Abstract
Background: Minimally invasive surgery (MIS) is increasingly adopted in pediatric surgical practice, yet it demands specific technical skills that require structured training. Simulation-based education offers a safe and effective environment for skill acquisition, especially in complex procedures such as thoracoscopic repair of esophageal atresia with tracheoesophageal fistula (EA-TEF). Objective: This study aimed to evaluate the effectiveness of a 3D-printed simulator for training pediatric surgeons in thoracoscopic EA-TEF repair, assessing improvements in operative time and technical performance. Methods: A high-fidelity, 3D-printed simulator replicating neonatal thoracic anatomy was developed. Six pediatric surgeons at different training levels performed eight simulation sessions, including fistula ligation and esophageal anastomosis. Operative time and technical skill were assessed using the Stanford Microsurgery and Resident Training (SMaRT) Scale. Results: All participants showed significant improvements. The average operative time decreased from 115.6 ± 3.51 to 90 ± 6.55 min for junior trainees and from 100.5 ± 3.55 to 77.5 ± 4.94 min for senior trainees. The mean SMaRT score increased from 23.8 ± 3.18 to 38.3 ± 3.93. These results demonstrate a clear learning curve and enhanced technical performance after repeated sessions. Conclusions: Such 3D-printed simulation models represent an effective tool for pediatric MIS training. Even within a short time frame, repeated practice significantly improves surgical proficiency, supporting their integration into pediatric surgical curricula as an ethical, safe, and efficient educational strategy.
1. Introduction
Esophageal atresia (EA) is a rare congenital anomaly occurring in approximately 1 in 3500 live births, frequently associated with tracheoesophageal fistula (TEF). Surgical repair is the mainstay of treatment, yet it presents significant technical challenges due to the small operative field, fragility of neonatal tissues, and associated comorbidities []. Despite advances, postoperative complications such as anastomotic strictures, recurrent fistulas, and gastroesophageal reflux disease remain common and often require additional interventions [].
In recent years, surgical education has undergone a significant paradigm shift from time-based models to competency-based frameworks. This evolution emphasizes the attainment of specific technical and cognitive skills before operating on real patients. Particularly in pediatric surgery, where many procedures are both complex and performed infrequently, simulation-based training offers a reproducible, risk-free, and ethically sound alternative to traditional methods such as live animal or cadaveric models. As recently highlighted, integrating simulation into structured surgical curricula helps bridge the gap between theoretical learning and operative performance, promoting both safety and proficiency among trainees [].
Minimally invasive surgery (MIS) has increasingly become the standard of care for a wide range of surgical conditions []. Numerous studies have shown that both laparoscopic and robotic approaches reduce postoperative pain, minimize the need for analgesics, accelerate recovery, shorten hospital stays, improve cosmetic outcomes, and lower the risk of adhesion formation [,,,]. These advantages are even more significant in pediatric patients, where minimizing surgical trauma is especially critical []. The growing application of MIS in pediatric surgery necessitates the use of specialized instruments and technologies, along with appropriate training for healthcare professionals [,,].
The transition from open surgery to MIS demands advanced technical abilities, such as depth perception, hand–eye coordination, and precise instrument manipulation. Simulation-based training, encompassing both basic and complex procedures, plays a vital role in educating surgical trainees and refining the skills of experienced surgeons [,]. Simulation provides a safe, controlled environment where learners can repeatedly practice without posing any risk to patients []. Evidence supports that competencies acquired through simulation translate into improved performance in the operating room []. Repetitive and structured training sessions significantly contribute to the development and enhancement of motor skills [,].
To address the limitations of traditional training—such as cadaveric dissection and live animal models, which raise ethical, financial, and logistical issues []—simulation-based education offers a viable, risk-free alternative. The advent of 3D printing technology has enabled the creation of high-fidelity anatomical models, providing an effective and ethically sustainable solution for surgical training [].
