Phase Angle Trajectory Among Critical Care Patients: Longitudinal Decline Predicts Mortality Independent of Clinical Severity Scores
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria
2.2. Measures
2.3. Primary and Secondary Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APACHE | Acute Physiology and Chronic Health Evaluation |
| BMI | Body Mass Index |
| BIA | Bioelectrical Impedance Analysis |
| COPD | Chronic Obstructive Pulmonary Disease |
| ICU | Intensive Care Unit |
| MUMC | Mid-upper arm circumference |
| PhA | Phase Angle |
| REE | Resting Energy Expenditure |
| SOFA | Sequential Organ Failure Assessment |
References
- Yamada, Y.; Yoshida, T.; Murakami, H.; Kawakami, R.; Gando, Y.; Ohno, H.; Tanisawa, K.; Konishi, K.; Julien, T.; Kondo, E.; et al. Phase angle obtained via bioelectrical impedance analysis and objectively measured physical activity or exercise habits. Sci. Rep. 2022, 12, 17274. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Di Gregorio, A.; Pasanisi, F.; Scalfi, L. Bioelectrical impedance analysis (BIA)-derived phase angle in sarcopenia: A systematic review. Clin. Nutr. 2021, 40, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ding, P.; Wu, J.; Yang, P.; Tian, Y.; Zhao, Q. Phase angle derived from bioelectrical impedance analysis as a marker for predicting sarcopenia. Front. Nutr. 2022, 9, 1060224. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.