Percutaneous Electrolysis for Musculoskeletal Disorders Management in Rehabilitation Settings: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
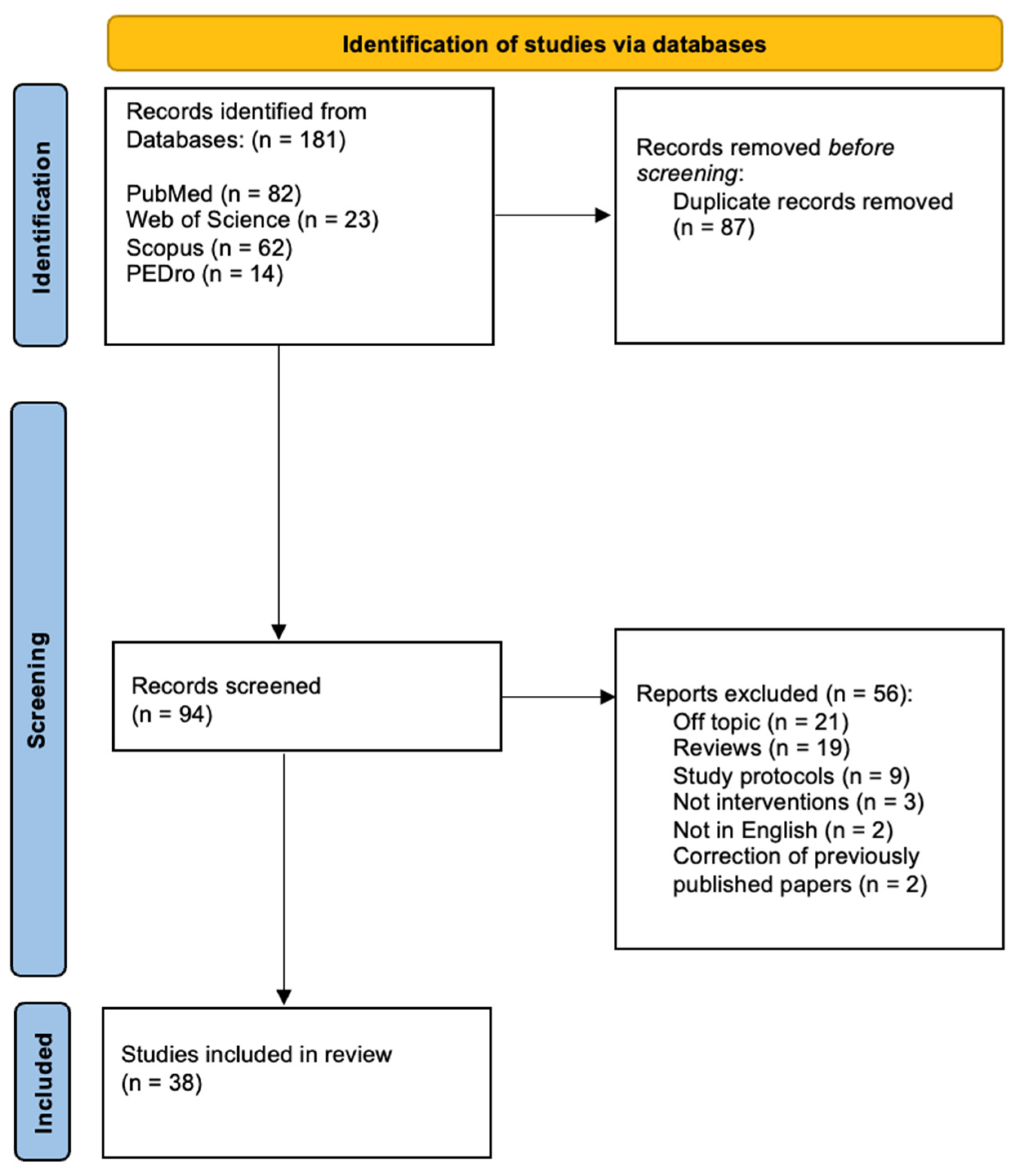
2.2. Screening Results and Eligibility
2.3. Data Extraction
- General characteristic of the paper: first author, year of publication, study design.
- Study population characteristics. Human/no human, patients or healthy volunteers, age, gender, and type of disease.
- Methods: type of US imaging, setting; use of the US guide; operator performing the procedure; type of disease; rehabilitation protocol applied.
- Times during rehabilitation: pre-, during, post-, and follow-up.
- Outcomes and results.
2.4. Risk of Bias
3. Result
3.1. Musculoskeletal Diseases
3.2. Animal Studies
3.3. Cadaveric Studies
3.4. Ultrasound Imaging and Percutaneous Electrolysis
3.5. Risk of Bias Assessment and Applicability Concerns
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varela-Rodríguez, S.; Sánchez-Sánchez, J.L.; Velasco, E.; Delicado-Miralles, M.; Sánchez-González, J.L. Endogenous Pain Modulation in Response to a Single Session of Percutaneous Electrolysis in Healthy Population: A Double-Blinded Randomized Clinical Trial. J. Clin. Med. 2022, 11, 2889. [Google Scholar] [CrossRef]
- Gómez-Chiguano, G.F.; Navarro-Santana, M.J.; Cleland, J.A.; Arias-Buría, J.L.; Fernández-de-las-Peñas, C.; Ortega-Santiago, R.; Plaza-Manzano, G. Effectiveness of Ultrasound-Guided Percutaneous Electrolysis for Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 1055–1071. [Google Scholar] [CrossRef]
- Abat, F.; Valles, S.L.; Gelber, P.E.; Polidori, F.; Stitik, T.P.; García-Herreros, S.; Monllau, J.C.; Sanchez-Ibánez, J.M. Mecanismos moleculares de reparación mediante la técnica Electrólisis Percutánea Intratisular en la tendinosis rotuliana. Rev. Esp. Cir. Ortopédica Traumatol. 2014, 58, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Pujalte, G.G.A.; Malone, M.; Mandavalli, A.; Phrathep, D.D.; Shah, N.P.; Perlman, A.I. Acupuncture in Sports Medicine. J. Acupunct. Meridian Stud. 2023, 16, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, J.L.; Calderón-Díez, L.; Herrero-Turrión, J.; Méndez-Sánchez, R.; Arias-Buría, J.L.; Fernández-de-las-Peñas, C. Changes in Gene Expression Associated with Collagen Regeneration and Remodeling of Extracellular Matrix after Percutaneous Electrolysis on Collagenase-Induced Achilles Tendinopathy in an Experimental Animal Model: A Pilot Study. J. Clin. Med. 2020, 9, 3316. [Google Scholar] [CrossRef] [PubMed]
- Margalef, R.; Muñoz, F.M.M.; Garrido, F.V.; Santafe, M.M. Vasodilation Secondary to Exposure to Galvanic Currents. Rev. Fisioter. Invasiva J. Invasive Technol. Phys. Ther. 2019, 2, 107. [Google Scholar] [CrossRef]
- Fakontis, C.; Iakovidis, P.; Lytras, D.; Kasimis, K.; Koutras, G.; Ntinou, S.R.; Kottaras, A.; Chatziprodromidou, I.P.; Chatzikonstantinou, P.; Apostolou, T. Efficacy of Percutaneous Needle Electrolysis versus Dry Needling in Musculoskeletal Pain: A Systematic Review and Meta-Analysis. J. Back Musculoskelet. Rehabil. 2023, 36, 1033–1046. [Google Scholar] [CrossRef]
- Berná-Serna, J.D.; García-Vidal, J.A.; Escolar-Reina, P.; Berná-Mestre, J.D. Ultrasound-Guided Percutaneous Electrolysis: A New Therapeutic Option for Mammary Fistulas. Med. Hypotheses 2018, 112, 35–36. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C.; Graven-Nielsen, T. Basic Aspects of Musculoskeletal Pain: From Acute to Chronic Pain. J. Man. Manip. Ther. 2011, 19, 186–193. [Google Scholar] [CrossRef]
- De La Corte-Rodríguez, H.; Román-Belmonte, J.M.; Rodríguez-Damiani, B.A.; Vázquez-Sasot, A.; Rodríguez-Merchán, E.C. Extracorporeal Shock Wave Therapy for the Treatment of Musculoskeletal Pain: A Narrative Review. Healthcare 2023, 11, 2830. [Google Scholar] [CrossRef]
- Aicale, R.; Oliva, F.; Maffulli, N. Achilles Tendinopathy. In Orthopaedic Sports Medicine: An Encyclopedic Review of Diagnosis, Prevention, and Management; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–13. [Google Scholar]
- Owoeye, O.B.A.; Palacios-Derflingher, L.; Pasanen, K.; HubkaRao, T.; Wiley, P.; Emery, C.A. The Burden and Risk Factors of Patellar and Achilles Tendinopathy in Youth Basketball: A Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 9480. [Google Scholar] [CrossRef]
- Jildeh, T.R.; Buckley, P.; Abbas, M.J.; Page, B.; Young, J.; Mehran, N.; Okoroha, K.R. Impact of Patellar Tendinopathy on Player Performance in the National Basketball Association. Orthop. J. Sports Med. 2021, 9, 23259671211025305. [Google Scholar] [CrossRef]
- Putra, K.A.W.; Kamayoga, I.D.G.A. Physiotherapy Interventions in Lateral Epicondylitis. Kinesiol. Physiother. Compr. 2022, 1, 11–13. [Google Scholar] [CrossRef]
- Bilgin, Y.; Birişik, F.; Guler, S.B. Kinesio Taping, Wrist Splinting, and Epicondylitis Bandaging in Managing Lateral Epicondylitis: A Prospective Comparative Study. Med. Sci. Monit. 2025, 31, e947642. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Ono-matsukubo, Q.; Nishigami, T.; Maitani, T.; Mibu, A.; Hirooka, T.; Machida, H. Generalizability of Eccentric Exercise for Patients with Subacromial Pain Syndrome to Real-World Clinical Practice: A Propensity Score-Based Analysis. Prog. Rehabil. Med. 2021, 6, 20210019. [Google Scholar] [CrossRef] [PubMed]
- Orandi, A.H.; Mansour, A.; Bagheri, N.; Majedi, H.; Meibodi, S.A.E.; Pestehei, S.K.; Saberian, P. The Comparison of the Efficacy of Intramuscular Tetracosactide and Subacromial Triamcinolone Injection in Rotator Cuff Tendinitis: A Randomized Trial. Rheumatol. Adv. Pract. 2024, 9, rkae150. [Google Scholar] [CrossRef]
- Cooper, K.; Alexander, L.; Brandie, D.; Brown, V.T.; Greig, L.; Harrison, I.; MacLean, C.; Mitchell, L.; Morrissey, D.; Moss, R.A.; et al. Exercise Therapy for Tendinopathy: A Mixed-Methods Evidence Synthesis Exploring Feasibility, Acceptability and Effectiveness. Health Technol. Assess. 2023, 27, 1. [Google Scholar] [CrossRef]
- Rhim, H.C.; Shin, J.; Kang, J.; Dyrek, P.; Crockett, Z.; Galido, P.; Wade, C.; Hollander, K.; Borg-Stein, J.; Sampson, S.; et al. Use of Extracorporeal Shockwave Therapies for Athletes and Physically Active Individuals: A Systematic Review. Br. J. Sports Med. 2024, 58, 154–163. [Google Scholar] [CrossRef]
- Manocchio, N.; Pirri, C.; Sorbino, A.; Giordani, L.; Vita, G.; Ljoka, C.; Foti, C. Shoulder Tendinopathy Induced by Statins: A Case Report and Systematic Review. J. Pers. Med. 2025, 15, 198. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Schäfer, L.; Manocchio, N.; Bossa, M.; Foti, C.; Betsch, M.; Kubach, J. Impact of Education in Patients Undergoing Physiotherapy for Lower Back Pain: A Level I Systematic Review and Meta-Analysis. Eur. J. Trauma Emerg. Surg. 2025, 51, 113. [Google Scholar] [CrossRef]
- Du, S.; Cui, Z.; Peng, S.; Wu, J.; Xu, J.; Mo, W.; Ye, J. Clinical Efficacy of Exercise Therapy for Lumbar Disc Herniation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2025, 12, 1531637. [Google Scholar] [CrossRef]
- Baroncini, A.; Maffulli, N.; Schäfer, L.; Manocchio, N.; Bossa, M.; Foti, C.; Klimuch, A.; Migliorini, F. Physiotherapeutic and Non-Conventional Approaches in Patients with Chronic Low-Back Pain: A Level I Bayesian Network Meta-Analysis. Sci. Rep. 2024, 14, 11546. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sung, S.-H.; Lee, S. The Effectiveness and Safety of Tai Chi on Knee Pain: A Systematic Review and Meta-Analysis. Healthcare 2025, 13, 1615. [Google Scholar] [CrossRef] [PubMed]
- Manocchio, N.; Ljoka, C.; Piacentini, N.; Sorge, R.; Vita, G.; Foti, C. Intra-Articular Injections with Carboxymethyl-Chitosan in Patients Affected by Knee Osteoarthritis Non-Responders to Hyaluronic Acid: A Pilot Study. Eur. J. Transl. Myol. 2024, 34, 12413. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Snichelotto, F.; Liguori, S.; Paoletta, M.; Toro, G.; Gimigliano, F.; Iolascon, G. The Challenge of Pharmacotherapy for Musculoskeletal Pain: An Overview of Unmet Needs. Ther. Adv. Musculoskelet. Dis. 2024, 16, 1759720X241253656. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; D’Amato, A.; Vita, G.; Giordani, L.; Foti, C. Sports Injuries and Re-Educational Treatments in Soccer: A Questionnaire Survey to Young Italian Athletes. Muscle Ligaments Tendons J. 2025, 15, 222. [Google Scholar] [CrossRef]