This study aims to assess the efficacy of a 3D-printed simulator designed for training in thoracoscopic repair of esophageal atresia with tracheoesophageal fistula (EA-TEF). Specifically, it evaluates the impact of repeated, short-term simulation on operative efficiency and skill acquisition among pediatric surgical trainees. Ultimately, this study underscores the critical importance of structured simulation training as an integral part of surgical education, ensuring that future surgeons acquire the necessary technical competencies and confidence to manage complex procedures effectively. In conclusion, the objective of the study was to assess the performance improvement of pediatric surgeons using a synthetic simulator specifically designed for thoracoscopic repair of esophageal atresia with tracheoesophageal fistula (EA-TEF).
2. Methods
This study was conducted at the Simulation Center for Minimally Invasive Pediatric Surgery of IRCCS Sant’Orsola-Malpighi, Bologna, from 27 March to 22 April 2024.
2.1. Simulator
The simulator consisted of a synthetic trainer model developed through 3D printing technology, replicating the neonatal thoracic cavity and EA-TEF anatomy (Figure 1).
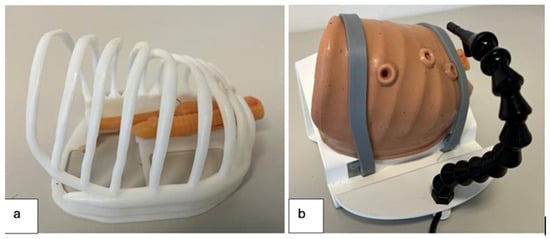
Figure 1.
Three-dimensional trainer model used for simulation of esophageal atresia repair; (a) Thoracic cage with 3D atretic esophagus with distal TEF model inside; (b) Silicon 3D model of neonate’s chest with access point for thoracoscopic procedures.
Its main components included:
- Thoracic cage: Modeled from a CT scan of a neonatal chest and scaled to 1:1.10.
- Silicone skin: A 3D-printed mold was used to produce a silicone layer that fits over the thoracic cage, with three access ports for thoracoscopic instruments and optics. The lateral ports allowed insertion of laparoscopic instruments (needle holders and scissors), while the central port was dedicated to the optical system.
- Optical sensor and lighting system: A PMW3360 optical sensor, connected via USB to a computer, provided real-time video feedback from within the thoracic cavity (Figure 2) [].
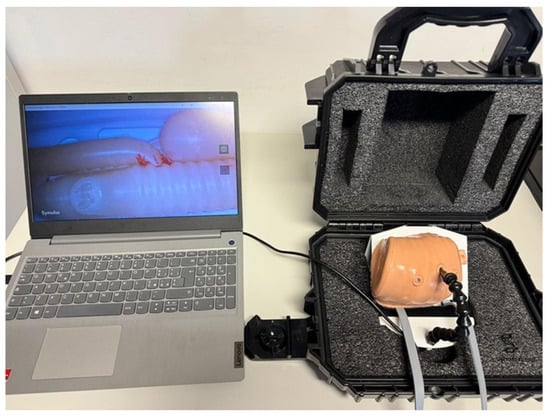 Figure 2. Trainer box with 3D neonatal chest connected to computer screen for the visualization of thoracoscopic images.
Figure 2. Trainer box with 3D neonatal chest connected to computer screen for the visualization of thoracoscopic images.
The three-dimensional model of the atretic esophagus with distal TEF was printed using the Ender 3 (Creality), a fused deposition modeling (FDM) printer. This process created a highly detailed, life-sized anatomical model, including both esophageal stumps, the distal fistula, and relevant tracheal anatomy (Figure 3).

Figure 3.
(A) The 3D model of atretic esophagus with the two esophageal stumps and fistula between the distal one and the trachea; (B) The 3D-printed model of trachea with thyroid and cricoid cartilages; (C) Posterior view of 3D-printed model of trachea and the two esophageal stumps; (D) The 3D printer used for the creation of the model applied in the study.
2.2. Surgical Instruments
The procedures were performed using the following instruments (Figure 4):
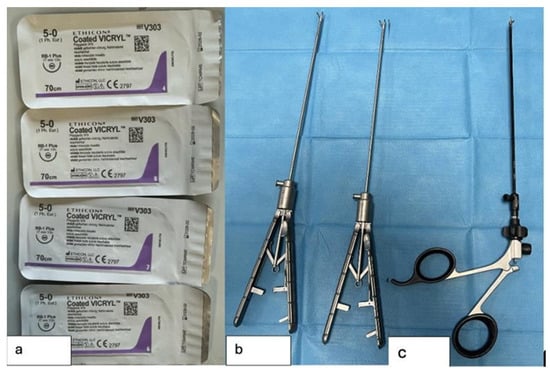
Figure 4.
