IL-6 Expression and the Confidence Interval-Based Estimation of Relevance (CIBER) Help Identify Persistent Inflammation and Cognitive Parameters of Executive Dysfunction in the Withdrawal Phase of Male Polydrug Abusers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. IL-6 Determination
2.3. Neuropsychological Evaluation
2.4. Data Analysis
3. Results
3.1. The Plasma Levels of IL-6 Are Increased in Polydrug Male Consumers During Abstinence
3.2. Executive Dysfunction Persists in Polydrug Males During Abstinence
3.3. Correlations Between Cognitive Task Performance and Reaction Time
3.4. Plasma IL-6 Levels Correlate to Working Memory Performance in Adult Males
3.5. Inhibition and Cognitive Flexibility Are the Most Sensitive Cognitive Parameters That Differentiate EFs’ Performance Between the Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aharonovich, E.; Hasin, D.S.; Brooks, A.C.; Liu, X.; Bisaga, A.; Nunes, E.V. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006, 81, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Aharonovich, E.; Nunes, E.; Hasin, D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003, 71, 207–211. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Editorial Panamericana; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Anthenelli, R.M.; Heffner, J.L.; Blom, T.J.; Daniel, B.E.; McKenna, B.S.; Wand, G.S. Sex differences in the ACTH and cortisol response to pharmacological probes are stressor-specific and occur regardless of alcohol dependence history. Psychoneuroendocrinology 2018, 94, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ashare, R.L.; Schmidt, H.D. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opin. Drug Discov. 2014, 9, 579–594. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Barker, M.J.; Greenwood, K.M.; Jackson, M.; Crowe, S.F. Cognitive Effects of Long-Term Benzodiazepine Use. CNS Drugs 2004, 18, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; McClellan, M.L.; Reed, B.G. Sex differences, gender and addiction. J. Neurosci. Res. 2017, 95, 136–147. [Google Scholar] [CrossRef]
- Blume, A.W.; Alan Marlatt, G. The Role of Executive Cognitive Functions in Changing Substance Use: What We Know and What We Need to Know. Ann. Behav. Med. 2009, 37, 117–125. [Google Scholar] [CrossRef]
- Brandt, L.; Chao, T.; Comer, S.D.; Levin, F.R. Pharmacotherapeutic strategies for treating cocaine use disorder—What do we have to offer? Addiction 2021, 116, 694–710. [Google Scholar] [CrossRef]