-C.; et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.K.; Berbigier, M.C.; de Almeida Rubin, B.; Moraes, R.B.; Corrêa Souza, G.; Schweigert Perry, I.D. Phase Angle as a Prognostic Marker in Patients with Critical Illness. Nutr. Clin. Pract. 2015, 30, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Eckert, I.; Gonzalez, M.C.; Silva, F.M. Prognostic value of phase angle and bioelectrical impedance vector in critically ill patients: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2022, 41, 2801–2816. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.S.; Gonzalez, M.C.; Freire, S.M.; Lourenço, R.A. Low phase angle in critically ill older patients is associated with late mortality: A prospective study. Nutrition 2023, 105, 111852. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, C.; Zhu, S.; Ge, Y.; Gong, D. The association between the change in severity score from baseline and the outcomes of critically ill patients was enhanced by integration of bioimpedance analysis parameters. Sci. Rep. 2024, 14, 14681. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score—Development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Paneru, H.; Ojha, R.; Karn, R.; Rajbhandari, R.; Gajurel, B.P. SOFA and APACHE II scoring systems for predicting outcome of neurological patients admitted in a tertiary hospital intensive care unit. Ann. Med. Surg. 2024, 86, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Beigmohammadi, M.T.; Amoozadeh, L.; Rezaei