Surgical instruments used in the study; (a) Sutures used for TEF ligation and esophageal anastomosis; (b) Laparoscopic needle holders; (c) Laparoscopic scissors used to dissect the TEF and opening the esophageal stumps.
- 4 Vicryl 5-0 sutures with a 17 mm needle
- 2 laparoscopic needle holders
- 1 laparoscopic scissors
2.3. Study Participants
Six pediatric surgeons from the Sant’Orsola Pediatric Surgery Unit were enrolled:
- 1 specialist pediatric surgeon with <5 years of experience
- 2 senior trainees (fourth-year residents in pediatric surgery)
- 3 junior trainees (third-year residents in pediatric surgery)
Participation was voluntary. All participants completed eight simulation sessions using the 3D-printed trainer.
2.4. Training Protocol
Prior to the simulation sessions, participants watched a standardized instructional video demonstrating the thoracoscopic EA-TEF repair. The video featured an expert pediatric surgeon performing the procedure under identical simulation conditions. This served to introduce the procedural steps, set performance expectations, and provide a benchmark for comparison.
Each participant performed the following four key steps:
- Closure of the tracheoesophageal fistula with a through-and-through suture
- Division of the fistula
- Opening of the proximal esophageal stump
- Esophageal anastomosis using interrupted sutures
Each session involved a total of 9 sutures: 1 for the fistula ligation and 8 for the anastomosis (2 at the 180° mark, 3 posterior, 3 anterior). Each suture was secured with 4 knots to ensure tension and stability.
2.5. Assessment and Data Collection
Two main metrics were recorded for each session:
- Operative time: From the beginning of the procedure to the final suture.
- SMaRT score (Stanford Microsurgery and Resident Training Scale): A validated tool assessing nine categories (instrument handling, tissue respect, efficiency, suture management, suturing technique, knot quality, final product, workflow, and overall performance), each scored from 1 to 5, for a maximum of 45 points (Table 1) [].
 Table 1. SMaRT Scale (Stanford Microsurgery and Resident Training Scale) [].
Table 1. SMaRT Scale (Stanford Microsurgery and Resident Training Scale) [].
An experienced minimally invasive pediatric surgeon observed all simulations and assigned the SMaRT scores to ensure objectivity. Data on both timing and scoring were entered into a purpose-built Excel database.
2.6. Study Duration and Objective
The training took place over 27 days. Each participant completed 8 simulations. The study aimed to track performance improvement in terms of time efficiency and technical precision across repeated sessions, providing a structured evaluation of surgical skill acquisition.
2.7. Statistics
Descriptive statistics were used to summarize operative times and SMaRT scores across simulation sessions. Continuous variables were expressed as mean ± standard deviation. To assess the improvement in operative times across simulations, data from the first and eighth sessions were compared. The Shapiro–Wilk test indicated a non-normal distribution of differences (p = 0.036). Therefore, in addition to the paired Student’s t-test (t = 13.70, p < 0.0001), the Wilcoxon signed-rank test was performed, confirming a statistically significant reduction in operative time (p = 0.031). Statistical analyses were performed using Microsoft Excel (Microsoft 365, Redmond, WA, USA) for descriptive statistics and paired t-test. The Shapiro–Wilk and Wilcoxon signed-rank tests were performed using Python (v3.10, SciPy library).
3. Results
The first simulation was performed by a specialist in minimally invasive pediatric surgery. The time required to complete the procedure—from the initial ligation of the tracheoesophageal fistula to the final suture of the esophageal anastomosis—was recorded as 61 min. All operative times were documented in minutes using a dedicated Excel database (Table 2).

Table 2.
Operative time per attempt for each participant (min = minutes).
3.1. Time Efficiency Improvement
A clear trend toward reduced operative time was observed over the course of the eight simulations for all participants. To quantify this improvement, the difference between the duration of the first and the eighth simulation was calculated for each surgeon (Figure 5).