- Anderson, C.P.; Pipinos, I.I.; Johanning, J.M.; Myers, S.A.; Rahman, H. Effects of Supervised Exercise Therapy on Muscle Function During Walking in Patients with Peripheral Artery Disease. Bioengineering 2024, 11, 1103. [Google Scholar] [CrossRef]
- López, I.; Mielgo-Ayuso, J.; Fernández-López, J.R.; Aznar, J.M.; Castañeda-Babarro, A. Protocol for a Trial to Assess the Efficacy and Applicability of Isometric Strength Training in Older Adults with Sarcopenia and Dynapenia. Healthcare 2025, 13, 1573. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 371, n71. [Google Scholar] [CrossRef]
- Abat, F.; Diesel, W.-J.; Gelber, P.-E.; Polidori, F.; Monllau, J.-C.; Sanchez-Ibañez, J.-M. Effectiveness of the Intratissue Percutaneous Electrolysis (EPI®) Technique and Isoinertial Eccentric Exercise in the Treatment of Patellar Tendinopathy at Two Years Follow-Up. Muscles Ligaments Tendons J. 2014, 4, 188. [Google Scholar] [CrossRef]
- Abat, F.; Gelber, P.E.; Polidori, F.; Monllau, J.C.; Sanchez-Ibañez, J.M. Clinical Results after Ultrasound-Guided Intratissue Percutaneous Electrolysis (EPI®) and Eccentric Exercise in the Treatment of Patellar Tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1046–1052. [Google Scholar] [CrossRef]
- López-Royo, M.P.; Ríos-Díaz, J.; Galán-Díaz, R.M.; Herrero, P.; Gómez-Trullén, E.M. A Comparative Study of Treatment Interventions for Patellar Tendinopathy: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 967–975. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz Torres, B.; Albornoz Cabello, M.; García Bermejo, P.; Naranjo Orellana, J. Autonomic Responses to Ultrasound-Guided Percutaneous Needle Electrolysis of the Patellar Tendon in Healthy Male Footballers. Acupunct. Med. 2016, 34, 275–279. [Google Scholar] [CrossRef] [PubMed]
- García Bermejo, P.; De La Cruz Torres, B.; Naranjo Orellana, J.; Albornoz Cabello, M. Autonomic Responses to Ultrasound-Guided Percutaneous Needle Electrolysis: Effect of Needle Puncture or Electrical Current? J. Altern. Complement. Med. 2018, 24, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanchis, D.; López-Royo, M.P.; Jiménez-Sánchez, C.; Herrero, P.; Gómez-Barrera, M.; Calvo, S. A Comparative Study of Treatment Interventions for Patellar Tendinopathy: A Secondary Cost-Effectiveness Analysis. Acupunct. Med. 2022, 40, 516–523. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Sánchez-Mayoral-Martín, A.; Varol, U. Short-Term Effectiveness of High- and Low-Intensity Percutaneous Electrolysis in Patients with Patellofemoral Pain Syndrome: A Pilot Study. World J. Orthop. 2021, 12, 781–790. [Google Scholar] [CrossRef]
- Iborra-Marcos, Á.; Ramos-Álvarez, J.J.; Rodriguez-Fabián, G.; Del Castillo-González, F.; López-Román, A.; Polo-Portes, C.; Villanueva-Martínez, M. Intratissue Percutaneous Electrolysis vs Corticosteroid Infiltration for the Treatment of Plantar Fasciosis. Foot Ankle Int. 2018, 39, 704–711. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, T.; Fernández-Rolle, Á.; Truyols-Domínguez, S.; Benítez-Martínez, J.C.; Casaña-Granell, J. Prospective Randomized Trial of Electrolysis for Chronic Plantar Heel Pain. Foot Ankle Int. 2018, 39, 1039–1046. [Google Scholar] [CrossRef]
- Al-Boloushi, Z.; Gómez-Trullén, E.M.; Arian, M.; Fernández, D.; Herrero, P.; Bellosta-López, P. Comparing Two Dry Needling Interventions for Plantar Heel Pain: A Randomised Controlled Trial. BMJ Open 2020, 10, e038033. [Google Scholar] [CrossRef]
- Fernández, D.; Al-Boloushi, Z.; Bellosta-López, P.; Herrero, P.; Gómez, M.; Calvo, S. Cost-Effectiveness of Two Dry Needling Interventions for Plantar Heel Pain: A Secondary Analysis of an RCT. Int. J. Environ. Res. Public Health 2021, 18, 1777. [Google Scholar] [CrossRef]
- Naranjo, J.G.; Rosa, S.B.; Ferrer, J.F.L.; Cañal, J.M.L.; Hernández, E.S. A Novel Approach in the Treatment of Acute Whiplash Syndrome: Ultrasound-Guided Needle Percutaneous Electrolysis. A Randomized Controlled Trial. Orthop. Traumatol. Surg. Res. 2017, 103, 1229–1234. [Google Scholar] [CrossRef]
- Moreno, C.; Mattiussi, G.; Núñez, F.J.; Messina, G.; Rejc, E. Intratissue Percutaneous Electolysis Combined with Active Physical Therapy for the Treatment of Adductor Longus Enthesopathy-Related Groin Pain: A Randomized Trial. J. Sports Med. Phys. Fit. 2017, 57, 1318–1329. [Google Scholar] [CrossRef]
- Sánchez-González, J.L.; Navarro-López, V.; Calderón-Díez, L.; Varela-Rodríguez, S.; Fernández-de-las-Peñas, C.; Sánchez-Sánchez, J.L. Effectiveness of Different Percutaneous Electrolysis Protocols in the Endogenous Modulation of Pain: A Double-Blinded Randomized Clinical Trial. Musculoskelet. Sci. Pract. 2023, 68, 102872. [Google Scholar] [CrossRef]
- Valera-Garrido, F.; Minaya-Muñoz, F.; Medina-Mirapeix, F. Ultrasound-Guided Percutaneous Needle Electrolysis in Chronic Lateral Epicondylitis: Short-Term and Long-Term Results. Acupunct. Med. 2014, 32, 446–454. [Google Scholar] [CrossRef]
- Yildizgoren, M.T.; Ekici, S.N.M.; Ekici, B. Biochemical Reactions and Ultrasound Insights in Percutaneous Needle Electrolysis Therapy. Interv. Pain Med. 2025, 4, 100593. [Google Scholar] [CrossRef] [PubMed]
- De-la-Cruz-Torres, B.; Barrera-García-Martín, I.; Valera-Garrido, F.; Minaya-Muñoz, F.; Romero-Morales, C. Ultrasound-Guided Percutaneous Needle Electrolysis in Dancers with Chronic Soleus Injury: A Randomized Clinical Trial. Evid. Based Complement. Alternat. Med. 2020, 2020, 4156258. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rubio, S.; Navandar, A.; Rivilla-García, J.; Paredes-Hernández, V. Validity of an On-Field Readaptation Program Following a Hamstring Injury in Professional Soccer. J. Sport Rehabil. 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huguet, M.; Góngora-Rodríguez, J.; Lomas-Vega, R.; Martín-Valero, R.; Díaz-Fernández, Á.; Obrero-Gaitán, E.; Ibáñez-Vera, A.J.; Rodríguez-Almagro, D. Percutaneous Electrolysis in the Treatment of Lateral Epicondylalgia: A Single-Blind Randomized Controlled Trial. J. Clin. Med. 2020, 9, 2068. [Google Scholar] [CrossRef]
- Rodríguez-Huguet, M.; Rodríguez-Almagro, D.; Rosety-Rodríguez, M.A.; Vinolo-Gil, M.J.; Molina-Jiménez, J.; Góngora-Rodríguez, J. Pulsed Negative Pressure Myofascial Vacuum Therapy and Percutaneous Electrolysis in the Treatment of Lateral Epicondylalgia: A Single-Blind Randomized Controlled Trial. J. Hand Ther. 2024, 37, 644–652. [Google Scholar] [CrossRef]
- Benito-de-Pedro, A.I.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Benito-de-Pedro, M. Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial. Life 2023, 13, 939. [Google Scholar] [CrossRef]
- Moreno, C.; Mattiussi, G.; Núñez, F.J. Therapeutic Results after Ultrasound-Guided Intratissue Percutaneous Electrolysis (EPI®) in the Treatment of Rectus Abdominis-Related Groin Pain in Professional Footballers: A Pilot Study. J. Sports Med. Phys. Fit. 2016, 56, 1171–1178. [Google Scholar]
- De-la-Cruz-Torres, B.; Romero-Rodríguez, B.; Romero-Morales, C. Ultrasound-Guided Percutaneous Needle Electrolysis Combined With Therapeutic Exercise May Add Benefit in the Management of Soleus Injury in Female Soccer Players: A Pilot Study. J. Sport Rehabil. 2023, 32, 265–271. [Google Scholar] [CrossRef]
- De Miguel Valtierra, L.; Moreno, J.S.; Fernández-de-las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L. Ultrasound-Guided Application of Percutaneous Electrolysis as an Adjunct to Exercise and Manual Therapy for Subacromial Pain Syndrome: A Randomized Clinical Trial. J. Pain 2018, 19, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Arias-Buría, J.L.; Truyols-Domínguez, S.; Valero-Alcaide, R.; Salom-Moreno, J.; Atín-Arratibel, M.A.; Fernández-de-las-Peñas, C. Ultrasound-Guided Percutaneous Electrolysis and Eccentric Exercises for Subacromial Pain Syndrome: A Randomized Clinical Trial. Evid. Based Complement Alternat. Med. 2015, 2015, 315219. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huguet, M.; Góngora-Rodríguez, J.; Rodríguez-Huguet, P.; Ibañez-Vera, A.J.; Rodríguez-Almagro, D.; Martín-Valero, R.; Díaz-Fernández, Á.; Lomas-Vega, R. Effectiveness of Percutaneous Electrolysis in Supraspinatus Tendinopathy: A Single-Blinded Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1837. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Rodríguez, J.; Rosety-Rodríguez, M.Á.; Rodríguez-Almagro, D.; Martín-Valero, R.; Góngora-Rodríguez, P.; Rodríguez-Huguet, M. Structural and Functional Changes in Supraspinatus Tendinopathy through Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation and Eccentric Exercise Combined Therapy: A Single-Blinded Randomized Clinical Trial. Biomedicines 2024, 12, 771. [Google Scholar] [CrossRef]