- Carroll, M.E.; Anker, J.J. Sex differences and ovarian hormones in animal models of drug dependence. Horm. Behav. 2010, 58, 44–56. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M. Neurobiology of the Adolescent Brain and Behavior: Implications for Substance Use Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Charlton, R.A.; Lamar, M.; Zhang, A.; Ren, X.; Ajilore, O.; Pandey, G.N.; Kumar, A. Associations between pro-inflammatory cytokines, learning, and memory in late-life depression and healthy aging. Int. J. Geriatr. Psychiatry 2018, 33, 104–112. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Tsai, H.-Y.; Cheng, H.-N. The effect of age on N2 and P3 components: A meta-analysis of Go/Nogo tasks. Brain Cogn. 2019, 135, 103574. [Google Scholar] [CrossRef]
- Colzato, L.S.; Huizinga, M.; Hommel, B. Recreational cocaine polydrug use impairs cognitive flexibility but not working memory. Psychopharmacology 2009, 207, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Crutzen, R.; Ygram-Peters, G.J.; Noijen, J. Using confidence interval-based estimation of relevance to select social-cognitive determinants for behavior change interventions. Front. Public Health 2017, 5, 165. [Google Scholar] [CrossRef]
- Crutzen, R.; Peters, G.J.Y. A lean method for selecting determinants when developing behavior change interventions. Health Psychol. Behav. Med. 2023, 11, 2167719. [Google Scholar] [CrossRef]
- Dean, A.C.; Kohno, M.; Hellemann, G.; London, E.D. Childhood maltreatment and amygdala connectivity in methamphetamine dependence: A pilot study. Brain Behav. 2014, 4, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.C.; Kohno, M.; Morales, A.M.; Ghahremani, D.G.; London, E.D. Denial in methamphetamine users: Associations with cognition and functional connectivity in brain. Drug Alcohol Depend. 2015, 151, 84–91. [Google Scholar] [CrossRef]
- Elenkov, I.J. Glucocorticoids and the Th1/Th2 Balance. Ann. N. Y. Acad. Sci. 2004, 1024, 138–146. [Google Scholar] [CrossRef]
- Fattore, L.; Marti, M.; Mostallino, R.; Castelli, M.P. Sex and Gender Differences in the Effects of Novel Psychoactive Substances. Brain Sci. 2020, 10, 606. [Google Scholar] [CrossRef]
- Felger, J.C.; Li, Z.; Haroon, E.; Woolwine, B.J.; Jung, M.Y.; Hu, X.; Miller, A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 2016, 21, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, M.T. Drug Abuse as a Problem of Impaired Control: Current Approaches and Findings. Behav. Cogn. Neurosci. Rev. 2003, 2, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bastías, O.; Karahanian, E. Neuroinflammation produced by heavy alcohol intake is due to loops of interactions between Toll-like 4 and TNF receptors, peroxisome proliferator-activated receptors and the central melanocortin system: A novel hypothesis and new therapeutic avenues. Neuropharmacology 2018, 128, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.C.; Sinha, R. Sex Differences in Drug-Related Stress-System Changes. Harv. Rev. Psychiatry 2009, 17, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.J.; Burgess, P.W. Executive function. Curr. Biol. 2008, 18, R110–R114. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.-K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Harrod, S.B.; Booze, R.M.; Welch, M.; Browning, C.E.; Mactutus, C.F. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol. Biochem. Behav. 2005, 82, 170–181. [Google Scholar] [CrossRef]