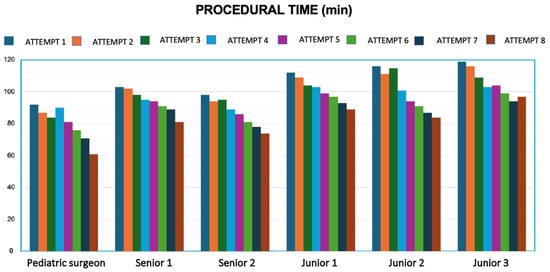
Figure 5.
Progression in operative time for each participant from the 1st to the 8th simulation session.
In the first simulation, the average times recorded were:
- Specialist surgeon: 92 min
- Senior trainees: 100.5 ± 3.55 min
- Junior trainees: 115.6 ± 3.51 min
By the eighth simulation, these times had significantly improved (Figure 6):
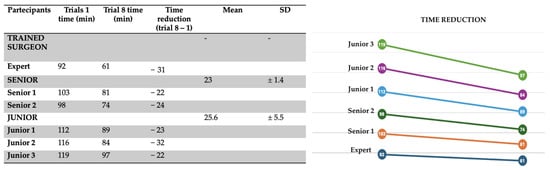
Figure 6.
Variation in execution times across the eight simulations, demonstrating a trend of progressive improvement.
- Specialist surgeon: 61 min
- Senior trainees: 77.5 ± 4.94 min
- Junior trainees: 90 ± 6.55 min
3.2. Technical Skill Assessment
Technical performance was evaluated using the SMaRT Scale, which includes nine parameters scored on a 5-point Likert scale (1 = failure, 5 = excellent). Each simulation was scored by the same experienced pediatric surgeon to ensure consistency and objectivity. As shown in Figure 7, a steady increase in the total SMaRT score was observed over time for all participants.
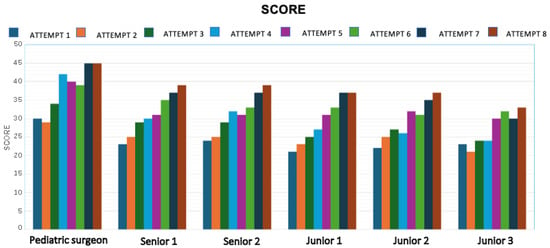
Figure 7.
Trend in SMaRT scores for each participant across simulations, indicating improvement in technical performance.
When comparing the average scores across all participants between the first and last simulations, the improvement is substantial (Figure 8):
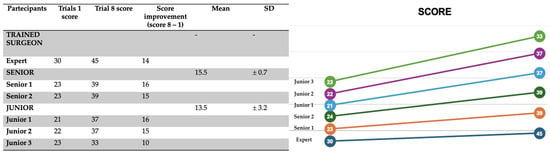
Figure 8.
Change in average SMaRT score from the 1st to the 8th simulation, illustrating a clear upward trend in technical proficiency.
- Simulation 1: 23.8 ± 3.18
- Simulation 8: 38.3 ± 3.93
4. Discussion
This study aimed to evaluate the effectiveness of a 3D-printed simulator specifically developed for training in thoracoscopic repair of esophageal atresia with tracheoesophageal fistula (EA-TEF). In particular, it focused on how repeated exposure to simulation over a short period could enhance operative efficiency and technical proficiency, especially among less experienced pediatric surgeons.
The findings confirm that repeated practice of a specific minimally invasive procedure leads to significant improvements in both operative time and procedural quality. These results are consistent with previous literature. For instance, Borselle et al. demonstrated that a short but intensive simulation program could significantly improve suturing skills for EA-TEF repair []. Similarly, Hong et al. created a 3D-printed model using both FDM and PolyJet technologies and validated its realism and training utility by comparing various materials []. Servi et al. reported the effectiveness of a 3D model for thoracoscopic pulmonary lobectomy training, highlighting anatomical accuracy and procedural fidelity as key benefits []. Deie et al. emphasized the added value of condition-specific simulators over traditional dry boxes, especially for rare neonatal conditions []. Youn et al. further confirmed the usefulness of patient-specific reconstructions, which enhanced tactile feedback and improved anastomotic technique []. Zahradnikovà et al. demonstrated the value of a 3D-printed model for thoracoscopic repair of esophageal atresia by analyzing the results achieved by medical students, pediatric surgical trainees, and expert surgeons [].