- Lopez-Martos, R.; Gonzalez-Perez, L.; Ruiz-Canela-Mendez, P.; Urresti-Lopez, F.; Gutierrez-Perez, J.; Infante-Cossio, P. Randomized, Double-Blind Study Comparing Percutaneous Electrolysis and Dry Needling for the Management of Temporomandibular Myofascial Pain. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e454. [Google Scholar] [CrossRef]
- López-Royo, M.P.; Bataller-Cervero, A.V. Functionality and Jump Performance in Patellar Tendinopathy with the Application of Three Different Treatments. J. Sci. Med. Sport 2024, 24, 702–707. [Google Scholar] [CrossRef]
- Doménech-García, V.; Pecos-Martín, D.; Blasco-Abadía, J.; Bellosta-López, P.; López-Royo, M.P. Placebo and Nocebo Effects of Percutaneous Needle Electrolysis and Dry-Needling: An Intra and Inter-Treatment Sessions Analysis of a Three-Arm Randomized Double-Blinded Controlled Trial in Patients with Patellar Tendinopathy. Front. Med. 2024, 11, 1381515. [Google Scholar] [CrossRef]
- Margalef, R.; Valera-Garrido, F.; Minaya-Muñoz, F.; Bosque, M.; Ortiz, N.; Santafe, M.M. Percutaneous Needle Electrolysis Reverses Neurographic Signs of Nerve Entrapment by Induced Fibrosis in Mice. Evid. Based Complement. Alternat. Med. 2020, 2020, 6615563. [Google Scholar] [CrossRef]
- Belón-Pérez, P.; Calderón-Díez, L.; Sánchez-Sánchez, J.L.; Robles-García, M.; Plaza-Manzano, G.; Fernández-de-las-Peñas, C. Cadaveric and Ultrasound Validation of Percutaneous Electrolysis Approaches at the Arcade of Frohse: A Potential Treatment for Radial Tunnel Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 2476. [Google Scholar] [CrossRef]
- Calderón-Díez, L.; Sánchez-Sánchez, J.L.; Robles-García, M.; Belón-Pérez, P.; Fernández-de-las-Peñas, C. Cadaveric and Ultrasound Validation of Percutaneous Electrolysis Approach at the Achilles Tendon as a Potential Treatment for Achilles Tendinopathy: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 11906. [Google Scholar] [CrossRef]
- Calderón-Díez, L.; Belón-Pérez, P.; Fernández-de-las-Peñas, C.; Sánchez-Sánchez, J.L. The Safety of Ultrasound-Guided Needle Approaches for Patellar Tendinopathy: A Theoretical Cadaveric Model. J. Funct. Morphol. Kinesiol. 2025, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Borrella-Andrés, S.; Malo-Urriés, M.; Pérez-Bellmunt, A.; Arias-Buría, J.L.; Rodríguez-Sanz, J.; Albarova-Corral, M.I.; González-Rueda, V.; Gallego-Sendarrubias, G.M.; Fernández-de-las-Peñas, C.; López-de-Celis, C. Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study. Int. J. Environ. Res. Public Health 2022, 19, 15738. [Google Scholar] [CrossRef] [PubMed]
- Malo-Urriés, M.; Rodríguez-Sanz, J.; Borrella-Andrés, S.; Ríos-Asín, I.; Albarova-Corral, I.; López-de-Celis, C. Quantitative Ultrasound Characterization of Intensity-Dependent Changes in Muscle Tissue During Percutaneous Electrolysis. J. Clin. Med. 2025, 14, 4064. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rodríguez, S.; Sánchez-González, J.L.; Sánchez-Sánchez, J.L.; Delicado-Miralles, M.; Velasco, E.; Fernández-de-las-Peñas, C.; Calderón-Díez, L. Effects of Percutaneous Electrolysis on Endogenous Pain Modulation: A Randomized Controlled Trial Study Protocol. Brain Sci. 2021, 11, 801. [Google Scholar] [CrossRef]
- Berná-Serna, J.D.D.; García-Vidal, J.A.; Escolar-Reina, M.P.; Medina-Mirapeix, F.; Guzmán-Aroca, F.; Piñero-Madrona, A.; Berná-Mestre, J.D.D. A New Treatment for Mammillary Fistulas Using Ultrasound-Guided Percutaneous Needle Electrolysis. J. Clin. Med. 2020, 9, 649. [Google Scholar] [CrossRef]
- Calderón-Díez, L.; Sánchez-Sánchez, J.L.; Belón-Pérez, P.; Robles-García, M.; Pérez-Robledo, F.; Fernández-de-las-Peñas, C. Cadaveric and Ultrasound Validation of Percutaneous Electrolysis Approach at the Distal Biceps Tendon: A Potential Treatment for Biceps Tendinopathy. Diagnostics 2022, 12, 3051. [Google Scholar] [CrossRef]
- Mattiussi, G.; Moreno, C. Percutaneous Electrochemical Debridement of the Plantaris Tendon. J. Am. Podiatr. Med. Assoc. 2018, 108, 437–441. [Google Scholar] [CrossRef]
- Mattiussi, G.; Moreno, C. Treatment of Proximal Hamstring Tendinopathy-Related Sciatic Nerve Entrapment: Presentation of an Ultrasound-Guided “Intratissue Percutaneous Electrolysis” Application. Muscle Ligaments Tendons J. 2019, 06, 248. [Google Scholar] [CrossRef]
- López-Royo, M.P.; Gómez-Trullén, E.M.; Ortiz-Lucas, M.; Galán-Díaz, R.M.; Bataller-Cervero, A.V.; Al-Boloushi, Z.; Hamam-Alcober, Y.; Herrero, P. Comparative Study of Treatment Interventions for Patellar Tendinopathy: A Protocol for a Randomised Controlled Trial. BMJ Open 2020, 10, e034304. [Google Scholar] [CrossRef] [PubMed]
- Arias-Buría, J.L.; Borrella-Andrés, S.; Rodríguez-Sanz, J.; López-de-Celis, C.; Malo-Urriés, M.; Fernández-de-las-Peñas, C.; Gallego-Sendarrubias, G.M.; González-Rueda, V.; Pérez-Bellmunt, A.; Albarova-Corral, I. Precision and Safety of Ultrasound-Guided versus Palpation-Guided Needle Placement on the Patellar Tendon: A Cadaveric Study. Life 2023, 13, 2060. [Google Scholar] [CrossRef] [PubMed]
- Al-Boloushi, Z.; Gómez-Trullén, E.M.; Bellosta-López, P.; López-Royo, M.P.; Fernández, D.; Herrero, P. Comparing Two Dry Needling Interventions for Plantar Heel Pain: A Protocol for a Randomized Controlled Trial. J. Orthop. Surg. 2019, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, L.-M.; Vera-Martin, R.; Montes-Latorre, E.; Torres-Carranza, E.; Infante-Cossio, P. Botulinum Toxin and Percutaneous Needle Electrolysis for the Treatment of Chronic Masticatory Myalgia. Toxins 2023, 15, 278. [Google Scholar] [CrossRef]
- García-Vidal, J.A.; Salinas, J.; Escolar-Reina, P.; Cuello, F.; Ortega, N.; De Dios Berná-Mestre, J.; López-Nicolás, M.; Valera-Garrido, F.; Medina-Mirapeix, F. Galvanic Current Dosage and Bacterial Concentration Are Determinants of the Bactericidal Effect of Percutaneous Needle Electrolysis: An in Vitro Study. Sci. Rep. 2021, 11, 18977. [Google Scholar] [CrossRef]
- García-Vidal, J.A.; Salinas, J.; Ortega, N.; Escolar-Reina, P.; Camacho-Alonso, F.; Medina-Mirapeix, F. In Vitro Bacteriological Effect of Tri-Beveled Needle Electrolysis against Staphylococcus aureus. Sci. Rep. 2022, 12, 11468. [Google Scholar] [CrossRef]
- Samuelsson, L.; Jönsson, L.; Ståhl, E. Percutaneous Treatment of Pulmonary Tumors by Electrolysis. Radiol. 1983, 23, 284–287. [Google Scholar]
- Samuelsson, L.; Jönsson, L. Electrolytic Destruction of Tissue in the Normal Lung of the Pig. Acta Radiol. Diagn. 1981, 22, 9–14. [Google Scholar] [CrossRef]
- Samuelsson, L.; Lamm, I.-L.; Mercke, C.E.; Ståhl, E.; Jönsson, L. Electrolytic Tissue Destruction and External Beam Irradiation of the Lung: An Experimental and Clinical Investigation. Acta Radiol. Diagn. 1985, 26, 521–524. [Google Scholar] [CrossRef]
- Garcea, G.; Lloyd, T.D.; Aylott, C.; Maddern, G.; Berry, D.P. The Emergent Role of Focal Liver Ablation Techniques in the Treatment of Primary and Secondary Liver Tumours. Eur. J. Cancer 2003, 39, 2150–2164. [Google Scholar] [CrossRef]
- Diolaiuti, S.; Iizuka, T.; Schroth, G.; Remonda, L.; Laedrach, K.; El-Koussy, M.; Frueh, B.E.; Goldblum, D. Orbital Venous Malformation: Percutaneous Treatment Using an Electrolytically Detachable Fibred Coil. Acta Ophthalmol. 2009, 87, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, S.H.; Kennedy, L.; Dexter, D.F.; Veinot, J.P. A Practical Method to Rapidly Dissolve Metallic Stents. Cardiovasc. Pathol. 2009, 18, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Cardella, J.F.; Hunter, D.W.; Castaneda-Zuniga, W.R.; Hulbert, J.; Young, A.T.; Coleman, C.C.; Mercado, S.; Amplatz, K. Electrolysis for Recanalization of Urinary Collecting System Obstructions: A Percutaneous Approach. Radiology 1985, 155, 87–90. [Google Scholar] [CrossRef]
- Ülger, Ö.; Demirel, A.; Oz, M.; Şahin, A. Effectiveness of Physiotherapy and Minimal Invasive Technics on Functional Status and Quality of Life in Geriatric Patients with Low Back Pain. J. Exerc. Rehabil. 2018, 14, 1048–1052. [Google Scholar] [CrossRef]
