Long-Term Trends in Laryngeal Cancer Incidence and Mortality in Central Serbia (1999–2023): A Joinpoint Regression Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
2.2. Data Sources
2.3. Rate Calculation and Standardization
2.4. Trend Analysis
2.5. Comparability Test and Statistical Software
3. Results
3.1. Position of LC Among Leading Cancer Sites
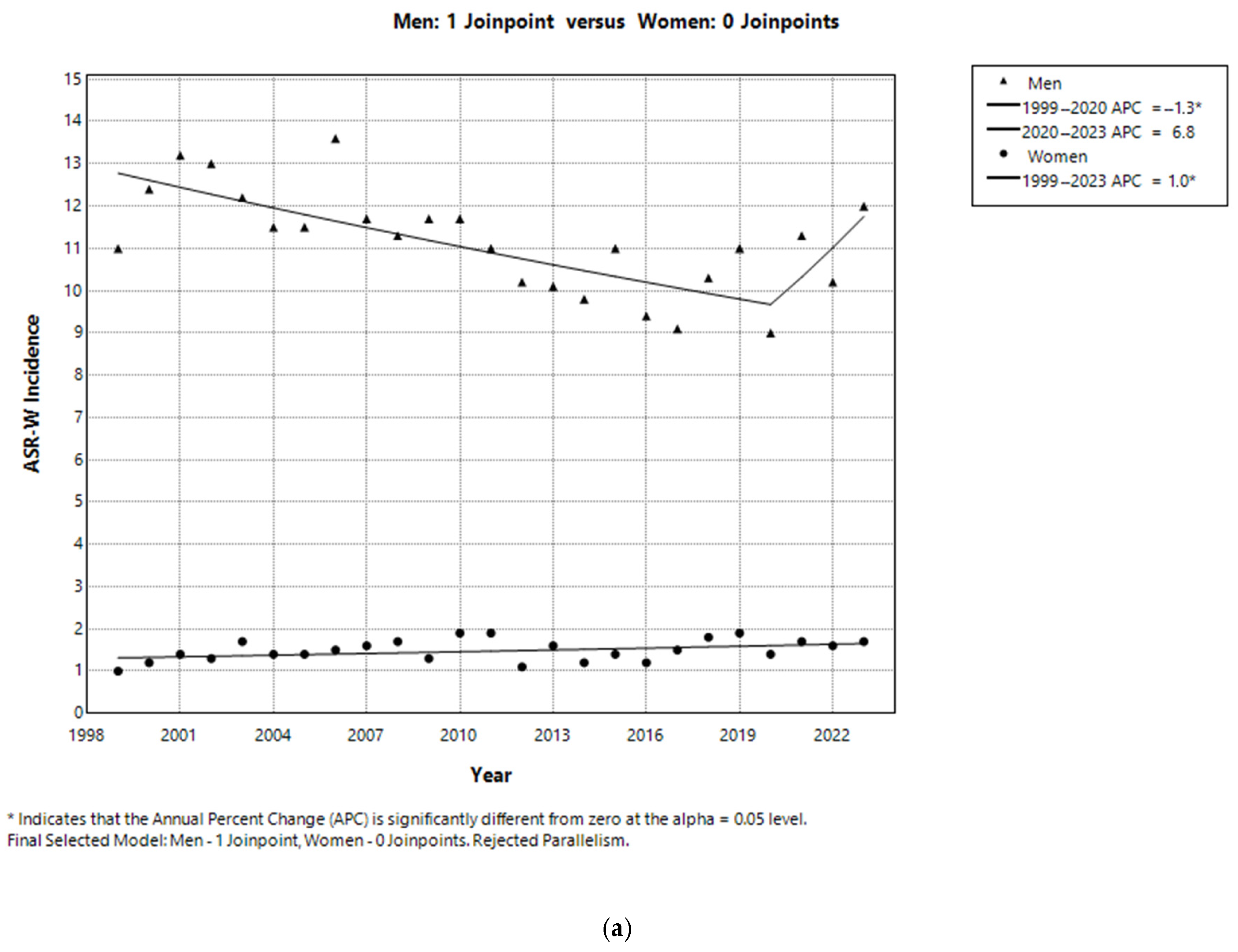
3.2. Sex-Specific Incidence and Trends in LC
3.3. Age-Specific Incidence and Trends in LC
3.4. Sex-Specific Mortality and Trends in LC
3.5. Age-Specific Mortality and Trends in LC
3.6. Sex-Based Comparison of Incidence and Mortality Trends
4. Discussion
4.1. Incidence and Mortality Rates
4.2. Epidemiological Trends
4.3. Risk Factors
4.4. Study Strengths and Limitations
4.5. Public Health Relevance and Prevention Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Amanian, A.; Anderson, D.W.; Durham, J.S.; Prisman, E.; Ng, T.; Hu, A. Treatment of Laryngeal Verrucous Carcinoma: 28-Year Retrospective Cohort Study and Literature Review. OTO Open 2023, 7, e50. [Google Scholar] [CrossRef] [PubMed]
- Cobanoglu, H.B.; Koprucu, E.R. Non-squamous Cancers of the Larynx. Curr. Oncol. Rep. 2024, 26, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S., Jr. Not your usual cancer case: Variants of laryngeal squamous cell carcinoma. Head Neck Pathol. 2011, 5, 23–30. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 1 May 2025).