Motlagh, F.; Rahimi, M.; Maghsoudloo, M.; Jafarnejad, B.; Eslami, B.; Salehi, M.R.; Zendehdel, K.; Zhu, Y. Mortality Predictive Value of APACHE II and SOFA Scores in COVID-19 Patients in the Intensive Care Unit. Can. Respir. J. 2022, 2022, 5129314. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerf, F.; Beumeler, L.F.E.; Rijnhart-de Jong, H.; Boerma, E.C.; Buter, H. The predictive value of phase angle on long-term outcome after ICU admission. Clin. Nutr. 2022, 41, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L.; Graves, J.E.; Mahar, M.T. Reliability and validity of bioelectrical impedance in determining body composition. J. Appl. Physiol. 1988, 64, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Denneman, N.; Hessels, L.; Broens, B.; Gjaltema, J.; Stapel, S.N.; Stohlmann, J.; Nijsten, M.W.; Straaten, H.M.O.-V. Fluid balance and phase angle as assessed by bioelectrical impedance analysis in critically ill patients: A multicenter prospective cohort study. Eur. J. Clin. Nutr. 2020, 74, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- García-Grimaldo, A.; Trujillo-Mercado, A.S.; Rodríguez-Moguel, N.C.; Rios-Ayala, M.A.; Hernandez-Cardenas, C.M.; Osuna-Padilla, I.A. Association between longitudinal changes in phase angle and mortality rate in adults critically ill with COVID-19: A retrospective cohort study. J. Parenter. Enter. Nutr. 2024, 48, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Stapel, S.N.; Looijaard, W.G.P.M.; Dekker, I.M.; Girbes, A.R.J.; Weijs, P.J.M.; Oudemans-van Straaten, H.M. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur. J. Clin. Nutr. 2018, 72, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Criteria |
|---|---|
| Participants | Adult critically ill patients admitted to the ICU at a tertiary hospital (Evangelismos Athens General Hospital), between May and November 2024. |
| Intervention/Exposure | Assessment of phase angle (PhA) on ICU admission and its changes over time |
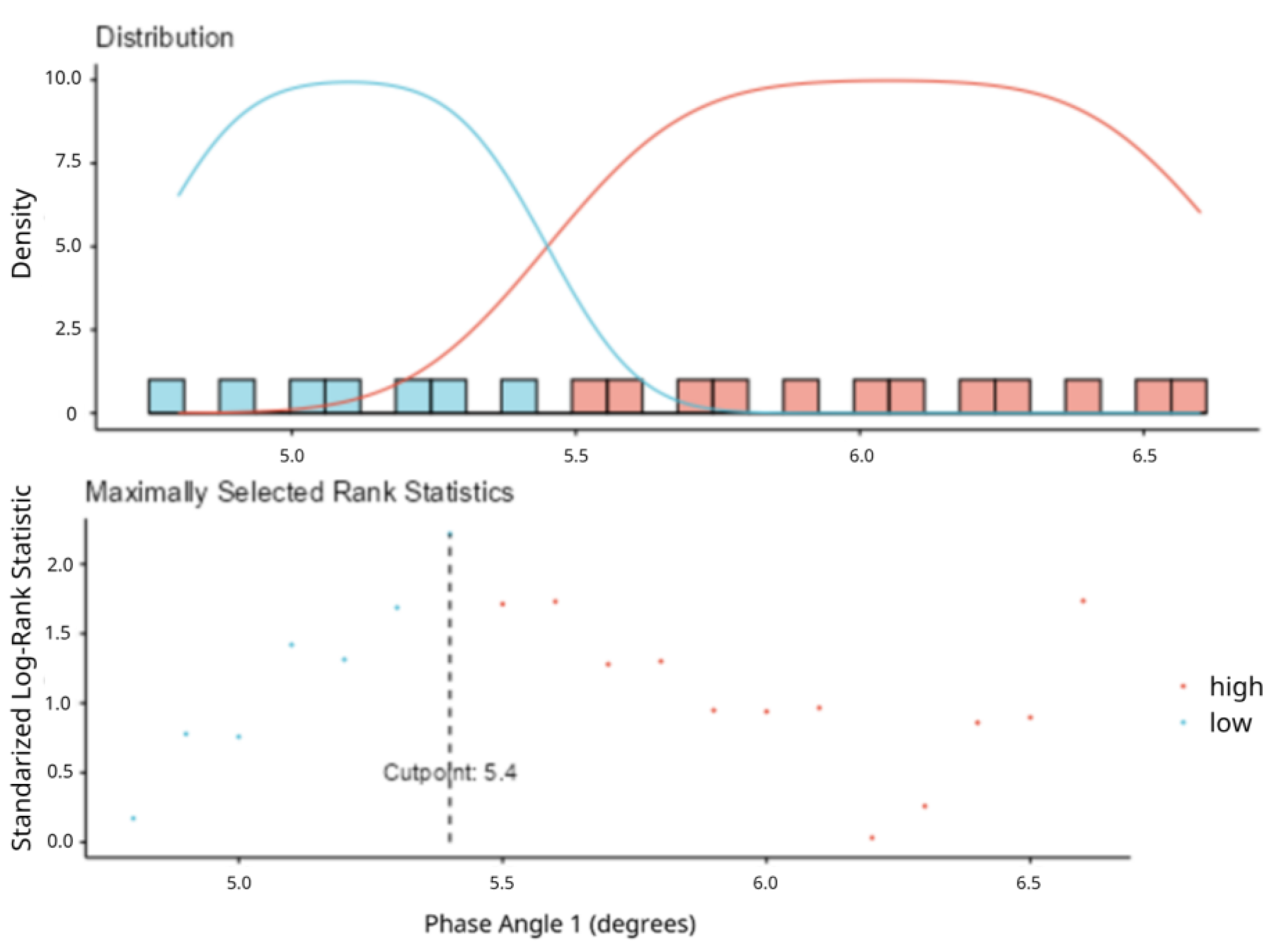
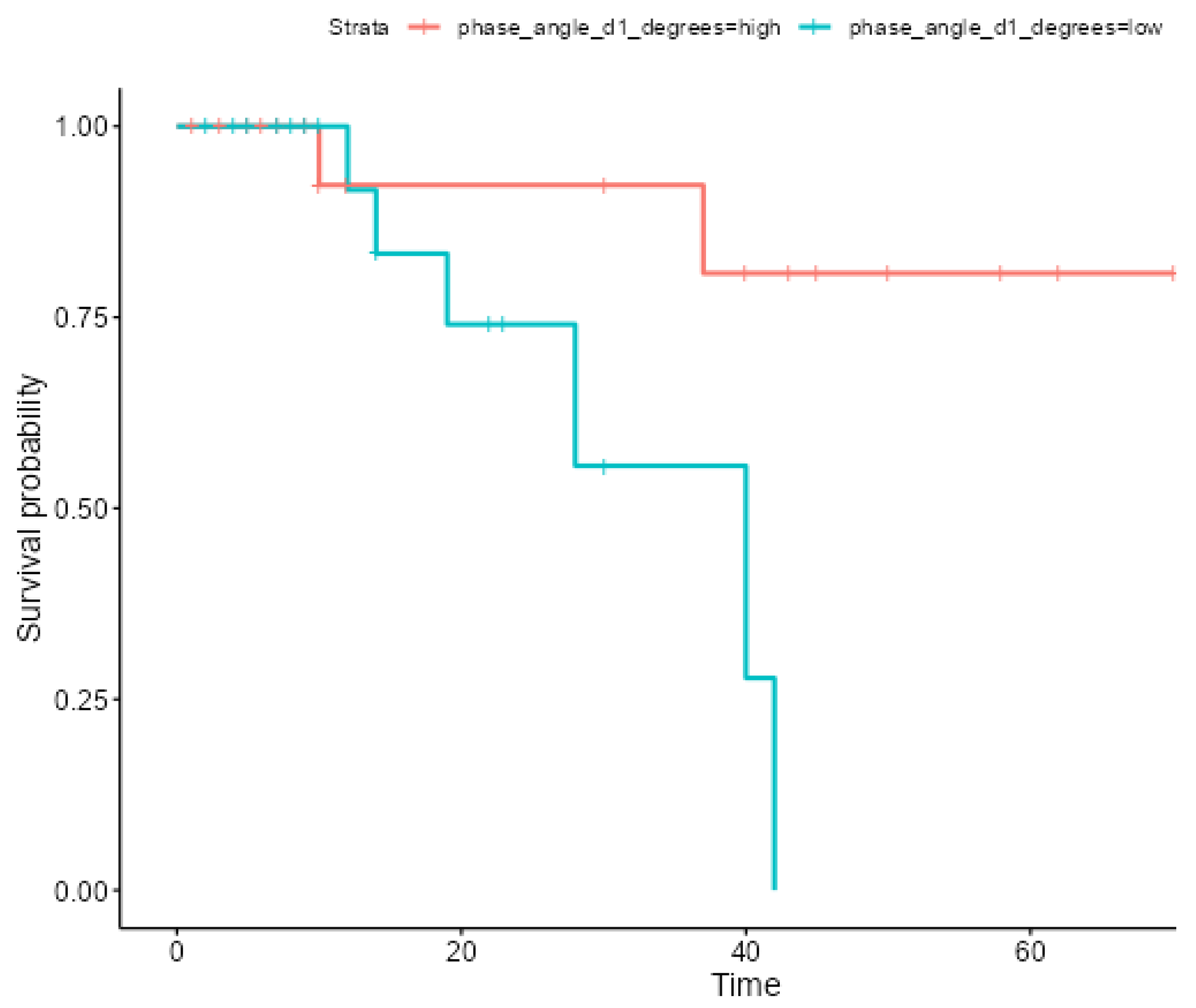
| Comparison | Patients stratified by PhA value:
|
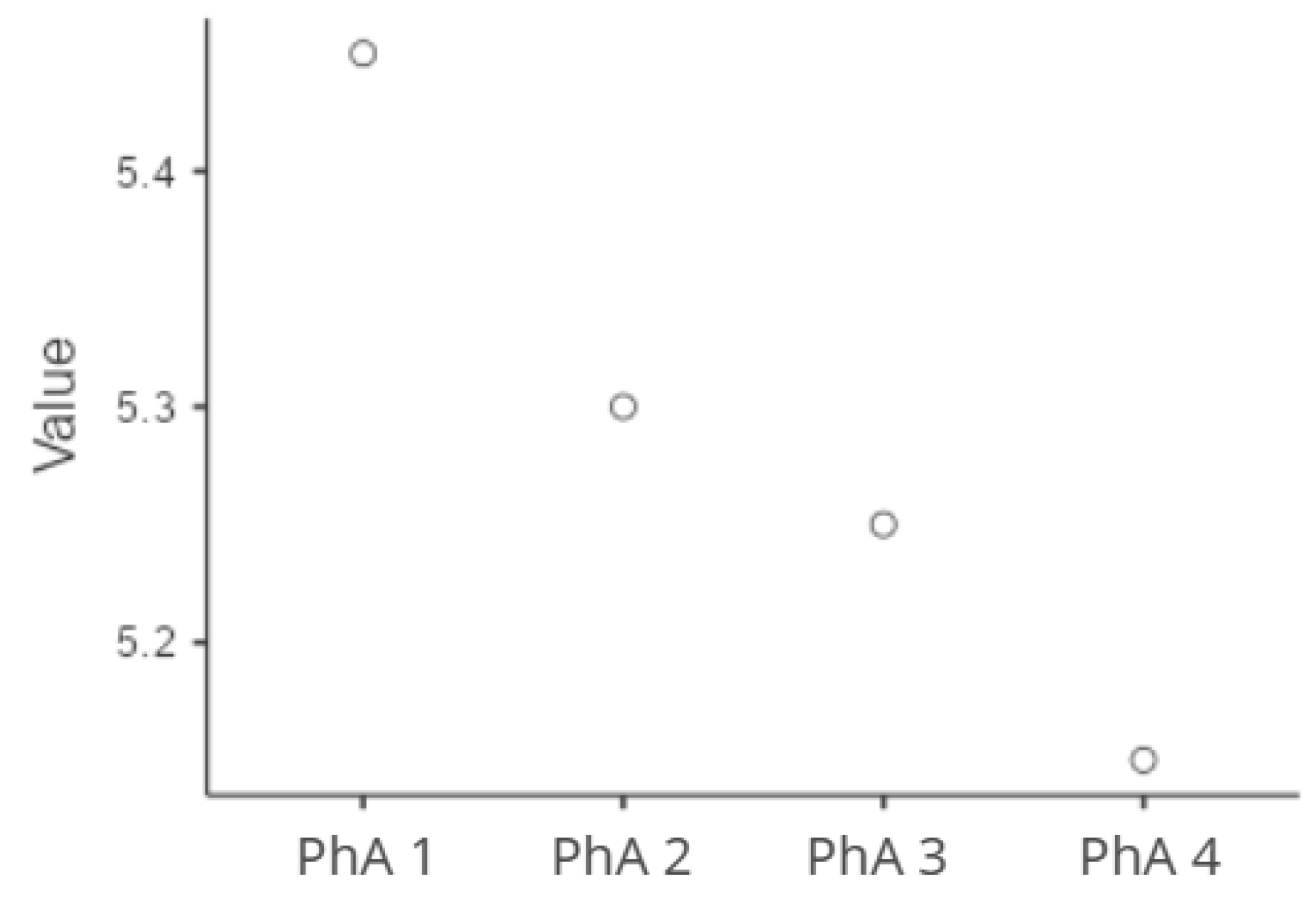
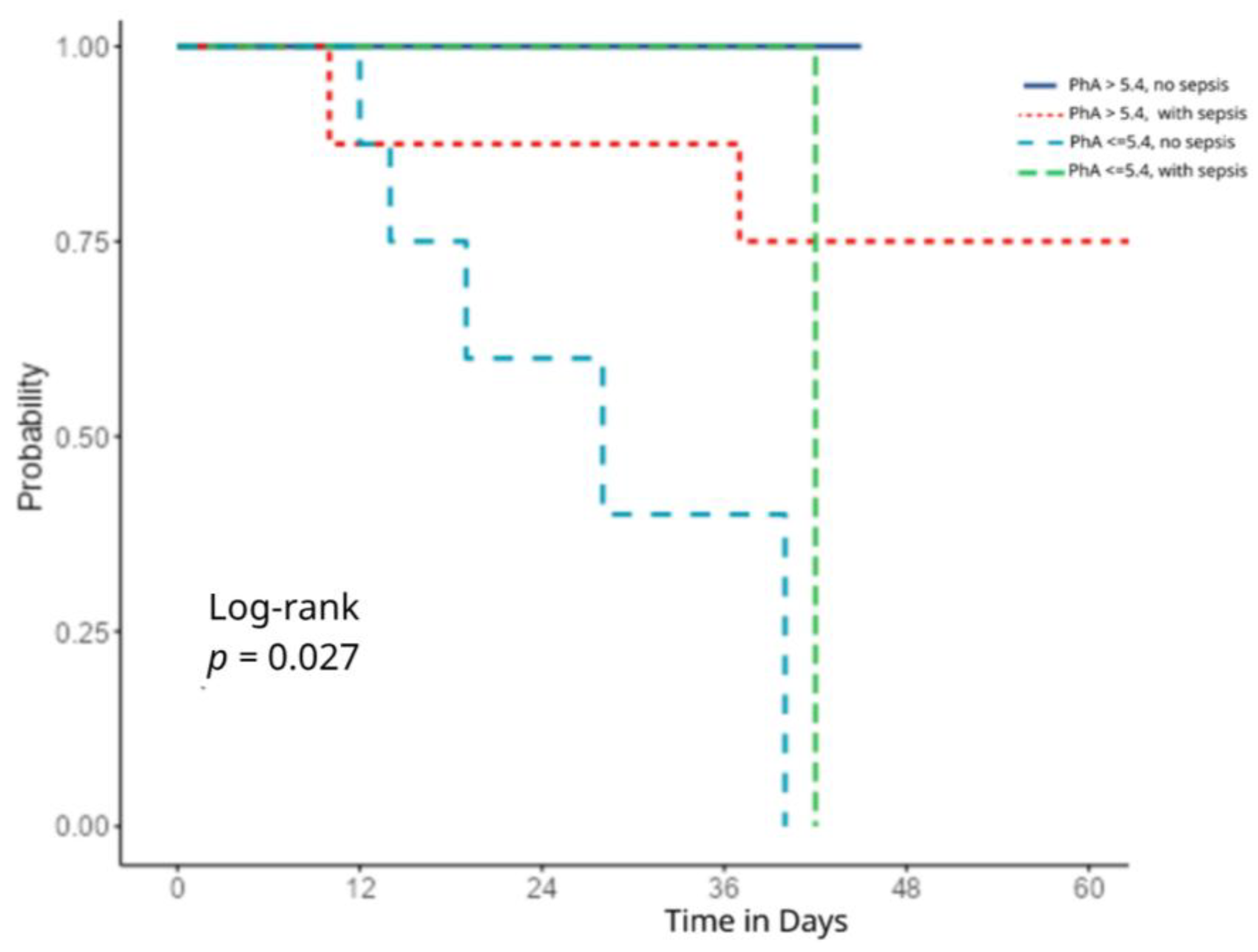
| Outcomes | Primary outcome: Association between PhA at ICU admission and in-hospital mortality. Secondary outcomes: Association of PhA with clinical severity scores (APACHE II and SOFA) on ICU admission; relationship between PhA and anthropometric/metabolic parameters; longitudinal changes in PhA during ICU stay, analyzed across four time-points; impact of PhA decline over time on survival outcomes; sepsis status |
| Phase Angle ≤ 5.4 (n = 22) | Phase Angle > 5.4 (n = 21) | p | |
|---|---|---|---|
| Age, years | 68 (13) | 49 (25) | <0.001 2 |
| Body Height, m | 1.70 (0.16) | 1.78 (0.07) | 0.08 2 |
| Body Weight, kg | 79.5 (16.5) | 82 (22) | 0.80 2 |
| BMI, kg/m2 | 26.5 (3.33) | 25.7 (4.1) | 0.11 2 |
| Body Fat, kg | 39.36 (12.7) | 31.45 (14.5) | 0.08 3 |