Multiple applications of 3D-printed models in the simulation of minimally invasive pediatric surgical procedures have been reported in the literature. For instance, Williams et al. [] reported results from a simulated training model for laparoscopic pyloromyotomy. Similarly, Cabarcas Maciá et al. [] developed a 3D model for pediatric laparoscopic pyeloplasty simulation, and Heo et al. [] designed a 3D-printed model for pediatric inguinal hernia repair training.
Similarly, recent developments by Petra et al. emphasized the potential of patient-specific anatomical models for enhancing procedural realism and preoperative planning []. However, our model uniquely integrates real-time optical feedback and a validated scoring system (SMaRT), allowing for structured assessment of technical progression.
Compared to traditional dry boxes, the 3D-printed model offers superior anatomical fidelity, particularly in replicating neonatal thoracic structures and the spatial constraints of thoracoscopic procedures []. While virtual simulators provide advanced visual guidance, they often lack the haptic feedback necessary for fine motor skill acquisition []. Unlike animal or cadaveric models, which pose ethical and logistical challenges, 3D printing enables reproducible, patient-specific simulations that are both cost-effective and customizable [].
In our study, the progression of both operative time and SMaRT scores clearly demonstrates the steep learning curve of thoracoscopic EA-TEF repair when supported by simulation. Initially, more experienced surgeons outperformed trainees in terms of time and quality. However, after only eight sessions, all participants—regardless of prior experience—showed substantial improvements. This suggests that simulation training can accelerate the acquisition of advanced surgical skills.
Several factors contributed to this learning curve:
- Increased familiarity with thoracoscopic instruments and port placement
- Improved hand–eye coordination and two-dimensional spatial interpretation
- Adaptation to the absence of tactile feedback, typical of MIS procedures
- Standardization of technique through visual and procedural benchmarking
The gradual increase in SMaRT scores—particularly in categories such as instrument handling, suturing technique, and workflow—indicates a qualitative enhancement of surgical execution. The results showed consistent improvements in both surgical technique and operative timing across all participants. Between the sixth and eighth sessions, further gains were noted, although not statistically significant. This plateau effect may be attributed to several factors, including ergonomic limitations, task repetition, or cognitive saturation. Importantly, all these improvements were achieved without any risk to patients and in a controlled, reproducible environment. Notably, even the experienced pediatric surgeon exhibited measurable performance gains over the course of the training. This suggests that simulation-based training may benefit not only trainees, but also experienced surgeons who may initially be challenged by the unfamiliar ergonomic constraints of the simulated setting.
These findings support the integration of simulation-based training into pediatric surgical education programs. The use of 3D-printed anatomical models offers several advantages: reproducibility, anatomical accuracy, and cost-effectiveness. Unlike cadaveric or animal models, 3D simulators can be tailored to represent patient-specific anatomy, making them valuable not only for training but also for preoperative planning.
Limitations of the Study
This study has several limitations. First, the small sample size limits the generalizability of the results and may not fully represent the variability in learning curves across different institutions. Second, performance was assessed in a simulated environment, which may not fully replicate the complexity and stress of real surgical settings. Third, the absence of inter-rater reliability assessment in the scoring process. Although a single experienced evaluator was used to ensure consistency, future studies should incorporate multiple independent raters to improve objectivity and allow statistical measurement of inter-rater agreement. Finally, the study evaluated only short-term improvements.
5. Conclusions
This study demonstrates that simulation-based training using 3D-printed models significantly improves technical skills and operative efficiency in pediatric minimally invasive surgery. Even after a limited number of sessions, participants showed marked progress in both precision and time management. These findings support the integration of high-fidelity simulation into surgical training programs as a safe, effective, and ethically sound method to enhance surgical competence and confidence.