- GLOBOCAN 2022. Global Cancer Observatory: Laryngeal Cancer Fact Sheet; International Agency for Research on Cancer: Lyon, France, 2022; Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/14-larynx-fact-sheet.pdf (accessed on 1 May 2025).
- Huang, J.; Chan, S.C.; Ko, S.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Updated disease distributions, risk factors, and trends of laryngeal cancer: A global analysis of cancer registries. Int. J. Surg. 2024, 110, 810–819. [Google Scholar] [CrossRef]
- Ramsey, T.; Guo, E.; Svider, P.F.; Lin, H.; Syeda, S.; Raza, S.N.; Fribley, A.M. Laryngeal cancer: Global socioeconomic trends in disease burden and smoking habits. Laryngoscope 2018, 128, 2039–2053. [Google Scholar] [CrossRef]
- Divakar, P.; Davies, L. Trends in Incidence and Mortality of Larynx Cancer in the US. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 34–41. [Google Scholar] [CrossRef]
- Nocini, R.; Molteni, G.; Mattiuzzi, C.; Lippi, G. Updates on larynx cancer epidemiology. Chin. J. Cancer Res. 2020, 32, 18–25. [Google Scholar] [CrossRef]
- Statistical Office of the Republic of Serbia. National Affiliation: Data by Municipalities and Cities, 2022 Census of Population, Households, and Dwellings; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2023. Available online: https://publikacije.stat.gov.rs/G2022/HtmlL/G20221350.html (accessed on 1 May 2025).
- Vukicevic, A.; Miljus, D.; Zivkovic Perisic, S.; Bozic, Z. Cancer Incidence and Mortality in Central Serbia, 1999–2015; Cancer Registry of Central Serbia; Institute of Public Health of Serbia: Belgrade, Serbia, 2017; Available online: https://www.batut.org.rs/index.php?content=185 (accessed on 1 May 2025).
- Miljus, D.; Zivkovic Perisic, S.; Bozic, Z. Malignant Tumours in Republic of Serbia, 2016–2023; Serbian Cancer Registry; Institute of Public Health of Serbia: Beograd, Serbia, 2024; Available online: https://www.batut.org.rs/index.php?content=2096 (accessed on 1 May 2025).
- Law on Health Documentation and Records in the Field of Healthcare. Official Gazette of the Republic of Serbia, No. 92/2023, Belgrade, Serbia, Available only in Serbian. 2023. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/skupstina/zakon/2023/92/15/reg (accessed on 1 July 2025).
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. (Eds.) Cancer Incidence in Five Continents, Vol. XI (Electronic Version); International Agency for Research on Cancer: Lyon, France, 2017; Available online: https://ci5.iarc.fr (accessed on 17 June 2025).
- European Network of Cancer Registries (ENCR). ENCR Recommendations. Available online: https://www.encr.eu/ENCR-Recommendations (accessed on 1 July 2025).
- World Health Organization. International Classification of Diseases—Eleventh Revision (ICD-11); World Health Organization: Geneva, Switzerland, 2022; Available online: https://icd.who.int/browse/2025-01/mms/en (accessed on 1 May 2025).
- Segi, M.; Fujisaku, S. Cancer Mortality for Selected Sites in 24 Countries (1950–1957); Department of Public Health, Tohoku University School of Medicine: Sendai, Japan, 1960. [Google Scholar]
- Lerman, P.M. Fitting Segmented Regression Models by Grid Search. J. R. Stat. Soc. C Appl. Stat. 1980, 29, 77–84. [Google Scholar] [CrossRef]
- Kim, H.J.; Fay, M.P.; Yu, B.; Barrett, M.J.; Feuer, E.J. Comparability of segmented line regression models. Biometrics 2004, 60, 1005–1014. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Joinpoint Regression Program, Version 5.4.0; National Cancer Institute: Bethesda, MD, USA, 2024. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 1 May 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, Y.D.; Park, C.S.; Han, K.D.; Joo, Y.H. Hypertension is associated with oral, laryngeal, and esophageal cancer: A nationwide population-based study. Sci. Rep. 2020, 10, 10291. [Google Scholar] [CrossRef] [PubMed]
- Menicagli, R.; Bolla, G.; Menicagli, L.; Esseridou, A. The Possible Role of Diabetes in the Etiology of Laryngeal Cancer. Gulf. J. Oncolog. 2017, 1, 44–51. [Google Scholar]
- Huang, J.; Chan, S.C.; Ko, S.; Tong, E.; Cheung, C.S.; Wong, W.N.; Cheung, N.T.; Wong, M.C. Associations between adoption of eHealth management module and optimal control of HbA1c in diabetes patients. NPJ Digit. Med. 2023, 6, 67. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Laryngeal and Hypopharyngeal Cancers. 2025. Available online: https://www.cancer.org/cancer/types/laryngeal-and-hypopharyngeal-cancer/about/key-statistics.html (accessed on 1 May 2025).
