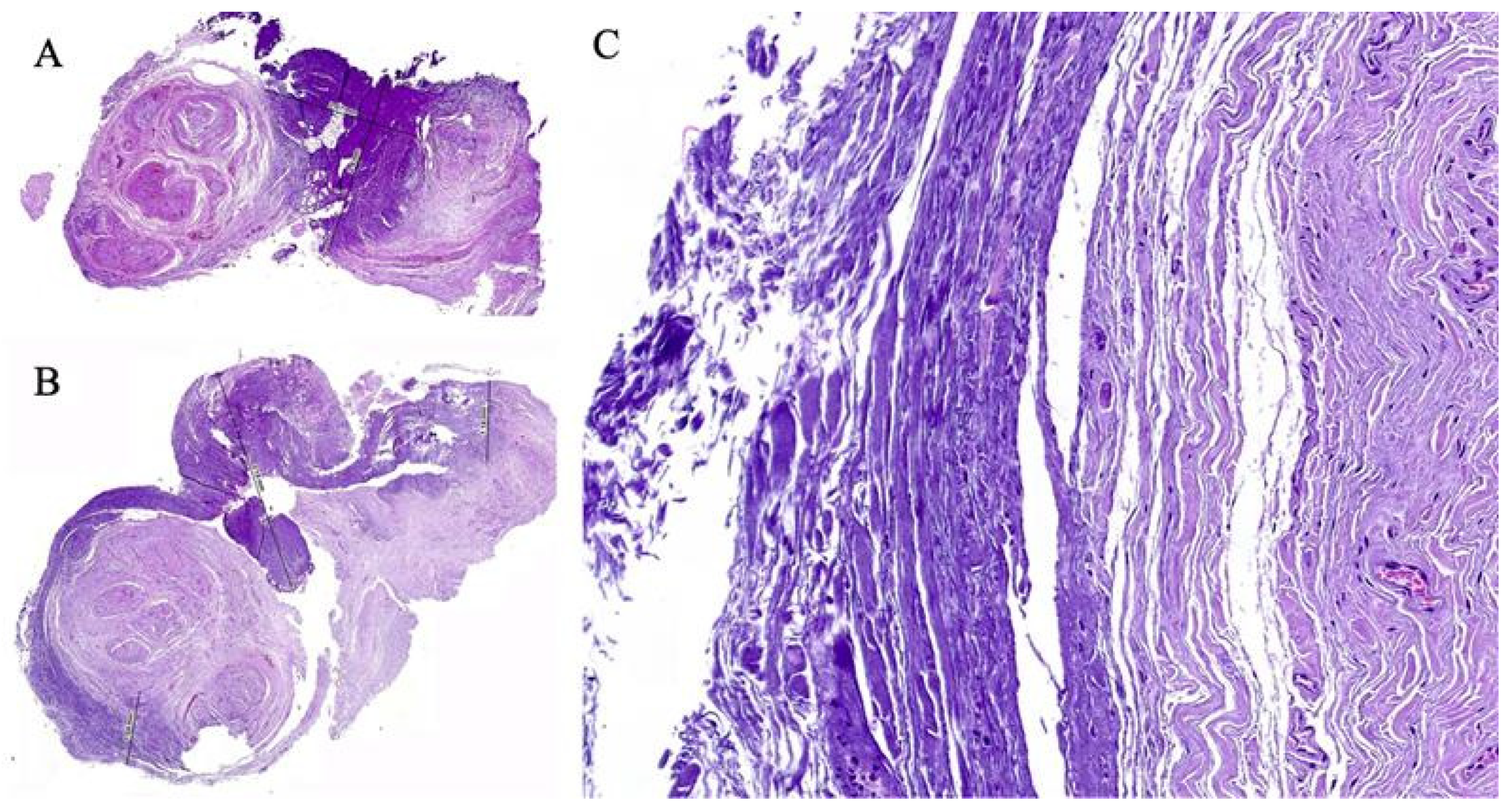
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, R.A.; Reynolds, J.C. Interdigital neuroma—A critical clinical analysis. Foot Ankle 1983, 3, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.R., 2nd. Morton’s neuroma. Clin. Podiatr. Med. Surg. 2010, 27, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K. Morton’s interdigital neuroma: A clinical review of its etiology, treatment, and results. J. Foot Ankle Surg. 1996, 35, 112–119, discussion 187–188. [Google Scholar] [CrossRef] [PubMed]
- Valisena, S.; Petri, G.J.; Ferrero, A. Treatment of Morton’s neuroma: A systematic review. Foot Ankle Surg. 2018, 24, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Santiago, F.; Prados Olleta, N.; Tomas Munoz, P.; Guzman Alvarez, L.; Martinez Martinez, A. Short term comparison between blind and ultrasound guided injection in morton neuroma. Eur. Radiol. 2019, 29, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Womack, J.W.; Richardson, D.R.; Murphy, G.A.; Richardson, E.G.; Ishikawa, S.N. Long-term evaluation of interdigital neuroma treated by surgical excision. Foot Ankle Int. 2008, 29, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Kasparek, M.; Schneider, W. Surgical treatment of Morton’s neuroma: Clinical results after open excision. Int. Orthop. 2013, 37, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Nieves, G.C.; Fernandez-Gibello, A.; Moroni, S.; Montes, R.; Marquez, J.; Ortiz, M.S.; Vázquez, T.; Duparc, F.; Moriggl, B.; Konschake, M. Anatomic basis for a new ultrasound-guided, mini-invasive technique for release of the deep transverse metatarsal ligament. Clin. Anat. 2021, 34, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.L. Endocsopic Decompression of Intermetatarsal Nerve (EDIN) for the Treatment of Morton’s Entrapment—Multicenter Retrospective Review. Open J. Orthop. 2012, 2, 19–24. [Google Scholar] [CrossRef]
- Chuter, G.S.; Chua, Y.P.; Connell, D.A.; Blackney, M.C. Ultrasound-guided radiofrequency ablation in the management of interdigital (Morton’s) neuroma. Skelet. Radiol. 2013, 42, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.L.; Rosen, R.; Cohen, J.; Rosen, B. Radiofrequency thermoneurolysis for the treatment of Morton’s neuroma. J. Foot Ankle Surg. 2012, 51, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Cabello, J.; Domínguez-Maldonado, G. Revisión narrativa sobre el uso y aplicaciones de la radiofrecuencia para el tratamiento del dolor musculoesquelético. Rev. Esp. Podol. 2021, 32, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cosman, E.R., Jr.; Cosman, E.R., Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005, 6, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, Y.; Yao, B.; Song, P.; Xu, R.; Li, R.; Liu, P.; Zhang, Y.; Mu, L.; Tong, X.; et al. Neuropathologic damage induced by radiofrequency ablation at different temperatures. Clinics 2022, 77, 100033. [Google Scholar] [CrossRef] [PubMed]