Author Contributions
Conceptualization, S.M.C., M.D.M., and M.L. (Mario Lima); Methodology, A.D.C., E.C. (Edoardo Collautti), S.D., and M.L. (Mario Lima); Software, S.M.C.; Validation, A.D.C., M.D.M., and C.B.; Formal analysis, A.D.C. and M.D.M.; Investigation, A.D.C. and M.L. (Mario Lima); Data curation, C.D.M. and S.D.; Writing—original draft, S.M.C., M.D.M., and M.L. (Mario Lima); Writing—review & editing, M.D.M., C.B., E.C. (Edoardo Collautti), and M.L. (Mario Lima); Visualization, C.B., M.L. (Michele Libri), and E.C. (Enrico Ciardini); Supervision, M.D.M., M.L. (Michele Libri), S.D., T.G., and M.L. (Mario Lima); Project administration, S.D., E.C. (Enrico Ciardini), and M.L. (Mario Lima). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Ethical review and approval were waived for this study because it did not involve experimental procedures or interventions.
Informed Consent Statement
Informed consent was obtained from all participants for the use of their results in this research. The data were anonymized to ensure confidentiality.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| MIS | Minimally Invasive Surgery |
| EA | esophageal atresia |
| TEF | Tracheoesophageal fistula |
| FDM | Fused Deposition Modeling |
References
- Kempker, T.; Peuterbaugh, J. Esophageal Atresia and Tracheoesophageal Fistula: Diagnosis, Management, and Outcomes. NeoReviews 2025, 26, e307–e315. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, T.; Polić, B.; Markić, J.; Ardalić Čatipović, T.; Bucat, M.; Mikulić, S.; Žuvan, L.; Pogorelić, Z.; Despot, R.; Žitko, V.; et al. Epidemiology and Treatment Outcomes in Neonates with Esophageal Atresia: A 30-Year Population-Based Study. Healthcare 2025, 13, 418. [Google Scholar] [CrossRef]
- Markel, M.; Lacher, M.; Hall, N.J.; Martynov, I.; Siles Hinojosa, A.; de Augustin Asensio, J.C.; Fortmann, C.; Hukkinen, M.; Mutanen, A.; Ford, K.; et al. Training in minimally invasive surgery: Experience of paediatric surgery trainees in Europe. Br. J. Surg. 2023, 110, 1397–1399. [Google Scholar] [CrossRef]
- St John, A.; Caturegli, I.; Kubicki, N.S.; Kavic, S.M. The Rise of Minimally Invasive Surgery: 16 Year Analysis of the Progressive Replacement of Open Surgery with Laparoscopy. JSLS J. Soc. Laparosc. Robot. Surg. 2020, 24, e2020.00076. [Google Scholar] [CrossRef]
- Blinman, T.; Ponsky, T. Pediatric minimally invasive surgery: Laparoscopy and thoracoscopy in infants and children. Pediatrics 2012, 130, 539–549. [Google Scholar] [CrossRef]
- Di Fabrizio, D.; Mastroberti, F.; Cruccetti, A.; Bindi, E.; Cobellis, G. Thoracotomy vs. Thoracoscopy for Esophageal Atresia with Tracheoesophageal Fistula Repair: Is There a Difference in Quality of Life? Child. Basel Switz. 2024, 11, 1340. [Google Scholar] [CrossRef]
- Borselle, D.; Davidson, J.; Loukogeorgakis, S.; De Coppi, P.; Patkowski, D. Thoracoscopic Stage Internal Traction Repair Reduces Time to Achieve Esophageal Continuity in Long Gap Esophageal Atresia. Eur. J. Pediatr. Surg. Off. J. Austrian Assoc. Pediatr. Surg. Al Z. Kinderchir. 2024, 34, 36–43. [Google Scholar] [CrossRef]
- Di Mitri, M.; Thomas, E.; Di Carmine, A.; Manghi, I.; Cravano, S.M.; Bisanti, C.; Collautti, E.; Ruspi, F.; Cordola, C.; Vastano, M.; et al. Intraoperative Ultrasound in Minimally Invasive Laparoscopic and Robotic Pediatric Surgery: Our Experiences and Literature Review. Children 2023, 10, 1153. [Google Scholar] [CrossRef]