- Peñin-Franch, A.; García-Vidal, J.A.; Martínez, C.M.; Escolar-Reina, P.; Martínez-Ojeda, R.M.; Gómez, A.I.; Bueno, J.M.; Minaya-Muñoz, F.; Valera-Garrido, F.; Medina-Mirapeix, F.; et al. Galvanic Current Activates the NLRP3 Inflammasome to Promote Type I Collagen Production in Tendon. eLife 2022, 11, e73675. [Google Scholar] [CrossRef]
- Margalef, R.; Bosque, M.; Minaya-Muñoz, F.; Valera-Garrido, F.; Santafe, M.M. Safety Analysis of Percutaneous Needle Electrolysis: A Study of Needle Composition, Morphology, and Electrical Resistance. Acupunct. Med. J. Br. Med. Acupunct. Soc. 2021, 39, 471–477. [Google Scholar] [CrossRef]
- Baltar, C.F.; Corral, M.E.M.; Fentes, D.P. Predicting and Avoiding Complications in Percutaneous Nephrolithotomy in the Era of Personalized Medicine: A Scoping Review. J. Pers. Med. 2024, 14, 962. [Google Scholar] [CrossRef]
- Cattaneo, A.; Bellenghi, M.; Ferroni, E.; Mangia, C.; Marconi, M.; Rizza, P.; Borghini, A.; Martini, L.; Luciani, M.N.; Ortona, E.; et al. Recommendations for the Application of Sex and Gender Medicine in Preclinical, Epidemiological and Clinical Research. J. Pers. Med. 2024, 14, 908. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Ferdinandi, V.; Cicchi, L.; Foti, C. Commentary on “The Learning Rehabilitation System: Strengthening an Intersectoral Strategy to Improve Functioning of an Ageing Population” by Bickenbach et al. Health Policy 2025, 155, 105303. [Google Scholar] [CrossRef]
- Zampolini, M.; Selb, M.; Boldrini, P.; Branco, C.A.; Golyk, V.; Hu, X.; Kiekens, C.; Negrini, S.; Nulle, A.; Oral, A.; et al. The Individual Rehabilitation Project as the Core of Person-Centered Rehabilitation: The Physical and Rehabilitation Medicine Section and Board of the European Union of Medical Specialists Framework for Rehabilitation in Europe. Eur. J. Phys. Rehabil. Med. 2022, 58, 503–510. [Google Scholar] [CrossRef]
- Magro, V.M.; Sorbino, A.; Manocchio, N.; Ljoka, C.; Foti, C. The Physiatrist in Intensive Care: Role, Tasks, and Critical Issues in a Clinical Case Report Analysis. Clin. Transl. Neurosci. 2025, 9, 11. [Google Scholar] [CrossRef]

| Population | Patients or Healthy Volunteers who Underwent Percutaneous Electrolysis for Musculoskeletal Diseases |
| Intervention | Percutaneous Electrolysis |
| Comparison | Not applicable |
| Outcome | Pain, function, mobility, thickness, quality of life, and ability to reach the target tissue. |
| Authors, Year | Title | Study Type | Human/Not Human | US Imaging | Sample Size | Sex | Age: Mean, (Range) | Operator | Type of Disease | Rehabilitation Pre | Rehabilitation During | Rehabilitation Post | Follow-Up | Setting | Outcomes | Results | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferran Abat, 2014 [31] | Effectiveness of the Intratissue Percutaneous Electrolysis (EPI®) technique and isoinertial eccentric exercise in the treatment of patellar tendinopathy at two years follow-up | Prospective Case Series | Human | YES | 33 | M: 29; F: 4 | 25.3 years (16–53) | / | patellar tendinopathy | not | Two weekly sessions of eccentric exercise using isoinertial resistance machines | / | up to two years | Weekly session of EPI® and two weekly sessions of eccentric exercise. Patients received the intratissue percutaneous electrolysis (EPI®) technique treatment until there was clinical improvement or no improvement in the symptomology was seen after 10 sessions, 3 milliamps echo-guided punctures, needles of from 0.30 to 0.32 mm in diameter and a modified electric scalpel | Victorian Institute of Sport Assessment for the patellar tendon (VISA-P), the Tegner scale, Roles and Maudsley scale | Average 35 points improvement in VISA-P | No adverse events |
| Ferran Abat, 2015 [32] | Clinical results after ultrasound-guided intratissue percutaneous electrolysis (EPI®) and eccentric exercise in the treatment of patellar tendinopathy. | Prospective Case Series | Human | YES | 40 | M: 35; F: 5 | 25.5 | / | Patellar Tendinopathy | NOT | YES (2 weekly sessions of eccentric exercise training using the resistance isoinertial leg-press machine) | NOT | 3 months, 2 years, 5 years, 10 years. | Session of PNE every 2 weeks up to a maximum of ten sessions. Acupuncture needles (0.3 mm in diameter) with different lengths. Intensity 3 mA | Victorian Institute of Sport Assessment–Patella (VISA-P); Tegner score; the Roles and Maudsley score | Treatment with the US-guided EPI technique and eccentric exercises in patellar tendinopathy resulted in a great improvement in knee function and a rapid return to the previous level of activity after few sessions | No adverse events |
| Fermín Valera-Garrido, 2014 [45] | Ultrasound-guided percutaneous needle electrolysis in chronic lateral epicondylitis: short-term and long-term results | Prospective Case Series | Human | YES | 36 | M: 19; F: 17 | 38 ± 6.4 | / | chronic lateral epicondylitis | NOT | Yes (home program consisting of eccentric exercise and stretching) | NOT | 6, 26 and 52 weeks | Session of PNE per week over 4 weeks. Intensity of 4–6 mA for 3 s approximately three times. A 0.3 × 25 mm (1 inch) acupuncture needle | VAS, DASH, US evaluation, patients’ perceptions of the overall outcome | Symptoms and degenerative structural changes of chronic lateral epicondylitis are reduced after US-guided PNE associated with EccEx and stretching | No adverse events |
| José L Arias-Buría, 2015 [55] | Ultrasound-Guided Percutaneous Electrolysis and Eccentric Exercises for Subacromial Pain Syndrome: A Randomized Clinical Trial | RCT | Human | YES | 36, randomly assigned into US-guided PE (n = 17) group or exercise (n = 19) group | M: 9; F: 27 | 58 ± 7 | clinician | Subacromial Pain Syndrome | / | Eccentric exercise program of the rotator cuff muscles | / | 1 week | Application of galvanic current through acupuncture needle on each session once a week (total of 4 sessions), acupuncture needles of different lengths (0.3 mm in diameter, intensity of 350 μA for 1.2 min) | Shoulder pain (NPRS) and disability (DASH) | US-guided percutaneous electrolysis combined with eccentric exercises resulted in slightly better outcomes in the short term compared to when only eccentric exercises were applied in subacromial pain syndrome | No adverse events |
| Blanca de la Cruz Torres, 2016 [34] | Autonomic responses to ultrasound-guided percutaneous needle electrolysis of the patellar tendon in healthy male footballers. | RCT | Human | YES | 22 | M: 22 | 23.5 | PT | patellar tendon in healthy male footballers | NOT | / | NOT | NOT | One session of PNE. Intensity of 3 mA. Acupuncture needles with 0.3 mm diameter and different lengths | Personal Psychological Apprehension Scale (PPAS), heart rate variability (HRV), the standard deviation of the RR intervals (SDNN), the square root of the mean of the sum of the squares of the differences between the adjacent RR intervals (rMSSD), and the number of adjacent RR interval (RRI) pairs that differ by >50 ms in the full register, divided by the total number of RRIs and expressed as a percentage (pNN50). The reverse axis (SD1), the longitudinal axis (SD2) | Significant increase in parasympathetic activity (in keeping with a potential vasovagal reaction) during application of the US-guided PNE technique on healthy patellar tendons of male football players | Measurable increase in parasympathetic activity (detected by HRV) |
| Carlos Moreno, 2016 [52] | Therapeutic results after ultrasound-guided intratissue percutaneous electrolysis (EPI®) in the treatment of rectus abdominis-related groin pain in professional footballers: a pilot study. | Prospective Case Series | Human | YES | 8 | M: 8 | 26.8 | PT | rectus abdominis-related groin pain | NOT | NOT | NOT | 24 h, 1 week, 1 month, 6 months | Four (from 2 to 6) sessions, once a week, with a needle 0.25 × 30 mm in diameter. Intensity of 3 mA for 4 s | Verbal Rating Scale (VRS), Patient-Specific Functional Scale | Treatment with ultrasound-guided EPI has shown encouraging clinical results for RAGP. | N.A. |