| Fat Free Mass, kg | 36.0 (2.75) | 44.0 (5.0) | <0.001 2 |
| Calf Circumference, cm | 32.0 (4.75) | 35.0 (1.0) | <0.001 2 |
| Extra Cellular Water, L | 26.0 (2.75) | 32.0 (3.0) | <0.001 2 |
| Mid-Upper Arm Muscle Circumference, cm | 38.0 (2.91) | 42.0 (6.4) | 0.009 2 |
| Resting Energy Expenditure, kcal | 1856 (560) | 2082 (377) | 0.07 2 |
| REE, kcal/kg | 23.13 (3.02) | 27.40 (5.88) | 0.002 2 |
| Respiratory Quotient, % | 0.82 (0.06) | 0.83 (0.04) | 0.24 2 |
| APACHE II Score | 10 (9.5) | 11 (8.0) | 0.97 2 |
| SOFA Score | 6.0 (5.7) | 7.5 (7.0) | 0.41 2 |
| Medication Number | 9.73 (2.88) | 9.05 (3.46) | 0.65 3 |
| Dyslipidemia | 8 (38.1) | 2 (9.5) | 0.03 4 |
| Hypertension | 8 (38.1) | 5 (23.8) | 0.317 4 |
| Diabetes | 3 (14.3 | 1 (4.8) | 0.29 4 |
| Coronary Disease | 3 (14.3) | 2 (9.5) | 0.63 4 |
| COPD | 2 (9.5) | 1 (4.8) | 0.54 4 |
| Chronic Renal Failure | 0 | 0 | - |
| Hepatic Failure | 0 | 1 (4.8) | 0.31 4 |
| Cancer | 2 (9.5) | 0 | 0.14 4 |
| COVID-19 | 1 (4.5) | 1 (4.8) | 0.97 4 |
| Independent Variables | HR | 95% CI | p |
|---|---|---|---|
| Sex | 0.16 | 0–18.4 | 0.45 |
| APACHE | 1.59 | 1.01–2.50 | 0.04 |
| SOFA | 0.54 | 0.24–1.22 | 0.13 |
| PhA | 0.10 | 0.01–0.76 | 0.02 |
| Sepsis | 2.92 | 0.36–24.05 | 0.31 |
| Body fat (kg) | 0.84 | 0.68–1.03 | 0.09 |