- Gazzini, L.; Fazio, E.; Dallari, V.; Accorona, R.; Abousiam, M.; Nebiaj, A.; Giorgetti, G.; Girolami, I.; Vittadello, F.; Magnato, R.; et al. Impact of the COVID-19 Pandemic on Head and Neck Cancer Diagnosis: Data from a Single Referral Center, South Tyrol, Northern Italy. Eur. Arch. Otorhinolaryngol. 2022, 279, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Muratovic, B.; Nikolic, A.; Sipetic Grujicic, S. Trends in incidence and mortality from pancreatic cancer in Central Serbia, in the period from 1999 to 2019. Health Care 2022, 51, 23–38. [Google Scholar] [CrossRef]
- Chatenoud, L.; Garavello, W.; Pagan, E.; Bertuccio, P.; Gallus, S.; La Vecchia, C.; Negri, E.; Bosetti, C. Laryngeal cancer mortality trends in European countries. Int. J. Cancer 2016, 138, 833–842. [Google Scholar] [CrossRef]
- Nocini, R.; Lippi, G.; Mattiuzzi, C. Mortality of laryngeal cancer before and during the COVID-19 pandemic. COVID 2024, 4, 652–657. [Google Scholar] [CrossRef]
- World Health Organization. Statement on the Fifteenth Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Coronavirus Disease (COVID-19) Pandemic; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 7 May 2025).
- Liberale, C.; Soloperto, D.; Marchioni, A.; Monzani, D.; Sacchetto, L. Updates on Larynx Cancer: Risk Factors and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 12913. [Google Scholar] [CrossRef]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An update on larynx cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed]
- Levesque, C.; Sanger, N.; Edalati, H.; Sohi, I.; Shield, K.D.; Sherk, A.; Stockwell, T.; Butt, P.R.; Paradis, C. A systematic review of relative risks for the relationship between chronic alcohol use and the occurrence of disease. Alcohol Clin. Exp. Res. 2023, 47, 1238–1255. [Google Scholar] [CrossRef] [PubMed]
- Bilano, V.; Gilmour, S.; Moffiet, T.; d’Espaignet, E.T.; Stevens, G.A.; Commar, A.; Tuyl, F.; Hudson, I.; Shibuya, K. Global trends and projections for tobacco use, 1990–2025: An analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet 2015, 385, 966–976. [Google Scholar] [CrossRef]
- Li, X.; Gao, L.; Li, H.; Gao, J.; Yang, Y.; Zhou, F.; Gao, C.; Li, M.; Jin, Q. Human papillomavirus infection and laryngeal cancer risk: A systematic review and meta-analysis. J. Infect. Dis. 2013, 207, 479–488. [Google Scholar] [CrossRef]
- Gama, R.R.; Carvalho, A.L.; Longatto Filho, A.; Scorsato, A.P.; López, R.V.; Rautava, J.; Syrjänen, S.; Syrjänen, K. Detection of human papillomavirus in laryngeal squamous cell carcinoma: Systematic review and meta-analysis. Laryngoscope 2016, 126, 885–893. [Google Scholar] [CrossRef]
- Erkul, E.; Yilmaz, I.; Narli, G.; Babayigit, M.A.; Gungor, A.; Demirel, D. The presence and prognostic significance of human papillomavirus in squamous cell carcinoma of the larynx. Eur. Arch. Otorhinolaryngol. 2017, 274, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Guillen, J.M.; Palacios-Saucedo, G.C.; Alanis-Valdez, A.Y.; Huerta-Escobedo, A.; Zavala-Pompa, A.; Rivera-Morales, L.G.; Martinez-Torres, A.C.; Gonzalez-Villasana, V.; Serna-Hernandez, J.C.; Hernandez-Martinez, S.J.; et al. p16INK4a and pRb expression in laryngeal squamous cell carcinoma with and without infection by EBV or different genotypes of HPV: A retrospective study. Infect. Agents Cancer 2023, 18, 43. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhao, X.; Xu, Z.; Dai, W.; Duan, W.; Huang, S.; Zhang, E.; Liu, J.; Zhang, S.; et al. Composition and function of oral microbiota between gingival squamous cell carcinoma and periodontitis. Oral Oncol. 2020, 107, 104710. [Google Scholar] [CrossRef]
- Milić, N.; Stanisavljević, D.; Krstić, M. (Eds.) The 2019 Serbian National Health Survey; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2021. Available online: https://www.stat.gov.rs/en-us/publikacije/?a=3037&s=0301 (accessed on 15 June 2025).