| Carlos Moreno, 2017 [43] | Intratissue percutaneous electolysis combined with active physical therapy for the treatment of adductor longus enthesopathy-related groin pain: a randomized trial | RCT | Human | YES | 24 | M: 24 | 26.1 | PT | Adductor longus enthesopathy-related groin pain (ALErGP) | NOT | YES | NOT | 6 months | Two sessions a week of PNE. Intensity of 3 mA. A 0.33 × 50 mm in diameter of s acupuncture needle. Intensity of 3 mA. 3 applications every session (3 right + 3 left if the ALErGP- adductor longus enthesopathy-related groin pain was present bilaterally), with a duration of 5 s each | Patient-Specific Functional Scale (PSFS), Numeric Rating Scale (NRS) | PE treatment in association with active physiotherapy ensured a greater and more rapid reduction of pain and tended to promote greater functional recovery in soccer players with ALErGP compared to active physiotherapy only. | No adverse events |
| García Naranjo J, 2017 [42] | A novel approach in the treatment of acute whiplash syndrome: ultrasound-guided needle percutaneous electrolysis. A randomized controlled trial. | RCT | Human | YES | 100, divided in two groups: physiotherapy and PE | M: 36; F: 64 | 38.1 | PT | acute whiplash syndrome | NOT | NOT | NOT | 5 Weeks | Weekly session for three weeks (3 sessions) with 25 × 0.16 mm acupuncture needles. Starting intensity was 2 mAmp, which was increased on a 1 mAmp/s speed to reach 4 mAmp, repeated three times per session, with a resting 210 interval of 1–2 min between shocks. | VAS, Northwick Park Neck Questionnaire (NPQ), pressure pain 167 threshold (PPT) with algometric assessment | Patients receiving the therapy substantially decreased their pain, pressure-pain threshold and quality-of-life measures, equally to standardized physiotherapy programs. Distinguishly, PNE protocol consists of only 3 application sessions of 15 min each one, with no added interventions | N.A. |
| Lorena de Miguel Valtierra, 2018 [54] | Ultrasound-Guided Application of Percutaneous Electrolysis as an Adjunct to Exercise and Manual Therapy for Subacromial Pain Syndrome: A Randomized Clinical Trial | RCT | Human | YES | 50 randomized into manual therapy/exercise (n = 25) or the manual therapy/exercise plus electrolysis (n = 25) | M: 23; F: 27 | 54 ± 7 years | PT | Subacromial Pain Syndrome | / | manual therapy and exercise: the program consisted of 3 exercises focusing on supraspinatus, infraspinatus, and scapular stabilizer muscles. Each exercise was performed in 3 sets of 12 repetitions | During the follow-up period, participants were asked to continue with the exercise program and this was monitored on subsequent follow-up assessments. | Up to 6 months | 0.30 mm × 25 mm needle, intensity of 350 µA for a total of 90 s | Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire. Secondary outcomes included pain, function (Shoulder Pain and Disability Index [SPADI]) pressure pain thresholds (PPTs) and Global Rating of Change (GROC) | The inclusion of US-guided PE in combination with manual therapy and exercise resulted in no significant differences for related disability compared with the application of manual therapy and exercise alone in patients with subacromial pain syndrome. Nevertheless, differences were reported for shoulder pain and function | No adverse events |
| Álvaro Iborra-Marcos, 2018 [38] | Intratissue Percutaneous Electrolysis vs. Corticosteroid Infiltration for the Treatment of Plantar Fasciosis | Retrospective Case-Control | Human | YES | 64 patients: 32 treated with ultrasound-guided EPI and 32 with ultrasound-guided Corticosteroid infiltration | M: 35; F: 29 | 46.4 ± 8.5 | / | plantar fasciosis | / | / | / | 1 year | G32 needle, 3 mA current was delivered for 5 s. The treatment was repeated 7 days later and then again for up to 10 sessions at weekly intervals as required | Visual analog scale (VAS) to record pain and the Foot and Ankle Disability Index (FADI) | Both techniques were effective in the treatment of PF, providing excellent VAS pain and FADI results at 12 months. However, CI required fewer patient visits and appeared to provide somewhat better VAS and FADI results | No adverse events |
| Ricardo Lopez-Martos, 2018 [58] | Randomized, double-blind study comparing percutaneous electrolysis and dry needling for the management of temporomandibular myofascial pain | RCT | Human | NOT | 60 | M: 8; F: 52 | 38.8 (18–62) | / | temporomandibular myofascial pain | NOT | / | YES (two weeks after each procedure, concentric exercises with the masticatory muscles) | 28, 42, and 70 days | Session of PNE once per week, for 3 consecutive weeks. Intensity of 2 mA for 3 s. A 0.25 × 40 mm acupuncture needle | VAS, maximum interincisal opening (MIO) without causing pain or discomfort, involvement of the TMJ, assessed by a 100-point questionnaire, Tolerability to the treatment was evaluated by the patient and the observer using a 5-point scale, ranging from 0 (very bad) to 4 (excellent) | greater and earlier relieving pain and improving MIO of patients treated with percutaneous needle electrolysis compared to deep dry needling and sham needling procedure | No adverse events |
| Paula Garcìa Bermejo, 2018 [35] | Autonomic Responses to Ultrasound-Guided Percutaneous Needle Electrolysis: Effect of Needle Puncture or Electrical Current? | Prospective Case–Control | Human | YES | 36 | M: 36 | 24.36 | PT | patellar tendinopathy | NOT | NOT | NOT | NOT | One session of PNE, with three applications with needles with 0.3 mm diameter and different lengths. Intensity of 3 mA for 3 s | Personal Psychological Apprehension Scale (PPAS), diameters of the Poincare’s plot (SD1, SD2), stress score, and sympathetic/parasympathetic ratio | The application of the US-guided PNE technique caused a measurable increase in parasympathetic activity (detected by heart-rate variability—HRV), which was due to the combination of needle puncture and electric current | Significant Autonomic Imbalance (In Keeping with A Potential Vasovagal Reaction) |
| Fernández-Rodríguez T, 2018 [39] | Prospective Randomized Trial of Electrolysis for Chronic Plantar Heel Pain. | RCT | Human | YES | 73 (PE group or placebo puncture) | M: 31, F: 42 | 46 | Clinician | chronic plantar heel pain | NOT | / | YES | 1, 12, and 24 weeks | Session of PE once per week, for 5 consecutive weeks. A 0.35 × 40 mm acupuncture needle. Intensity of 28 mC of cathodal PNE. | VAS, 21-item activities of daily living subscale of the Foot and Ankle Ability Measure questionnaire, and plantar fascia thickness measured by ultrasound. | Improved pain and function. This treatment may also decrease fascia thickness (but further studies are needed for this last point). | No adverse events |
| Jiménez-Rubio S, 2019 [48] | Validity of an On-Field Readaptation Program Following a Hamstring Injury in Professional Soccer. | Prospective Case Series | Human | YES | 19 | M: 19 | 24.23 ± 5.36 | / | Hamstring Injury | NOT | YES | / | / | / | Aiken’s V for each item of the program and number of days taken by the players to return to play | The program proposed for the rehabilitation and readaptation phase following an injury to the hamstring muscle complex was determined to be valid by the panel of experts, given its soccer-specific context and that the entire program was carried out on the field. | N.A. |
| Manuel Rodríguez-Huguet, 2020 [49] | Percutaneous Electrolysis in the Treatment of Lateral Epicondylalgia: A Single-Blind Randomized Controlled Trial | RCT | Human | YES | 32: trigger point dry needling (n = 16) and PE group (n = 16) | M: 20; F: 12 | 38.16 ± 13.89 | PT | Lateral Epicondylalgia | / | Eccentric exercise program to be performed daily (three series of ten repetitions of eccentric work twice daily (morning and afternoon) with 1 kg weights) | / | up to three months | EPTE® percutaneous electrolysis device (Ionclinics & A. Deionic SL, Valencia, Spain) for 1.2 min at an intensity of 350 µA in the insertional tendon of the muscles of the epicondyle using a 0.3 mm needle guided by ultrasound (Voluson 730 pro, General Electric®, Boston, MA, USA) and forming an angle of between 30° and 45° with the axis. The treatment was performed once a week for four weeks | Numerical pain rating scale (NPRS), pressure pain thresholds (PPT), SF-12, and elbow range of motion | Ultrasound-guided percutaneous electrolysis as an adjunct to an eccentric exercise program is more effective for pain and range of movement than trigger point dry needling | No adverse events |