| Medication | 0.99 | 0.53–1.87 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanastasiou, P.; Chaloulakou, S.; Karayiannis, D.; Almperti, A.; Poupouzas, G.; Vrettou, C.S.; Issaris, V.; Jahaj, E.; Vassiliou, A.G.; Dimopoulou, I. Phase Angle Trajectory Among Critical Care Patients: Longitudinal Decline Predicts Mortality Independent of Clinical Severity Scores. Healthcare 2025, 13, 1463. https://doi.org/10.3390/healthcare13121463
Papanastasiou P, Chaloulakou S, Karayiannis D, Almperti A, Poupouzas G, Vrettou CS, Issaris V, Jahaj E, Vassiliou AG, Dimopoulou I. Phase Angle Trajectory Among Critical Care Patients: Longitudinal Decline Predicts Mortality Independent of Clinical Severity Scores. Healthcare. 2025; 13(12):1463. https://doi.org/10.3390/healthcare13121463
Chicago/Turabian StylePapanastasiou, Pantelis, Stavroula Chaloulakou, Dimitrios Karayiannis, Avra Almperti, Georgios Poupouzas, Charikleia S. Vrettou, Vasileios Issaris, Edison Jahaj, Alice G. Vassiliou, and Ioanna Dimopoulou. 2025. "Phase Angle Trajectory Among Critical Care Patients: Longitudinal Decline Predicts Mortality Independent of Clinical Severity Scores" Healthcare 13, no. 12: 1463. https://doi.org/10.3390/healthcare13121463
APA StylePapanastasiou, P., Chaloulakou, S., Karayiannis, D., Almperti, A., Poupouzas, G., Vrettou, C. S., Issaris, V., Jahaj, E., Vassiliou, A. G., & Dimopoulou, I. (2025). Phase Angle Trajectory Among Critical Care Patients: Longitudinal Decline Predicts Mortality Independent of Clinical Severity Scores. Healthcare, 13(12), 1463. https://doi.org/10.3390/healthcare13121463