- Masala, S.; Cuzzolino, A.; Morini, M.; Raguso, M.; Fiori, R. Ultrasound-Guided Percutaneous Radiofrequency for the Treatment of Morton’s Neuroma. Cardiovasc. Interv. Radiol. 2018, 41, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Cosman, E.R., Jr.; Dolensky, J.R.; Hoffman, R.A. Factors that affect radiofrequency heat lesion size. Pain Med. 2014, 15, 2020–2036. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Parr, A.; Bryceson, W. Three Cycles of Radiofrequency Ablation Are More Efficacious Than Two in the Management of Morton’s Neuroma. Foot Ankle Spec. 2018, 11, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Perez, M.; Oller-Boix, A.; Perez-Lorensu, P.J.; de Bergua-Domingo, J.; Gonzalez-Casamayor, S.; Marquez-Marfil, F.; Díaz-Flores, L.; Pais-Brito, J. Intraoperative neurophysiological monitoring in peripheral nerve surgery: Technical description and experience in a centre. Rev. Esp. Cir. Ortop. Traumatol. 2015, 59, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Karri, J.; Singh, M.; Abd-Elsayed, A. Incidence, Diagnosis, and Management of Neuromas Following Radiofrequency Ablation Treatment: A Narrative Review. Curr. Pain Headache Rep. 2021, 25, 45. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camuñas-Nieves, G.; Fernández-Gibello, A.; Moroni, S.; Galluccio, F.; Fajardo-Pérez, M.; Martínez-Pérez, F.; Simón-Pérez, E.; Martínez-Nova, A. Continuous Radiofrequency for Morton’s Neuroma: Is There Complete Ablation? A Preliminary Report. Healthcare 2025, 13, 1838. https://doi.org/10.3390/healthcare13151838
Camuñas-Nieves G, Fernández-Gibello A, Moroni S, Galluccio F, Fajardo-Pérez M, Martínez-Pérez F, Simón-Pérez E, Martínez-Nova A. Continuous Radiofrequency for Morton’s Neuroma: Is There Complete Ablation? A Preliminary Report. Healthcare. 2025; 13(15):1838. https://doi.org/10.3390/healthcare13151838
Chicago/Turabian StyleCamuñas-Nieves, Gabriel, Alejandro Fernández-Gibello, Simone Moroni, Felice Galluccio, Mario Fajardo-Pérez, Francisco Martínez-Pérez, Eduardo Simón-Pérez, and Alfonso Martínez-Nova. 2025. "Continuous Radiofrequency for Morton’s Neuroma: Is There Complete Ablation? A Preliminary Report" Healthcare 13, no. 15: 1838. https://doi.org/10.3390/healthcare13151838
APA StyleCamuñas-Nieves, G., Fernández-Gibello, A., Moroni, S., Galluccio, F., Fajardo-Pérez, M., Martínez-Pérez, F., Simón-Pérez, E., & Martínez-Nova, A. (2025). Continuous Radiofrequency for Morton’s Neuroma: Is There Complete Ablation? A Preliminary Report. Healthcare, 13(15), 1838. https://doi.org/10.3390/healthcare13151838