- Law on the Protection of the Population from Exposure to Tobacco Smoke. Official Gazette of the Republic of Serbia, No. 30/2010, Belgrade, Serbia, Available only in Serbian. 2010. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/skupstina/zakon/2010/30/6/reg (accessed on 1 July 2025).
- International Agency for Research on Cancer (IARC). CanReg5 Software for Cancer Registries Version 5.00.44k; IARC: Lyon, France, 2023; Available online: https://www.iacr.com.fr/index.php?option=com_content&view=category&layout=blog&id=68&Itemid=445 (accessed on 1 July 2025).
- Tobacco Control Strategy. Official Gazette of the Republic of Serbia, No. 8/2007, Belgrade, Serbia, Available only in Serbian. 2007. Available online: http://demo.paragraf.rs/demo/combined/Old/t/t2007_01/t01_0256.htm (accessed on 1 July 2025).
- The National Programme for the Prevention of Cervical Cancer. Official Gazette of the Republic of Serbia, No. 54/2008, Belgrade, Serbia, Available only in Serbian. 2008. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/vlada/uredba/2013/83/2 (accessed on 1 July 2025).
- Strategy for the Prevention and Control of Non-Communicable Diseases in Serbia. Official Gazette of the Republic of Serbia, No. 22/2009, Belgrade, Serbia, Available only in Serbian. 2009. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/vlada/strategija/2009/22/2 (accessed on 1 July 2025).
- National Program for the Prevention of Harmful Use of Alcohol and Alcohol-Related Disorders. Official Gazette of the Republic of Serbia, No. 115/2017, Belgrade, Serbia, Available only in Serbian. 2017. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/vlada/uredba/2017/115/1/reg (accessed on 1 July 2025).
- Strategy of Public Health in the Republic of Serbia, 2018–2026. Official Gazette of the Republic of Serbia, No. 61/2018, Belgrade, Serbia, Available only in Serbian. 2018. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/vlada/strategija/2018/61/1/reg (accessed on 1 July 2025).
- Program for the Improvement of Cancer Control in the Republic of Serbia for the Period 2020–2022. Official Gazette of the Republic of Serbia, No. 105/2020, Belgrade, Serbia, Available only in Serbian. 2020. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/vlada/drugiakt/2020/105/1 (accessed on 1 July 2025).
- Rulebook on Healthcare Quality Indicators and Quality Assessment of Professional Work. Official Gazette of the Republic of Serbia, No. 123/2021, Belgrade, Serbia, Available only in Serbian. 2021. Available online: https://pravno-informacioni-sistem.rs/eli/rep/sgrs/ministarstva/pravilnik/2021/123/1 (accessed on 1 July 2025).

| Year | Crude Incidence Rate | Standardized Incidence Rate | ||||
|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | |
| 1999 | 17.3 | 1.4 | 9.4 | 10.9 | 0.9 | 5.9 |
| 2000 | 19.8 | 1.9 | 10.8 | 12.3 | 1.1 | 6.7 |
| 2001 | 21.1 | 2.2 | 11.6 | 13.1 | 1.3 | 7.2 |
| 2002 | 21.0 | 2.1 | 11.6 | 12.9 | 1.2 | 7.0 |
| 2003 | 19.8 | 2.6 | 11.2 | 12.1 | 1.6 | 6.8 |
| 2004 | 18.8 | 2.2 | 10.5 | 11.4 | 1.3 | 6.4 |
| 2005 | 18.5 | 2.1 | 10.3 | 11.4 | 1.3 | 6.4 |
| 2006 | 22.0 | 2.4 | 12.2 | 13.5 | 1.4 | 7.4 |
| 2007 | 19.5 | 2.8 | 11.2 | 11.6 | 1.5 | 6.6 |
| 2008 | 18.8 | 2.6 | 10.7 | 11.2 | 1.6 | 6.4 |
| 2009 | 19.4 | 2.0 | 10.7 | 11.6 | 1.2 | 6.4 |
| 2010 | 19.5 | 3.2 | 11.1 | 11.6 | 1.8 | 6.7 |
| 2011 | 18.9 | 3.1 | 10.8 | 10.9 | 1.8 | 6.4 |
| 2012 | 17.8 | 2.0 | 9.7 | 10.1 | 1.0 | 5.6 |
| 2013 | 18.3 | 3.0 | 10.5 | 10.0 | 1.5 | 5.8 |
| 2014 | 18.1 | 2.3 | 10.2 | 9.7 | 1.1 | 5.4 |
| 2015 | 20.3 | 2.5 | 11.4 | 10.9 | 1.3 | 6.1 |
| 2016 | 18.0 | 2.1 | 9.9 | 9.3 | 1.1 | 4.9 |
| 2017 | 17.3 | 3.0 | 10.0 | 9.0 | 1.4 | 5.0 |
| 2018 | 19.9 | 3.4 | 11.4 | 10.2 | 1.7 | 5.6 |
| 2019 | 21.6 | 3.6 | 12.4 | 10.9 | 1.8 | 6.0 |
| 2020 | 18.6 | 3.2 | 10.7 | 8.9 | 1.3 | 4.8 |
| 2021 | 22.8 | 3.6 | 12.9 | 11.2 | 1.6 | 6.0 |
| 2022 | 20.9 | 3.7 | 12.1 | 10.1 | 1.5 | 5.5 |
| 2023 | 24.8 | 3.6 | 13.9 | 11.9 | 1.6 | 6.4 |
| Average ** | 19.7 | 2.7 | 11.1 | 11.1 | 1.4 | 6.2 |
| Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Group (Years) | Incidence Rate | Period | APC (95% CI) | AAPC (95% CI) | Age Group (Years) | Incidence Rate | Period | APC (95% CI) | AAPC (95% CI) |
| 0–39 | 0.5 | 1999–2023 | −5.6 * (−8.7, −2.5) | −5.6 * (−8.7, −2.5) | 0–49 | 0.7 | 1999–2023 1999–2002 2002–2023 | 62.9 * (6.4, 277.3) −7.7 * (−11.4, −5.4) | −0.9 (−4.8, 4.2) |
| 40–49 | 10.4 | 1999–2023 1999–2010 2010–2019 2019–2023 | 0.1 (−3.1, 5.4) −14.7 * (−29.5, −10.5) 19.4 * (2.5, 63.4) | −2.8 * (−4.4, −1.2) | |||||
| 50–59 | 39.4 | 1999–2023 | −2.6 * (−3.5, −1.9) | −2.6 * (−3.5, −1.9) | 50–59 | 4.6 | 1999–2023 | 0.5 (−1.4, 2.6) | 0.5 (−1.4, 2.6) |
| 60–69 | 61.1 | 1999–2023 | 0.9 * (0.1, 1.8) | 0.9 * (0.1, 1.8) | 60–69 | 7.4 | 1999–2023 | 2.7 * (1.6, 4.5) | 2.7 * (1.6, 4.5) |
| 70+ | 47.9 | 1999–2023 1999–2017 2017–2023 | −0.4 (−5.0, 0.8) 7.8 * (1.6, 25.4) | 1.6 * (0.2, 2.6) | 70+ | 5.1 | 1999–2023 | 2.8 * (1.5, 5.0) | 2.8 * (1.5, 5.0) |
| Average Standardized Rate | 11.1 | 1999–2023 1999–2017 2017–2023 | −1.6 * (−3.9, −1.1) 2.0 (−0.7, 10.5) | −0.7 * (−1.4, −0.2) | Average Standardized Rate | 1.4 | 1999–2023 | 0.9 (−0.2, 2.0) | 0.9 (−0.2, 2.0) |
| Age (Years) | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70+ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | W | M | W | M | W | M | W | M | W | M | W |
| Years | ||||||||||||
| 1999 | 0.0 | 0.0 | 1.1 | 0.0 | 13.0 | 0.9 | 42.9 | 4.5 | 53.4 | 5.4 | 40.8 | 1.5 |