- Harrod, S.B.; Mactutus, C.F.; Bennett, K.; Hasselrot, U.; Wu, G.; Welch, M.; Booze, R.M. Sex differences and repeated intravenous nicotine: Behavioral sensitization and dopamine receptors. Pharmacol. Biochem. Behav. 2004, 78, 581–592. [Google Scholar] [CrossRef]
- Horner, M.D.; Harvey, R.T.; Denier, C.A. Self-report and objective measures of cognitive deficit in patients entering substance abuse treatment. Psychiatry Res. 1999, 86, 155–161. [Google Scholar] [CrossRef]
- Jacobskind, J.S.; Rosinger, Z.J.; Zuloaga, D.G. Hypothalamic-pituitary-adrenal axis responsiveness to methamphetamine is modulated by gonadectomy in males. Brain Res. 2017, 1677, 74–85. [Google Scholar] [CrossRef]
- Jankord, R.; Turk, J.R.; Schadt, J.C.; Casati, J.; Ganjam, V.K.; Price, E.M.; Keisler, D.H.; Laughlin, M.H. Sex Difference in Link between Interleukin-6 and Stress. Endocrinology 2007, 148, 3758–3764. [Google Scholar] [CrossRef] [PubMed]
- Joutsa, J.; Moussawi, K.; Siddiqi, S.H.; Abdolahi, A.; Drew, W.; Cohen, A.L.; Ross, T.J.; Deshpande, H.U.; Wang, H.Z.; Bruss, J.; et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat. Med. 2022, 28, 1249–1255. [Google Scholar] [CrossRef]
- Kampman, K.M. The treatment of cocaine use disorder. Sci. Adv. 2019, 5, eaax 1532. [Google Scholar] [CrossRef]
- Kohno, M.; Loftis, J.M.; Huckans, M.; Dennis, L.E.; McCready, H.; Hoffman, W.F. The relationship between interleukin-6 and functional connectivity in methamphetamine users. Neurosci. Lett. 2018, 677, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Morales, A.M.; Ghahremani, D.G.; Hellemann, G.; London, E.D. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry 2014, 71, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of Addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Kubera, M.; Filip, M.; Budziszewska, B.; Basta-Kaim, A.; Wydra, K.; Leskiewicz, M.; Regulska, M.; Jaworska-Feil, L.; Przegalinski, E.; Machowska, A.; et al. Immunosuppression Induced by a Conditioned Stimulus Associated with Cocaine Self-Administration. J. Pharmacol. Sci. 2008, 107, 361–369. [Google Scholar] [CrossRef]
- Lakens, D. Sample Size Justification. Collabra Psychol. 2022, 8, 33267. [Google Scholar] [CrossRef]
- Levandowski, M.L.; Hess, A.R.B.; Grassi-Oliveira, R.; de Almeida, R.M.M. Plasma interleukin-6 and executive function in crack cocaine-dependent women. Neurosci. Lett. 2016, 628, 85–90. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.; Bigler, E.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press Inc.: New York, NY, USA, 2012. [Google Scholar]