- Lerwick, J.L. Minimizing pediatric healthcare-induced anxiety and trauma. World J. Clin. Pediatr. 2016, 5, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Rebello, L.; Livergant, R.; Khanbadr, P.; Bednarek, O.; Joharifard, S. Simulation Models for Training in Pediatric General, Thoracic, Plastic, and Urologic Surgery in Low-resource Settings: A Scoping Review. J. Pediatr. Surg. 2025, 60, 162183. [Google Scholar] [CrossRef] [PubMed]
- Parente, G.; Thomas, E.; Cravano, S.; Di Mitri, M.; Vastano, M.; Gargano, T.; Cerasoli, T.; Ruspi, F.; Libri, M.; Lima, M. ArtiSential® Articulated Wrist-Like Instruments and Their First Application in Pediatric Minimally Invasive Surgery: Case Reports and Literature Review of the Most Commonly Available Robot-Inspired Devices. Child. Basel Switz. 2021, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Di Mitri, M.; Di Carmine, A.; D’Antonio, S.; Capobianco, B.M.; Bisanti, C.; Collautti, E.; Cravano, S.M.; Ruspi, F.; Libri, M.; Gargano, T.; et al. Advancing Pediatric Surgery: The Use of HoloLens 2 for 3D Anatomical Reconstructions in Preoperative Planning. Children 2025, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.L.; Haddad, M.; Clarke, S.A. Learning Curves in Pediatric Minimally Invasive Surgery: A Systematic Review of the Literature and a Framework for Reporting. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Shahrezaei, A.; Sohani, M.; Taherkhani, S.; Zarghami, S.Y. The impact of surgical simulation and training technologies on general surgery education. BMC Med. Educ. 2024, 24, 1297. [Google Scholar] [CrossRef]
- Sadideen, H.; Hamaoui, K.; Saadeddin, M.; Kneebone, R. Simulators and the simulation environment: Getting the balance right in simulation-based surgical education. Int. J. Surg. Lond. Engl. 2012, 10, 458–462. [Google Scholar] [CrossRef]
- Badash, I.; Burtt, K.; Solorzano, C.A.; Carey, J.N. Innovations in surgery simulation: A review of past, current and future techniques. Ann. Transl. Med. 2016, 4, 453. [Google Scholar] [CrossRef]
- Parente, G.; Di Mitri, M.; Gargano, T.; Lima, M. Training in Pediatric Gastrointestinal Endoscopy during Surgery Residency: Can Training Programs Currently Educate Proficient Pediatric Endoscopists? A Nationwide Assessment. Gastrointest. Disord. 2023, 5, 356–366. [Google Scholar] [CrossRef]
- Spruit, E.N.; Band, G.P.H.; Hamming, J.F.; Ridderinkhof, K.R. Optimal training design for procedural motor skills: A review and application to laparoscopic surgery. Psychol. Res. 2014, 78, 878–891. [Google Scholar] [CrossRef]
- Moorhead, A.A.; Nair, D.; Morison, C.; Cook, N.J.; Beasley, S.W.; Wells, J.M. Development of an instrumented thoracoscopic surgical trainer for objective evaluation of esophageal atresia/tracheoesophageal fistula repair. Med. Biol. Eng. Comput. 2020, 58, 601–609. [Google Scholar] [CrossRef]
- Ali, F.; Kalva, S.N.; Koc, M. Advancements in 3D printing techniques for biomedical applications: A comprehensive review of materials consideration, post processing, applications, and challenges. Discov. Mater. 2024, 4, 53. [Google Scholar] [CrossRef]
- Satterwhite, T.; Son, J.; Carey, J.; Echo, A.; Spurling, T.; Paro, J.; Gurtner, G.; Chang, J.; Lee, G.K. The Stanford Microsurgery and Resident Training (SMaRT) Scale: Validation of an on-line global rating scale for technical assessment. Ann. Plast. Surg. 2014, 72, S84–S88. [Google Scholar] [CrossRef]