| 2000 | 0.0 | 0.6 | 1.2 | 0.0 | 12.6 | 0.8 | 47.2 | 3.6 | 64.8 | 4.3 | 47.5 | 5.9 |
| 2001 | 0.0 | 0.0 | 1.4 | 0.3 | 13.8 | 3.5 | 58.2 | 3.4 | 63.6 | 6.2 | 44.9 | 3.3 |
| 2002 | 0.6 | 0.0 | 1.4 | 0.3 | 15.4 | 2.2 | 51.1 | 3.4 | 63.6 | 4.8 | 46.0 | 5.7 |
| 2003 | 0.0 | 0.0 | 2.0 | 0.6 | 13.4 | 4.0 | 52.1 | 3.4 | 49.3 | 7.6 | 52.0 | 4.2 |
| 2004 | 0.5 | 0.2 | 0.9 | 0.3 | 15.1 | 3.0 | 43.9 | 4.6 | 54.2 | 5.6 | 51.8 | 3.4 |
| 2005 | 0.2 | 0.0 | 0.3 | 0.0 | 17.6 | 3.1 | 42.2 | 5.6 | 52.8 | 4.5 | 42.0 | 2.7 |
| 2006 | 0.6 | 0.2 | 1.2 | 0.0 | 18.4 | 2.4 | 47.2 | 5.4 | 68.8 | 7.4 | 51.2 | 3.0 |
| 2007 | 0.8 | 0.0 | 1.7 | 0.8 | 13.8 | 2.2 | 39.6 | 5.8 | 57.3 | 5.6 | 52.2 | 5.9 |
| 2008 | 0.0 | 0.0 | 1.4 | 0.6 | 12.0 | 1.7 | 48.1 | 5.2 | 52.4 | 9.2 | 40.3 | 3.7 |
| 2009 | 0.0 | 0.6 | 1.1 | 0.0 | 14.0 | 2.5 | 44.0 | 3.5 | 60.1 | 4.9 | 43.2 | 3.4 |
| 2010 | 0.0 | 0.0 | 0.6 | 0.6 | 12.8 | 2.0 | 49.2 | 7.1 | 60.6 | 10.0 | 34.6 | 4.7 |
| 2011 | 0.0 | 0.6 | 0.8 | 0.2 | 10.0 | 2.0 | 39.6 | 6.0 | 63.0 | 8.4 | 42.5 | 7.0 |
| 2012 | 0.0 | 0.0 | 0.2 | 0.0 | 14.4 | 1.7 | 34.2 | 3.3 | 53.6 | 5.6 | 41.0 | 3.4 |
| 2013 | 0.3 | 0.0 | 0.6 | 0.0 | 7.9 | 1.4 | 35.8 | 6.0 | 57.2 | 7.0 | 42.0 | 7.2 |
| 2014 | 0.3 | 0.0 | 1.1 | 0.2 | 6.2 | 0.8 | 33.6 | 4.8 | 58.6 | 6.3 | 45.4 | 3.2 |
| 2015 | 0.3 | 0.3 | 0.6 | 0.0 | 8.2 | 2.2 | 35.1 | 2.8 | 67.2 | 7.8 | 47.6 | 4.5 |
| 2016 | 0.0 | 0.0 | 0.3 | 0.3 | 6.6 | 0.8 | 28.5 | 4.9 | 57.2 | 5.2 | 47.1 | 3.1 |
| 2017 | 0.3 | 0.0 | 0.3 | 0.3 | 5.7 | 1.1 | 30.2 | 4.2 | 57.4 | 8.3 | 38.7 | 6.7 |
| 2018 | 0.7 | 0.3 | 0.8 | 0.3 | 2.8 | 2.0 | 31.5 | 5.1 | 70.8 | 10.1 | 45.6 | 5.6 |
| 2019 | 0.0 | 0.0 | 0.9 | 0.0 | 3.4 | 2.0 | 35.1 | 6.3 | 72.5 | 10.1 | 52.7 | 6.3 |
| 2020 | 0.0 | 0.0 | 0.0 | 0.0 | 5.3 | 0.3 | 23.0 | 2.9 | 51.0 | 11.0 | 64.4 | 7.1 |
| 2021 | 0.0 | 0.0 | 0.3 | 0.0 | 4.7 | 0.6 | 33.3 | 5.9 | 70.2 | 9.4 | 67.0 | 8.2 |
| 2022 | 0.0 | 0.0 | 0.3 | 0.3 | 5.4 | 0.6 | 30.3 | 3.5 | 64.1 | 10.5 | 54.6 | 9.3 |
| 2023 | 0.0 | 0.0 | 0.3 | 0.3 | 7.7 | 0.6 | 28.5 | 4.7 | 83.8 | 10.7 | 62.8 | 7.5 |
| Average * | 0.2 | 0.1 | 0.8 | 0.2 | 10.4 | 1.8 | 39.4 | 4.6 | 61.1 | 7.4 | 47.9 | 5.1 |
| Year | Crude Mortality Rate | Standardized Mortality Rate | ||||
|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | |
| 1999 | 11.7 | 1.0 | 6.4 | 7.0 | 0.5 | 3.8 |
| 2000 | 11.8 | 1.0 | 6.4 | 7.1 | 0.5 | 3.8 |
| 2001 | 10.5 | 1.0 | 5.8 | 6.2 | 0.5 | 3.4 |
| 2002 | 10.1 | 1.1 | 5.6 | 5.9 | 0.6 | 3.2 |
| 2003 | 11.7 | 0.9 | 6.3 | 6.7 | 0.5 | 3.6 |
| 2004 | 10.5 | 1.1 | 5.8 | 6.0 | 0.6 | 3.3 |
| 2005 | 10.3 | 1.2 | 5.8 | 5.6 | 0.6 | 3.1 |
| 2006 | 10.4 | 1.1 | 5.8 | 5.9 | 0.5 | 3.2 |
| 2007 | 10.5 | 1.1 | 5.8 | 5.9 | 0.5 | 3.2 |
| 2008 | 10.2 | 1.4 | 5.8 | 5.5 | 0.7 | 3.1 |
| 2009 | 11.2 | 0.8 | 6.0 | 6.1 | 0.3 | 3.2 |