- Liu, P.; Qin, D.; Lv, H.; Fan, W.; Zhou, F.; Gao, Z.; Tao, Z.; Xu, Y. Activation of Dopamine D2 Receptor Alleviates Neuroinflammation in a Mouse Model of Allergic Rhinitis with Olfactory Dysfunction. Allergy Asthma Immunol. Res. 2021, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, K.G.; Marsland, A.L.; Cohen, S.; Gianaros, P.J. Sex differences in the association between stressor-evoked interleukin-6 reactivity and C-reactive protein. Brain Behav. Immun. 2016, 58, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Loeber, S.; Duka, T.; Welzel Marquez, H.; Nakovics, H.; Heinz, A.; Mann, K.; Flor, H. Effects of Repeated Withdrawal from Alcohol on Recovery of Cognitive Impairment under Abstinence and Rate of Relapse. Alcohol Alcohol. 2010, 45, 541–547. [Google Scholar] [CrossRef]
- Loftis, J.M.; Choi, D.; Hoffman, W.; Huckans, M.S. Methamphetamine causes persistent immune dysregulation: A cross-species, translational report. Neurotox. Res. 2011, 20, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lugones-Sanchez, C.; Crutzen, R.; Recio-Rodriguez, J.I.; Garcia-Ortiz, L. Establishing the relevance of psychological determinants regarding physical activity in people with overweight and obesity. Int. J. Clin. Health Psychol. IJCHP 2021, 21, 100250. [Google Scholar] [CrossRef]
- Luo, Y.; He, H.; Ou, Y.; Zhou, Y.; Fan, N. Elevated serum levels of TNF-α, IL-6, and IL-18 in chronic methamphetamine users. Hum. Psychopharmacol. Clin. Exp. 2022, 37, e2810. [Google Scholar] [CrossRef]
- Namba, M.D.; Leyrer-Jackson, J.M.; Nagy, E.K.; Olive, M.F.; Neisewander, J.L. Neuroimmune Mechanisms as Novel Treatment Targets for Substance Use Disorders and Associated Comorbidities. Front. Neurosci. 2021, 15, 650785. [Google Scholar] [CrossRef]
- McGregor, C.; Srisurapanont, M.; Jittiwutikarn, J.; Laobhripatr, S.; Wongtan, T.; White, J.M. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005, 100, 1320–1329. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Morein-Zamir, S.; Robbins, T.W. Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Res. 2015, 1628, 117–129. [Google Scholar] [CrossRef]
- Munro, C.A.; Saxton, J.; Butters, M.A. The neuropsychological consequences of abstinence among older alcoholics: A cross-sectional study. Alcoholism, Clin. Exp. Res. 2000, 24, 1510–1516. [Google Scholar]
- Nikbakhtzadeh, M.; Ranjbar, H.; Moradbeygi, K.; Zahedi, E.; Bayat, M.; Soti, M.; Shabani, M. Cross-talk between the HPA axis and addiction-related regions in stressful situations. Heliyon 2023, 9, e15525. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, P.; Liao, K.; Kook, Y.H.; Niu, F.; Callen, S.E.; Guo, M.-L.; Buch, S. Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol. Neurobiol. 2018, 55, 3196–3210. [Google Scholar] [CrossRef]