| Manuel Rodríguez-Huguet, 2020 [56] | Effectiveness of Percutaneous Electrolysis in Supraspinatus Tendinopathy: A Single-Blinded Randomized Controlled Trial | RCT | Human | YES | 36: PE group (n = 18) or a trigger point dry needling group (n = 18) | M: 27; F: 9 | 25–60 (40.04 ± 9.88) | / | Supraspinatus tendinopathy | / | Eccentric exercises for the supraspinatus to be performed daily at home from the first to the last day of treatment (3 × 10 repetitions): | / | 1 year | PE group: One treatment per week over four weeks (four sessions in total) using a percutaneous electrolysis EPTE® device (Ionclinics & Deionic S.L., Valencia, Spain) at an intensity of 350 μA for 1.2 min was performed. PE was applied on the injured zone of the supraspinatus tendon, which was located by ultrasound Trigger point dry needling group: A weekly session for four weeks (four sessions in total) of dry needling of the upper trapezius muscle towards supraspinatus | Numerical Pain Rating Scale (NPRS) but the shoulder range of motion (ROM) and trigger point pressure pain threshold (PPT) | PE seems to be more effective than TDN in relieving pain and improving ROM and PPT supraspinatus values in patients with supraspinatus tendinopathy, both right after treatment and at one-year follow-up. | No adverse events |
| B De-la-Cruz-Torres, 2020 [47] | Ultrasound-Guided Percutaneous Needle Electrolysis in Dancers with Chronic Soleus Injury: A Randomized Clinical Trial | RCT | Human | YES | 30 dancers randomly allocated to a PE group (n = 10), an eccentric exercise group (n = 10), or a combined group (n = 10) | M: 27; F: 3 | 21.03 ± 2.88 (16–26) | PT | Chronic soleus injury | / | Eccentric exercise program | / | 4 weeks | Two sessions of US-guided PE therapy (one session per week), acupuncture needle measuring 0.30 mm × 40 mm, intensity of 2.5 mA, during 3 s, 3 times (2.5 : 3: 3) | Pain (NRS), ankle dorsiflexion range of motion (DROM) measured using the weight-bearing lunge test (WBLT), endurance, the heel raise test, the Dance Functional Outcome Survey (DFOS) questionnaire, and the minimal clinically important difference (MCID) | US-guided PE, combined with an eccentric exercise program, is a useful therapeutic tool for the treatment of chronic soleus injury | N.A. |
| Al-Boloushi Z, 2020 [40] | Comparing two dry needling interventions for plantar heel pain: a randomised controlled trial | RCT | Human | YES | 102 | M: 30; F: 72 | 48.8 ± 8.8, (24–60) | PT | Plantar heel pain | NOT | stretching | NOT | 4, 8, 12, 26 and 52 weeks | Four sessions of DN or PNE once a week; needle from 30 to 75 mm in length and 0.25 to 0.30 mm in diameter. Intensity of 1.5 mA (intensity was adapted to patients’ characteristics according to their pain tolerance) | Foot Pain domain of the FHSQ, VAS, Quality of life (QoL) was assessed with the EQ-5D-5L | Both PNE and DN were effective for PHP management, reducing mean and maximum pain since the first treatment session, with long-lasting effects (52 weeks) and significant differences between groups in the case of QoL at 52 weeks in favour of the PNE group | No adverse events |
| María Pilar López-Royo, 2021 [33] | A Comparative Study of Treatment Interventions for Patellar Tendinopathy: A Randomized Controlled Trial | RCT | Human | YES | 48 (19 for each group: control group, DN intervention combined with EE group, or PNE intervention combined with EE) | M: 42; F: 6 | 18–45, (32.46) | PT | patellar tendinopathy | / | Eccentric exercise | / | up to 22 weeks | An intensity of 3 mA galvanic current was used during the 3 s that the procedure lasted | Disability was measured using the Victorian Institute of Sports Assessment Questionnaire, patellar tendon. VAS, Short Form-36. Ultrasound was used to measure structural abnormalities | DN or PNE combined with an EE program has not shown to be more effective than a program of only EE to improve disability and pain in patients with Patellar tendinopathy in the short (10 wk) and medium (22 wk) terms. Clinical improvements were not associated with structural changes in the tendon. | N.A. |
| Fernández D, 2021 [41] | Cost-Effectiveness of Two Dry Needling Interventions for Plantar Heel Pain: A Secondary Analysis of an RCT. | Secondary analysis of RCT | Human | / | 102 | M: 30; F: 72 | 48.8 ± 8.8, (24–60) | PT | Plantar heel pain | NOT | YES | NOT | 52 Weeks | One session a week of PNE or DN for 4 weeks. Procedure not described in detail | EQ-5D-5L (EuroQoL), the quality-adjusted life years (QALYs) | PNE treatment was more cost-effective than DN, with significant differences at 52 weeks. In the comparisons made according to the cost-effectiveness analysis, this translated into an 86% probability that PNE was more cost-effective compared to DN at 52 weeks | N.A. |
| Juan Antonio Valera-Calero, 2021 [37] | Short-term effectiveness of high- and low-intensity percutaneous electrolysis in patients with patellofemoral pain syndrome: A pilot study | RCT | Human | / | 15 equally randomized to the high-intensity percutaneous electrolysis (HIPE) experimental group, low-intensity percutaneous electrolysis (LIPE) experimental group or Dry Needling active control group | / | 25.6 ± 1.9 | PT | Patellofemoral pain syndrome | / | / | / | 1 week | The HIPE group received a 660 mA galvanic current for 10 s, the LIPE group 220 mA × 30 s, and the Dry Needling group received no galvanic current; 0.30 × 40 needle | Myofascial trigger points (MTrPs), patellar tendon pain pressure thresholds (PPTs), and subjective anterior knee pain perception (SAKPP) | HIPE and LIPE induce PPT changes in MTrPs and patellar tendon and improvements in SAKPP and seem to produce less pain during the intervention compared with DN | No adverse events |
| Sergio Varela-Rodríguez, 2022 [1] | Endogenous Pain Modulation in Response to a Single Session of Percutaneous Electrolysis in Healthy Population: A Double-Blinded Randomized Clinical Trial | RCT | Human | YES | 54 asymptomatic subjects randomized into three groups: sham (without electrical current), low-intensity, and high-intensity | M: 34; F: 20 | 22.96 ± 3.63 (18–40) | PT | asymptomatic | / | / | / | / | low-intensity (0.3 mA, 90 s), and high-intensity (three pulses of 3 mA, 3 s) 0.3 × 25 mm acupuncture needle | Widespread pressure pain thresholds (PPTs), conditioned pain modulation (CPM), and temporal summation (TS) were assessed in the elbow, shoulder, and leg | A single PE intervention modulated pain processing in local and widespread areas, implying an endogenous pain modulation. The pain processing effect was independent of the dosage administrated | No adverse events |
| Daniel Fernández-Sanchis, 2022 [36] | A comparative study of treatment interventions for patellar tendinopathy: a secondary cost-effectiveness analysis | Secondary analysis of RCT | Human | YES | 48 randomly divided into three groups: PE, dry needling and sham needling | M: 42; F: 6 | 32.5 ± 7.14 | PT | patellar tendinopathy | / | Eccentric exercise: three sets of fifteen repetitions of single-leg squats on a decline board twice a day | / | up to 22 weeks | Percutaneous needle electrolysis (three needle insertions 0.25 mm × 25 mm, intensity of 3 mA, 3 s), dry needling (idem as PE), or sham needling. Four treatment sessions, once every 2 weeks over 8 weeks | Costs, quality-adjusted life years and incremental cost-effectiveness ratio, SF-36 transformed to QoL values using SF-6D scores | The total cost per session was similar in the three groups: EUR 9.46 for the percutaneous needle electrolysis group; EUR 9.44 for the dry needling group; and EUR 8.96 for the sham group. The percutaneous needle electrolysis group presented better cost-effectiveness in terms of quality-adjusted life years and 96% and 93% probability of being cost-effective compared to the sham and dry needling groups, respectively. Percutaneous needle electrolysis has a greater probability of being cost-effective than sham or dry needling treatment | N.A. |
| Blanca De-la-Cruz-Torres, 2023 [53] | Ultrasound-Guided Percutaneous Needle Electrolysis Combined with Therapeutic Exercise May Add Benefit in the Management of Soleus Injury in Female Soccer Players: A Pilot Study | Prospective Case–Control | Human | YES | 20: an experimental group (exercise program + US-guided PNE; n = 10) or a control group (exercise program + sham stimulation; n = 10) | F: 20 | PE: 21.30 ± 3.80 Control: 23.40 ± 5.98 | PT | Soleus Injury in Female Soccer Players | / | Specific exercise program (4 sessions per week during 4 weeks) based on strength gluteus exercise, eccentric hamstring exercise, eccentric gastrocnemius exercise, and eccentric soleus exercise | / | 4 weeks | PE: needle 0.30 mm × 40 mm, intensity of 1.5 mA, during 3 s, 3 times Control: idem without current | Pain intensity, dorsiflexion range of motion, knee-flexion heel raise test, curve sprint test, and the global rating of change scale | The application of the US-guided PNE combined with a specific exercise program may cause clinical benefits in the treatment of female soccer players with soleus injury | N.A. |