| 2010 | 9.8 | 1.3 | 5.6 | 5.0 | 0.6 | 2.8 |
| 2011 | 10.8 | 1.2 | 6.0 | 5.9 | 0.5 | 3.2 |
| 2012 | 10.7 | 1.4 | 6.0 | 5.5 | 0.6 | 3.0 |
| 2013 | 9.6 | 1.2 | 5.4 | 4.9 | 0.5 | 2.7 |
| 2014 | 9.6 | 0.9 | 5.2 | 4.9 | 0.3 | 2.6 |
| 2015 | 9.6 | 1.2 | 5.4 | 4.7 | 0.5 | 2.6 |
| 2016 | 9.6 | 1.3 | 5.3 | 4.7 | 0.5 | 2.4 |
| 2017 | 10.6 | 0.9 | 5.6 | 5.2 | 0.4 | 2.6 |
| 2018 | 9.9 | 1.3 | 5.5 | 4.8 | 0.6 | 2.5 |
| 2019 | 10.6 | 0.8 | 5.6 | 4.9 | 0.3 | 2.4 |
| 2020 | 9.5 | 1.3 | 5.3 | 4.3 | 0.4 | 2.2 |
| 2021 | 9.3 | 1.1 | 5.1 | 4.2 | 0.4 | 2.1 |
| 2022 | 8.8 | 1.6 | 5.1 | 4.1 | 0.6 | 2.2 |
| 2023 | 9.1 | 1.1 | 5.0 | 4.0 | 0.4 | 2.1 |
| Average ** | 10.3 | 1.1 | 5.7 | 5.4 | 0.5 | 2.9 |
| Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Group (Years) | Incidence Rate | Period | APC (95% CI) | AAPC (95% CI) | Age Group (Years) | Incidence Rate | Period | APC (95% CI) | AAPC (95% CI) |
| 0–49 | 1.3 | 1999–2023 | −4.9 * (−7.2, −3.8) | −4.9 * (−7.2, −3.8) | 0–59 | 0.7 | 1999–2023 | −1.2 (−3.5, 0.5) | −1.2 (−3.5, 0.5) |
| 50–59 | 17.1 | 1999–2023 | −2.5 * (−4.5, −1.1) | −2.5 * (−4.5, −1.1) | |||||
| 60–69 | 29.9 | 1999–2023 | −1.3 * (−1.9, −0.6) | −1.3 * (−1.9, −0.6) | 60–69 | 4.6 | 1999–2023 | −0.4 (−2.3, 1.3) | −0.4 (−2.3, 1.3) |
| 70+ | 35.7 | 1999–2023 1999–2010 2010–2015 2015–2020 2020–2023 | −0.8 * (−1.5, −0.3) | 70+ | 7.4 | 1999–2023 | 1.5 * (0.3, 3.1) | 1.5 * (0.3, 3.1) | |
| 1.0 * (0.1, 2.7) | |||||||||
| −4.6 * (−10.0, −1.8) | |||||||||
| 4.8 * (1.9, 11.8) | |||||||||
| −9.4 * (−19.8, −4.2) | |||||||||
| Average Standardized Rate | 5.4 | 1999–2023 | −2.0 * (−2.5, −1.6) | −2.0 * (−2.5, −1.6) | Average Standardized Rate | 1.4 | 1999–2023 | −0.7 (−1.9, 0.4) | −0.7 (−1.9, 0.4) |
| Age (Years) | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70+ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | W | M | W | M | W | M | W | M | W | M | W |
| Years | ||||||||||||
| 1999 | 0.0 | 0.0 | 0.2 | 0.0 | 7.8 | 0.9 | 20.6 | 1.2 | 40.9 | 2.7 | 36.2 | 3.7 |
| 2000 | 0.0 | 0.0 | 0.3 | 0.2 | 7.1 | 0.7 | 23.4 | 1.6 | 37.4 | 1.6 | 38.4 | 2.9 |
| 2001 | 0.0 | 0.0 | 0.6 | 0.0 | 4.6 | 0.4 | 20.8 | 1.8 | 32.8 | 2.4 | 35.2 | 3.3 |
| 2002 | 0.0 | 0.0 | 0.0 | 0.0 | 3.7 | 0.4 | 24.2 | 1.8 | 31.3 | 3.4 | 29.5 | 3.4 |
| 2003 | 0.0 | 0.0 | 0.0 | 0.6 | 4.1 | 0.7 | 25.2 | 1.0 | 34.4 | 2.0 | 40.3 | 2.2 |
| 2004 | 0.0 | 0.0 | 0.3 | 0.0 | 5.3 | 0.0 | 18.2 | 2.2 | 31.8 | 2.2 | 38.0 | 3.2 |
| 2005 | 0.0 | 0.0 | 0.3 | 0.0 | 3.2 | 1.0 | 20.1 | 1.7 | 28.0 | 2.2 | 39.6 | 3.8 |
| 2006 | 0.0 | 0.3 | 0.0 | 0.0 | 5.5 | 0.5 | 19.4 | 1.2 | 31.2 | 2.4 | 34.4 | 4.0 |
| 2007 | 0.0 | 0.0 | 0.3 | 0.0 | 5.5 | 0.6 | 20.1 | 1.8 | 28.4 | 3.0 | 36.6 | 2.7 |
| 2008 | 0.0 | 0.0 | 0.3 | 0.0 | 3.6 | 0.3 | 15.6 | 2.4 | 30.2 | 4.6 | 39.0 | 3.4 |