- Peters, G.-J.Y.; Crutzen, R. Establishing Determinant Relevance Using CIBER: An Introduction and Tutorial. 2018. Available online: https://osf.io/preprints/psyarxiv/5wjy4_v1 (accessed on 1 June 2025). [CrossRef]
- Proebstl, L.; Krause, D.; Kamp, F.; Hager, L.; Manz, K.; Schacht-Jablonowsky, M.; Straif, M.; Riebschläger, M.; Neumann, S.; Schreiber, A.; et al. Methamphetamine withdrawal and the restoration of cognitive functions—A study over a course of 6 months abstinence. Psychiatry Res. 2019, 281, 112599. [Google Scholar] [CrossRef]
- Rossi, J.-F.; Lu, Z.-Y.; Jourdan, M.; Klein, B. Interleukin-6 as a Therapeutic Target. Clin. Cancer Res. 2015, 21, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Sekine, Y.; Iyo, M.; Ouchi, Y.; Matsunaga, T.; Tsukada, H.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Takei, N.; Mori, N. Methamphetamine-Related Psychiatric Symptoms and Reduced Brain Dopamine Transporters Studied with PET. Am. J. Psychiatry 2001, 158, 1206–1214. [Google Scholar] [CrossRef]
- Sewpaul, R.; Crutzen, R.; Reddy, P. Psychosocial determinants of the intention and self-efficacy to attend antenatal appointments among pregnant adolescents and young women in Cape Town, South Africa: A cross-sectional study. BMC Public Health 2022, 22, 1809. [Google Scholar] [CrossRef]
- Sjoberg, E.A.; Cole, G.G. Sex Differences on the Go/No-Go Test of Inhibition. Arch. Sex. Behav. 2018, 47, 537–542. [Google Scholar] [CrossRef]
- Srisurapanont, M.; Lamyai, W.; Pono, K.; Indrakamhaeng, D.; Saengsin, A.; Songhong, N.; Khuwuthyakorn, P.; Jantamo, P. Cognitive impairment in methamphetamine users with recent psychosis: A cross-sectional study in Thailand. Drug Alcohol Depend. 2020, 210, 107961. [Google Scholar] [CrossRef]
- Stoet, G. PsyToolkit: A software package for programming psychological experiments using Linux. Behav. Res. Methods 2010, 42, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Teubner-Rhodes, S.; Vaden, K.I.; Dubno, J.R.; Eckert, M.A. Cognitive persistence: Development and validation of a novel measure from the Wisconsin Card Sorting Test. Neuropsychologia 2017, 102, 95–108. [Google Scholar] [CrossRef]
- Trevizol, A.P.; Brietzke, E.; Grigolon, R.B.; Subramaniapillai, M.; McIntyre, R.S.; Mansur, R.B. Peripheral interleukin-6 levels and working memory in non-obese adults: A post-hoc analysis from the CALERIE study. Nutrition 2019, 58, 18–22. [Google Scholar] [CrossRef]
- Uddin, L.Q. Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Valentine, G.; Sofuoglu, M. Cognitive Effects of Nicotine: Recent Progress. Curr. Neuropharmacol. 2018, 16, 403–414. [Google Scholar] [CrossRef]