| Ana Isabel Benito-de-Pedro, 2023 [51] | Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial | RCT | Human | YES | 52—intervention (PE; n = 26) and control (DDN; n = 26) groups | PE: M: 8 F: 19–DDN: M: 8 F: 19 | 38.77 (36.15–41.38) | PT | non-specific neck pain lasting more than 3 months | / | / | / | 14 days | Single treatment session, needle (0.30 mm × 30 or 0.30 mm × 40), 3–5 applications of 5 s at an intensity of 1.5 mA. | Pain intensity: modified visual numeric pain scale (VNPS), pressure pain threshold (PPT), cervical range of motion (CROM), neck disability: Northwick Park Pain Questionnaire (NPQ) and post-needling soreness | PE and DDN appear to have similar short-term effects. PE proved to be a more painful treatment than DDN | No adverse events |
| Juan L Sánchez-González, 2023 [44] | Effectiveness of different percutaneous electrolysis protocols in the endogenous modulation of pain: A Double-Blinded Randomized Clinical Trial | RCT | Human | YES | 46 (three groups receiving a single ultrasound-guided PE intervention: sham (without electrical current), low-intensity (0.3 mA, 90 s), or high-intensity (three pulses of 3 mA, 3 s) PE | Sham: M: 5; F: 11 Low Int: M: 5; F: 10 High Int: M: 5; F: 10 | 18–40 | PT | asymptomatic | / | / | / | / | A 0.3 × 25 mm acupuncture needle at 45° to the skin in the direction of the lateral epicondyle. The sham group did not receive any electrical current, just the needle insertion; the low-intensity group received an electrical galvanic current at an intensity of 0.3 mA for 90 s, whereas the high-intensity groups received three pulses of 3 mA for 3 s each of electrical galvanic current | Widespread pressure pain thresholds (PPT), conditioned pain modulation (CPM), and temporal summation (TS) were bilaterally assessed in the lateral epicondyle, bicipital groove, transverse process of C5 and the tibialis anterior muscle | One session of PE is able to slightly stimulate modulatory pathways related to nociceptive gain, particularly pressure pain sensitivity and temporal summation | No adverse events |
| Jorge Góngora-Rodríguez, 2024 [57] | Structural and Functional Changes in Supraspinatus Tendinopathy through Percutaneous Electrolysis, Percutaneous Peripheral Nerve Stimulation and Eccentric Exercise Combined Therapy: A Single-Blinded Randomized Clinical Trial | RCT | Human | YES | 50 randomized in two groups: PE + peripheral nerve stimulation (n = 25) and conventional electrotherapy treatment (TENS + therapeutic US) | M: 36; F: 14 | 44.24 ± 11.80 | PT | Supraspinatus tendinopathy | / | Eccentric exercise program consisting of 3 sets of 10 repetitions of each of the 3 exercises, twice a day, during the 4 weeks | / | up to 24 weeks | Four treatment sessions, one per week, intensity: 350 µA for 72 s, 0.30 × 40 mm acupuncture needles; PNS is carried out after the application of PE, with the same treatment frequency | pain (NPRS), strength, electromyographic activity, ultrasound characteristics of the tendon (echogenicity, thickness, and hypervascularization) and functionality (DASH and SPADI) | Combined treatment with PE, Peripheral Nerve Stimulation, and EE is an effective option, with positive results in the short and long terms | N.A. |
| Manuel Rodríguez-Huguet, 2024 [50] | Pulsed negative pressure myofascial vacuum therapy and percutaneous electrolysis in the treatment of lateral epicondylalgia: A single-blind randomized controlled trial | RCT | Human | YES | 40 | M: 25, F: 15 | 40.1 | PT | Lateral Epicondylalgia | NOT | YES | NOT | 1 and 3 months | One weekly session of PNE, for 4 weeks, with a needle 0.3 mm in diameter. Intensity of 3.5 mA for 80 s | Pain, Range of Motion (ROM), pressure pain threshold (PPT), Patient-Rated Tennis Elbow Evaluation Questionnaire (PRTEE) | Pulsed negative pressure myofascial vacuum therapy and ultrasound-guided percutaneous electrolysis, as an adjunct to an eccentric exercise program, is more effective for pain, range of movement, pressure pain threshold, and functionality than manual therapy and ultrasound treatment as an adjunct to the same exercise program in patients with lateral epicondylalgia | No adverse events |
| López-Royo MP, 2024 [59] | Functionality and jump performance in patellar tendinopathy with the application of three different treatments | RCT | Human | YES | 48 randomized into groups: DN, percutaneous electrolysis (PNE), and sham needling as the control group (CG) | M: 42, F: 6 | 32.46 ± 7.14 | PT | Lateral Epicondylalgia | NOT | Eccentric exercises (three sets of fifteen single-leg squat repetitions on a decline board twice a day) | NOT | Up to 3 months | One treatment session every 2 weeks. Needles (0.25 × 25 mm), for 2 s at 3 mA galvanic current | Spanish version of VISA-P and a jump protocol to assess participants’ performance | Eccentric exercise could be effective in improving functionality in patellar tendinopathy and DN could improve eccentric power in jumps performance. Moreover, the DN group experienced an increase in functionality that correlated with the improvements found in jump performance in eccentric power and concentric strength | N.A. |
| Doménech-García V, 2024 [60] | Placebo and nocebo effects of percutaneous needle electrolysis and dry-needling: an intra and inter-treatment sessions analysis of a three-arm randomized double-blinded controlled trial in patients with patellar tendinopathy | Secondary analysis of RCT | Human | YES | 48 divided into 3 parallel groups: “no-sham group” (PNE intervention), “single-sham group” (sham PNE by using dry needling), and “double-sham group” (sham PNE by using sham needles). | M: 42, F: 6 | 35.16 (28–43.5) | PT | Patellar Tendinopathy | NOT | unilateral eccentric exercise program of the quadriceps muscle on the affected side (3 sets of 15 repetitions daily on a decline board) | NOT | / | Every group received four sessions of the needling therapies targeting the patellar tendon over 8 weeks | Clinical pain reduction after needle intervention (placebo) and needle-related pain intensity after needle intervention (nocebo) | Needling therapies for individuals with patellar tendinopathy are prone to elicit placebo effects regarding clinical pain and nocebo effects regarding needling-related pain | No adverse events |
| Mustafa Turgut Yildizgoren et al., 2025 [46] | Biochemical reactions and ultrasound insights in percutaneous needle electrolysis therapy | Case Report | Human | YES | 1 | F | 45 | / | Lateral epicondylitis | / | / | / | 4 weeks post-treatment | US-guided PNE, three sessions, 350 μA, 80 sec each | Pain (VAS), function (QuickDASH), ultrasound visualization of gas formation | VAS decreased from 8/10 to 2/10; QuickDASH improved from 56 to 18; hyperechoic foci (gas) seen on US | No adverse events |
| Authors, Year | Title | Study Type | Human/Not Human | US Imaging | Sample Size | Sex | Age: Mean, (Range) | Operator | Type of Disease | Rehabilitation Pre | Rehabilitation During | Rehabilitation Post | Follow-Up | Setting | Outcomes | Results | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R Margalef, 2020 [61] | Percutaneous Needle Electrolysis Reverses Neurographic Signs of Nerve Entrapment by Induced Fibrosis in Mice | / | Animal | / | 46 | / | / | / | Mouse model of sciatic nerve entrapment | / | / | / | 1.5 mA for 3 s and 3 repetitions in the immediacy of perineural fibrosis | amplitude (peak-to-peak) of the compound muscle action potential (CMAPs) | sciatic nerve was definitively released from its fibrous entrapment | N.A. | |