| 2009 | 0.0 | 0.0 | 0.0 | 0.0 | 5.2 | 0.0 | 19.4 | 1.2 | 29.4 | 1.6 | 40.9 | 2.8 |
| 2010 | 0.0 | 0.0 | 0.0 | 0.3 | 1.8 | 0.0 | 16.4 | 1.6 | 26.8 | 3.7 | 39.8 | 4.0 |
| 2011 | 0.0 | 0.0 | 0.0 | 0.0 | 3.8 | 0.3 | 18.4 | 1.2 | 34.0 | 2.4 | 36.2 | 4.8 |
| 2012 | 0.0 | 0.0 | 0.2 | 0.0 | 4.4 | 0.8 | 31.9 | 2.4 | 32.0 | 1.2 | 35.2 | 4.6 |
| 2013 | 0.0 | 0.0 | 0.2 | 0.0 | 3.6 | 0.6 | 15.0 | 1.0 | 25.0 | 2.0 | 33.8 | 4.2 |
| 2014 | 0.0 | 0.0 | 0.6 | 0.0 | 2.6 | 0.0 | 15.2 | 0.6 | 26.1 | 2.4 | 32.8 | 3.2 |
| 2015 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.3 | 15.2 | 1.0 | 29.2 | 3.0 | 30.2 | 4.1 |
| 2016 | 0.0 | 0.0 | 0.3 | 0.0 | 2.9 | 0.3 | 11.6 | 0.8 | 28.3 | 2.7 | 32.9 | 4.9 |
| 2017 | 0.0 | 0.0 | 0.0 | 0.3 | 2.6 | 0.0 | 14.5 | 0.3 | 30.0 | 2.4 | 36.1 | 3.4 |
| 2018 | 0.0 | 0.0 | 0.6 | 0.0 | 2.0 | 0.8 | 12.9 | 1.7 | 29.7 | 2.5 | 32.2 | 3.6 |
| 2019 | 0.0 | 0.0 | 0.3 | 0.0 | 0.6 | 0.0 | 13.4 | 0.0 | 27.3 | 2.0 | 41.4 | 3.2 |
| 2020 | 0.0 | 0.0 | 0.3 | 0.0 | 2.0 | 0.3 | 6.8 | 0.6 | 28.3 | 1.8 | 36.1 | 5.6 |
| 2021 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.3 | 9.3 | 1.2 | 23.3 | 1.8 | 37.7 | 4.1 |
| 2022 | 0.0 | 0.0 | 0.0 | 0.0 | 2.6 | 0.3 | 10.8 | 2.1 | 22.5 | 3.4 | 30.3 | 4.8 |
| 2023 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 7.8 | 0.6 | 29.2 | 2.4 | 30.1 | 4.0 |
| Average * | 0.0 | 0.0 | 0.2 | 0.1 | 3.5 | 0.4 | 17.1 | 1.3 | 29.9 | 2.5 | 35.7 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nešić, V.; Krstić Nešić, D.; Šipetić Grujičić, S.; Bukurov, B.; Miljuš, D.; Živković Perišić, S.; Nikolić, A. Long-Term Trends in Laryngeal Cancer Incidence and Mortality in Central Serbia (1999–2023): A Joinpoint Regression Analysis. Healthcare 2025, 13, 1633. https://doi.org/10.3390/healthcare13131633
Nešić V, Krstić Nešić D, Šipetić Grujičić S, Bukurov B, Miljuš D, Živković Perišić S, Nikolić A. Long-Term Trends in Laryngeal Cancer Incidence and Mortality in Central Serbia (1999–2023): A Joinpoint Regression Analysis. Healthcare. 2025; 13(13):1633. https://doi.org/10.3390/healthcare13131633
Chicago/Turabian StyleNešić, Vladimir, Dragana Krstić Nešić, Sandra Šipetić Grujičić, Bojana Bukurov, Dragan Miljuš, Snežana Živković Perišić, and Aleksandra Nikolić. 2025. "Long-Term Trends in Laryngeal Cancer Incidence and Mortality in Central Serbia (1999–2023): A Joinpoint Regression Analysis" Healthcare 13, no. 13: 1633. https://doi.org/10.3390/healthcare13131633
APA StyleNešić, V., Krstić Nešić, D., Šipetić Grujičić, S., Bukurov, B., Miljuš, D., Živković Perišić, S., & Nikolić, A. (2025). Long-Term Trends in Laryngeal Cancer Incidence and Mortality in Central Serbia (1999–2023): A Joinpoint Regression Analysis. Healthcare, 13(13), 1633. https://doi.org/10.3390/healthcare13131633