- Vervoort, L.; Naets, T.; De Guchtenaere, A.; Tanghe, A.; Braet, C. Using confidence interval-based estimation of relevance to explore bottom-up and top-down determinants of problematic eating behavior in children and adolescents with obesity from a dual pathway perspective. Appetite 2020, 150, 104676. [Google Scholar] [CrossRef]
- Volkow, N.D.; Chang, L.; Wang, G.-J.; Fowler, J.S.; Ding, Y.-S.; Sedler, M.; Logan, J.; Franceschi, D.; Gatley, J.; Hitzemann, R.; et al. Low Level of Brain Dopamine D2 Receptors in Methamphetamine Abusers: Association with Metabolism in the Orbitofrontal Cortex. Am. J. Psychiatry 2001, 158, 12. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J. The addicted human brain: Insights from imaging studies. J. Clin. Investig. 2003, 111, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Chen, L.; Zhang, J.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in substance use disorders: A meta-analysis of 74 studies. Addiction 2020, 115, 2257–2267. [Google Scholar] [CrossRef]
- Wilson, C.J.; Finch, C.E.; Cohen, H.J. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J. Am. Geriatr. Soc. 2002, 50, 2041–2056. [Google Scholar] [CrossRef]
- Wisor, J.P.; Schmidt, M.A.; Clegern, W.C. Cerebral microglia mediate sleep/wake and neuroinflammatory effects of methamphetamine. Brain Behav. Immun. 2011, 25, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Yaple, Z.A.; Stevens, W.D.; Arsalidou, M. Meta-analyses of the n-back working memory task: fMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. NeuroImage 2019, 196, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Sutherland, S.C.; McCoy, A.W. Optimal go/no-go ratios to maximize false alarms. Behav. Res. Methods 2018, 50, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Zorick, T.; Nestor, L.; Miotto, K.; Sugar, C.; Hellemann, G.; Scanlon, G.; Rawson, R.; London, E.D. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction 2010, 105, 1809–1818. [Google Scholar] [CrossRef]
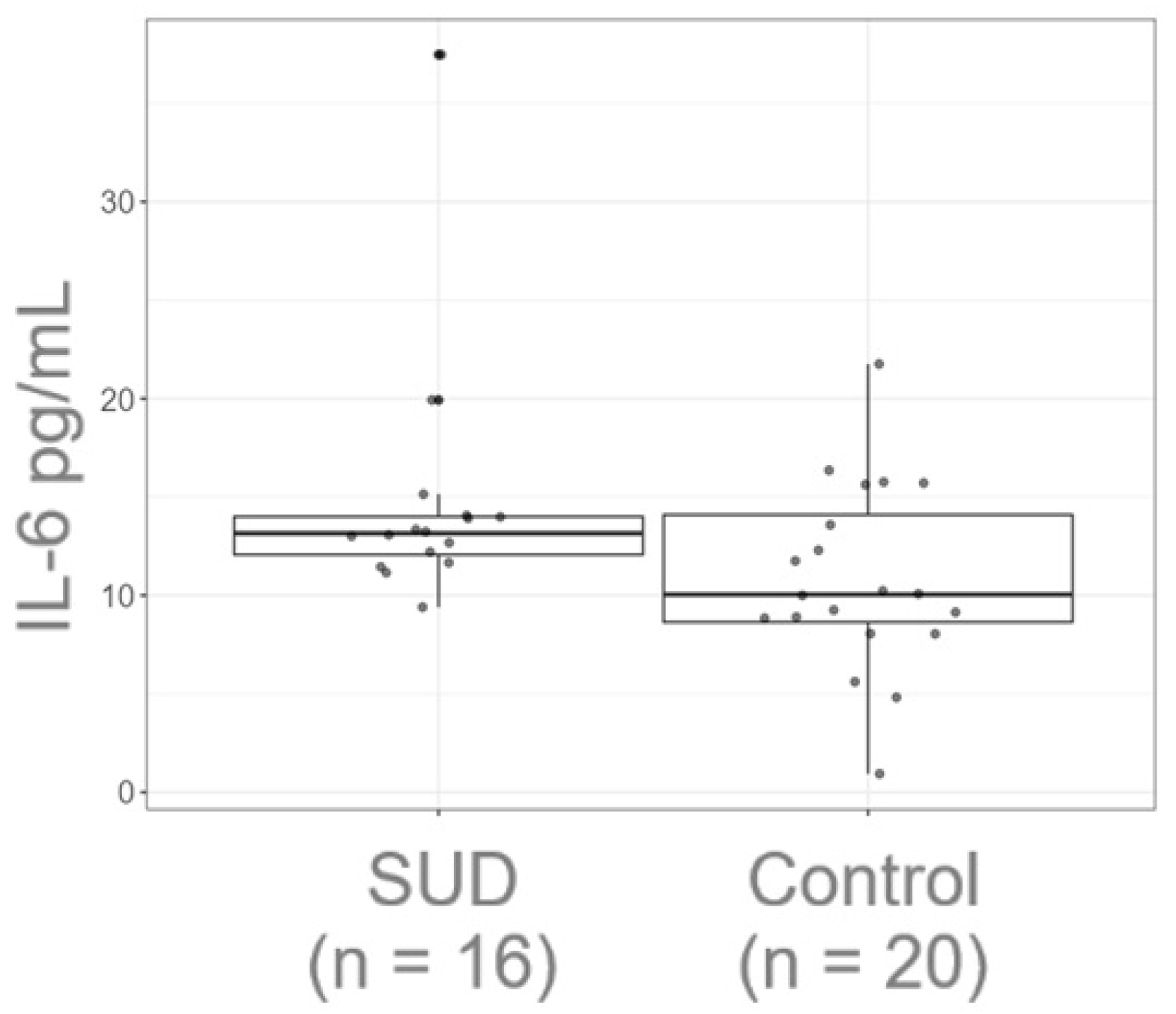
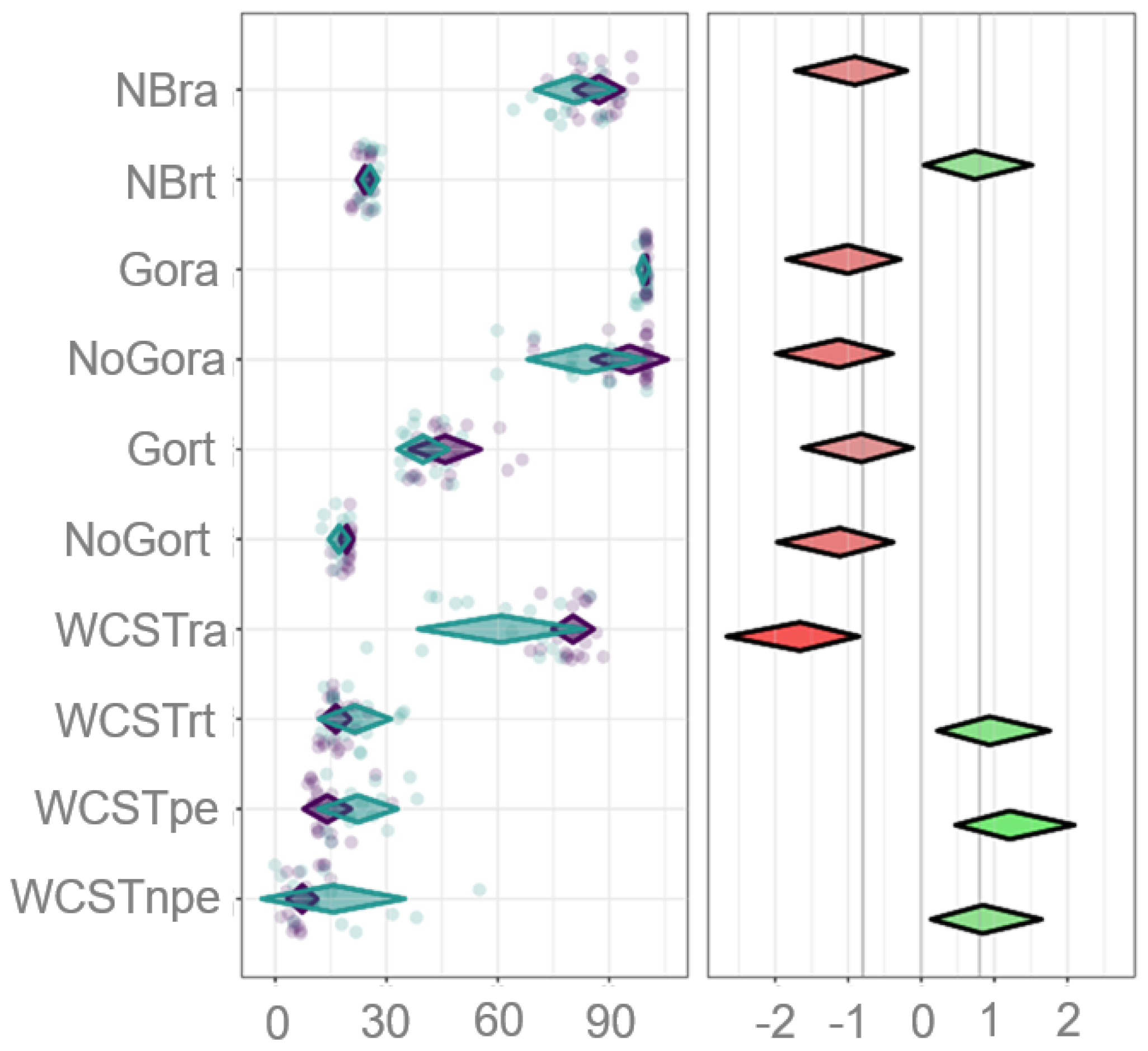
| Control Med (IQR) | SUD Med (IQR) | Statistical Test | |
|---|---|---|---|
| Age | 21.0 (2.0) | 24.5 (7.75) | U = 108.5, δ = 0.48, p = 0.15 |
| Year of schooling | 12.0 (0.0) | 12.0 (0.0) | U = 186.5, δ = 0.27, p = 0.29 |
| Days with abstinence | 171 (59.8) | ||
| Consumption type | f (%) | ||
| Alcohol | 16 (100%) | ||
| Tobacco | 16 (100%) | ||
| Inhalants | 2 (10.53%) | ||
| Cannabinoids | 6 (31.58%) | ||
| Benzodiazepines | 1 (5.26%) | ||
| Methamphetamines | 16 (100%) |
| Test | Control Med (IQR) | SUD Med (IQR) | U | p |
|---|---|---|---|---|
| NBack right answer | 88.66 (82.33–92.00) | 80 (74.67–88.00) | 86 | 0.019 * |
| NBack reaction time | 25.00 (22.36–25.59) | 25.96 (24.17–26.66) | 94 | 0.037 * |
| Go-no-go right answer Go | 100 (100–100) | 100 (97.50–100) | 110 | 0.009 ** |