- Borselle, D.; Więckowski, A.; Patkowski, D. Evaluating suturing skill improvement for pediatric minimally invasive esophageal anastomosis model: An observational cohort study based on simulator training. Sci. Rep. 2025, 15, 7692. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Kim, H.; Kim, T.; Kim, Y.-H.; Kim, N. Development of patient specific, realistic, and reusable video assisted thoracoscopic surgery simulator using 3D printing and pediatric computed tomography images. Sci. Rep. 2021, 11, 6191. [Google Scholar] [CrossRef] [PubMed]
- Servi, M.; Lo Piccolo, R.; Dalle Mura, F.; Mencarelli, M.; Puggelli, L.; Facchini, F.; Severi, E.; Martin, A.; Volpe, Y. Advanced physical simulator for pediatric minimally invasive thoracoscopy training in the treatment of pulmonary sequestration. Comput. Biol. Med. 2025, 188, 109847. [Google Scholar] [CrossRef] [PubMed]
- Deie, K.; Nakagawa, Y.; Uchida, H.; Hinoki, A.; Shirota, C.; Tainaka, T.; Sumida, W.; Yokota, K.; Makita, S.; Fujiogi, M.; et al. Evaluation of minimally invasive surgical skills training: Comparing a neonatal esophageal atresia/tracheoesophageal fistula model with a dry box. Surg. Endosc. 2022, 36, 6035–6048. [Google Scholar] [CrossRef]
- Youn, J.K.; Ko, D.; Yang, H.-B.; Kim, H.-Y. A 3D printed esophageal atresia–tracheoesophageal fistula thorascopy simulator for young surgeons. Sci. Rep. 2024, 14, 11489. [Google Scholar] [CrossRef]
- Zahradniková, P.; Babala, J.; Pechanová, R.; Smrek, M.; Vitovič, P.; Laurovičová, M.; Bernát, T.; Nedomová, B. Inanimate 3D printed model for thoracoscopic repair of esophageal atresia with tracheoesophageal fistula. Front. Pediatr. 2023, 11, 1286946. [Google Scholar] [CrossRef]
- Williams, A.; McWilliam, M.; Ahlin, J.; Davidson, J.; Quantz, M.A.; Bütter, A. A simulated training model for laparoscopic pyloromyotomy: Is 3D printing the way of the future? J. Pediatr. Surg. 2018, 53, 937–941. [Google Scholar] [CrossRef]
- Cabarcas Maciá, L.; Marmolejo Franco, F.; Siu Uribe, A.; Palomares Garzón, C.; Rojo Díez, R. Pilot study for low-cost model validation in laparoscopic pediatric pyeloplasty simulation. Cirugia Pediatr. Organo Of. Soc. Espanola Cirugia Pediatr. 2022, 35, 141–145. [Google Scholar] [CrossRef]
- Heo, K.; Greaney, E.; Haehl, J.; Stunden, C.; Lindner, A.; Malik, P.R.A.; Rosenbaum, D.G.; Muensterer, O.; Zakani, S.; Jacob, J.; et al. Iterative Design and Manufacturing of a 3D-Printed Pediatric Open and Laparoscopic Integrated Simulator for Hernia Repair (POLISHeR). J. Pediatr. Surg. 2025, 60, 162232. [Google Scholar] [CrossRef]
- Petra, Z.; Silvia, H.; Martin, L.; Rebeka, P.; Dominika, Š.; Pavol, V.; Miroslava, L.; František, H.; Tomáš, T.; Jozef, B. Validation study of synthetic models for esophageal atresia with tracheoesophageal fistula (EA/TEF): A simulation-based training in pediatric surgery. J. Pediatr. Surg. Open 2025, 9, 100180. [Google Scholar] [CrossRef]
- Nair, D.; Wells, J.M.; Cook, N.; Yi, M.; Scott, V.; Beasley, S.W. Construct validation of a 3D printed neonatal thoracoscopic simulator: Can it measure expertise? J. Pediatr. Surg. 2021, 56, 1962–1965. [Google Scholar] [CrossRef]
- Radhakrishnan, U.; Kuang, L.; Koumaditis, K.; Chinello, F.; Pacchierotti, C. Haptic Feedback, Performance and Arousal: A Comparison Study in an Immersive VR Motor Skill Training Task. IEEE Trans. Haptics 2024, 17, 249–262. [Google Scholar] [CrossRef]
- Gund, M.P.; Strähle, U.T.; Naim, J.; Waldmeyer, M.; Hannig, M.; Rupf, S. Comparison of 3D-Printed Patient Model Versus Animal Cadaveric Model in Periodontal Surgery Block Course-What Is More Feasible for Beginners? A Pilot Study. Eur. J. Dent. Educ. Off. J. Assoc. Dent. Educ. Eur. 2025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).