| José Luis Sánchez-Sánchez, 2020 [5] | Changes in Gene Expression Associated with Collagen Regeneration and Remodeling of Extracellular Matrix after Percutaneous Electrolysis on Collagenase-Induced Achilles Tendinopathy in an Experimental Animal Model: A Pilot Study | / | Animal | / | 15 divided into three different groups (no treatment vs. percutaneous electrolysis vs. needling) | / | 8 weeks | / | Achilles tendon tendinopathy | / | / | / | / | 3 sessions (one per week) Each percutaneous electrolysis session consisted of three punctures targeting the Achilles intra-tendon 2 mm away from the osteotendinous junction. The intensity of the continuous (galvanic) electrical current was set at 3 mA and applied for 4 s on each puncture | genes involved in tendon repair and remodeling and histological tissue changes | percutaneous electrolysis increases the expression of some genes associated with collagen regeneration and remodeling of extracellular matrix | N.A. |
| Authors, Year | Title | Human/Not Human | US Imaging | Sample Size | Sex | Age: Mean, (Range) | Operator | Type of Disease | Follow-Up | Setting | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sergio Borrella-Andrés, 2022 [65] | Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study | Cadaver | YES | 10 cryopreserved knees | M: 5; F: 5 | 67–85 | / | / | / | Three applications for 3 s of 3 mA of intensity (3:3:3) when the tendon was the targeted tissue, three applications for 3 s of 1.5 mA of intensity (1.5:3:3) when the fat or muscle was the targeted tissue, and 24 s of 1 mA of intensity (1:24:1) in all tissues | Temperature changes in target tissues after PE application | The application of two different PE protocols did not produce appreciable thermal changes in the tendon, fat, and muscle tissues of human cadavers |
| Pedro Belón-Pérez, 2022 [62] | Cadaveric and Ultrasound Validation of Percutaneous Electrolysis Approaches at the Arcade of Frohse: A Potential Treatment for Radial Tunnel Syndrome | Five healthy volunteers (ultrasound study) and three Thiel-embalmed cadaver forearms | YES (Human) | 5 humans + 3 cadavers | / | / | PT | Radial tunnel syndrome | / | Human: Two approaches were taken, the first one with the forearm in supination and the second one with the forearm in pronation, both with the elbow straight Cadaver: The needle was inserted into the cadaver with all the tissues overlaid and left in situ during the anatomical dissection to determine if the tip of the needle properly reached the supinator muscle | Ability to reach the target tissue | Accurate needle penetration of the supinator muscle was observed in 100% in both US-imaging and cadaveric studies. No neurovascular bundle of the radial-nerve deep branch was pierced in any insertion |
| Laura Calderón-Díez, 2022 [63] | Cadaveric and Ultrasound Validation of Percutaneous Electrolysis Approach at the Achilles Tendon as a Potential Treatment for Achilles Tendinopathy: A Pilot Study | human + cadaver | YES | 10 healthy volunteers + 10 fresh cadaver legs | M: 4; F: 6 | 45 ± 14 years | PT | Achilles tendon tendinopathy | / | A needle was inserted from the medial to the lateral side under the body of the Achilles tendon, just between the tendon and the Kager’s triangle, about 5 cm from the insertion of tendon in the calcaneus Humans: 25 × 0.3 mm filiform solid needle | Ability to reach the target tissue | Percutaneous electrolysis can be safely performed at the Kager’s fat-Achilles tendon interphase if it is US-guided. |
| Laura Calderón-Díez, 2025 [64] | The Safety of Ultrasound-Guided Needle Approaches for Patellar Tendinopathy: A Theoretical Cadaveric Model | human + cadaver | YES (Human) | 10 healthy humans, 10 knees from 5 cadavers | Humans: M: 7; F: 3 Cadavers: M: 2; F: 3 | Humans: mean 42 (males), 33 (females); Cadavers: mean 69 (males), 75 (females) | PT | Patellar tendinopathy | / | Cadaveric dissection (non-US-guided) Ultrasound-guided needling in healthy volunteers | Anatomical safety of needle approach, visualization of infrapatellar nerve branches, safety of lateral approach | No neurovascular bundle of infrapatellar nerve branches was pierced in any insertion from the lateral side; lateral approach considered safe; medial approach vulnerable to nerve injury |
| Miguel Malo-Urriés, 2025 [66] | Quantitative Ultrasound Characterization of Intensity-Dependent Changes in Muscle Tissue During Percutaneous Electrolysis | Cadaver | Yes (quantitative ultrasound imaging) | 29 | M: 50% | 73.7 ± 9.94 | Not specified | None | None (cadaveric medial gastrocnemius muscle) | / | 0.00–10.00 mA. Initial intensity increments were set at 0.10 mA, followed by 0.50 mA increments up to the maximum intensity (10.00 mA). Each application lasted exactly one second | Dose-dependent changes in ultrasound parameters; Muscle_Electrolysis_Dose variable explained 66.7% of dose variance; significant differences between low, medium, and high doses; identified a 1–4 mA therapeutic window for muscle response |
| References | Selection | Comparability of Cohorts | Ascertainment of Exposure | Outcomes | Non Response Rate | NOS Score | |||
|---|---|---|---|---|---|---|---|---|---|
| Adequate Case Definition | Representativeness of Cases | Selection of Controls | Definition of Controls | Same Method of Ascertainment | |||||
| Iborra-Marcos [38] | ** | * | * | ** | * | * | ** | - | 10 |
| De-la-Cruz-Torres [53] | ** | * | * | ** | * | * | * | - | 9 |
| García Bermejo [35] | * | * | * | * | * | ** | ** | - | 9 |
| References | Were Patient’s Demographic Characteristics Clearly Described? | Was the Patient’s History Clearly Described and Presented as a Timeline? | Was the Current Clinical Condition of the Patient on Presentation Clearly Described? | Were Diagnostic Tests or Assessment Methods and the Results Clearly Described? | Was the Intervention(s) or Treatment Procedure(s) Clearly Described? | Was the Post-Intervention Clinical Condition Clearly Described? | Were Adverse Events (Harms) or Unanticipated Events Identified and Described? | Does the Case Report Provide Takeaway Lessons? |
|---|---|---|---|---|---|---|---|---|
| Abat [31] | Y | - | Y | - | Y | Y | Y | Y |
| Valera-Garrido [45] | Y | - | Y | - | Y | Y | Y | Y |
| Abat [32] | Y | Y | Y | - | Y | Y | Y | Y |
| Moreno [52] | Y | Y | Y | Y | Y | - | Y | - |
| Jiménez-Rubio [48] | Y | - | - | - | Y | - | - | - |
| Yildizgoren [46] | Y | Y | Y | Y | Y | Y | Y | Y |
| References | Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding of Caregivers/Investigators | Random Outcome Assessment | Blinding of Outcome Assessor | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Sánchez-Sánchez [5] | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Margalef [61] | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| References | Clear Aim of the Study | Adequate Sample Description | Inclusion/Exclusion Criteria | Detailed Dissection Procedure | Appropriate Instrumentation | Anatomical Landmarks Described | Intra-Observer Variability Controlled | Inter-Observer Variability Controlled | Outcome Measurement Described | Procedural Reproducibility | Statistical Analysis Performed | Sample Size Justification | Conflict of Interest Disclosed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrella-Andrés [65] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Belón-Pérez [62] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Calderón-Díez [63] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Calderón-Díez [64] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Malo-Urriés [66] | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Manocchio, N.; Sorbino, A.; Pirri, N.; Foti, C. Percutaneous Electrolysis for Musculoskeletal Disorders Management in Rehabilitation Settings: A Systematic Review. Healthcare 2025, 13, 1793. https://doi.org/10.3390/healthcare13151793
Pirri C, Manocchio N, Sorbino A, Pirri N, Foti C. Percutaneous Electrolysis for Musculoskeletal Disorders Management in Rehabilitation Settings: A Systematic Review. Healthcare. 2025; 13(15):1793. https://doi.org/10.3390/healthcare13151793
Chicago/Turabian StylePirri, Carmelo, Nicola Manocchio, Andrea Sorbino, Nina Pirri, and Calogero Foti. 2025. "Percutaneous Electrolysis for Musculoskeletal Disorders Management in Rehabilitation Settings: A Systematic Review" Healthcare 13, no. 15: 1793. https://doi.org/10.3390/healthcare13151793
APA StylePirri, C., Manocchio, N., Sorbino, A., Pirri, N., & Foti, C. (2025). Percutaneous Electrolysis for Musculoskeletal Disorders Management in Rehabilitation Settings: A Systematic Review. Healthcare, 13(15), 1793. https://doi.org/10.3390/healthcare13151793