| Go-no-go right answer no-go | 100 (97.50–100) | 90 (80.00–90.00) | 58.5 | 0.001 * |
| Go-no-go reaction time Go | 43.91 (38.39–48.13) | 37–56 (36.50–44.03) | 88 | 0.023 * |
| Go-no-go reaction time no-go | 20.00 (19.59–20.00) | 18.27(16.49–18.31) | 60 | 0.001 *** |
| WCST right answer | 81.66 (78.33–83.33) | 65.83 (47.08–73.75) | 35 | 0.001 *** |
| WCST reaction time | 15.82 (13.65–17.96) | 20.59 (15.36–24) | 101 | 0.063 |
| WCST perseverative errors | 11.66 (10–15) | 20 (15.00–27.08) | 53.5 | 0.001 *** |
| WCST non-perseverative errors | 6.66 (5.00–8.75) | 10 (6.25–19.17) | 99 | 0.053 |
| Dimension | SUD | Control | Correlation |
|---|---|---|---|
| Interleukin 6 | Impaired | Preserved | |
| Working memory | Impaired | Preserved | |
| Reaction times | + | ||
| Right Answers | − | ||
| Inhibitory control | Impaired | Preserved | |
| Reaction times | + | ||
| Right Answers | − | ||
| Cognitive flexibility | Impaired | Preserved | |
| Reaction times | − | ||
| Right Answers | + | ||
| Non perseverative errors | + | ||
| Perseverative errors | + |
| Dependent Variable | Sum of Squares | df | Mean Square | F | p | |
|---|---|---|---|---|---|---|
| Group | NBra | 360.29 | 1 | 360.287 | 6.98 | 0.012 |
| NBrt | 15.74 | 1 | 15.737 | 4.56 | 0.04 | |
| Gora | 5.43 | 1 | 5.425 | 8.59 | 0.006 | |
| NoGora | 1227.22 | 1 | 1227.222 | 10.78 | 0.002 | |
| Gort | 320.49 | 1 | 320.494 | 5.76 | 0.022 | |
| NoGort | 35.84 | 1 | 35.841 | 10.54 | 0.003 | |
| WCSTra | 3351.17 | 1 | 3351.173 | 23.4 | <.001 | |
| WCSTrt | 236.16 | 1 | 236.156 | 7.42 | 0.01 | |
| WCSTpe | 611.13 | 1 | 611.127 | 12.46 | 0.001 | |
| WCSTnpe | 623.47 | 1 | 623.472 | 6.01 | 0.02 | |
| Residuals | NBra | 1753.85 | 34 | 51.584 | ||
| NBrt | 117.28 | 34 | 3.449 | |||
| Gora | 21.48 | 34 | 0.632 | |||
| NoGora | 3870 | 34 | 113.824 | |||
| Gort | 1890.5 | 34 | 55.603 | |||
| NoGort | 115.58 | 34 | 3.4 | |||
| WCSTra | 4868.19 | 34 | 143.182 | |||
| WCSTrt | 1081.98 | 34 | 31.823 | |||
| WCSTpe | 1668.19 | 34 | 49.065 | |||
| WCSTnpe | 3528.61 | 34 | 103.783 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-González, J.; Galvez-Contreras, A.; Jimenez-Navarro, I.; Perez-Alcaraz, I.; Gonzalez-Perez, O.; Gonzalez-Castañeda, R.E. IL-6 Expression and the Confidence Interval-Based Estimation of Relevance (CIBER) Help Identify Persistent Inflammation and Cognitive Parameters of Executive Dysfunction in the Withdrawal Phase of Male Polydrug Abusers. Healthcare 2025, 13, 1462. https://doi.org/10.3390/healthcare13121462
Guzmán-González J, Galvez-Contreras A, Jimenez-Navarro I, Perez-Alcaraz I, Gonzalez-Perez O, Gonzalez-Castañeda RE. IL-6 Expression and the Confidence Interval-Based Estimation of Relevance (CIBER) Help Identify Persistent Inflammation and Cognitive Parameters of Executive Dysfunction in the Withdrawal Phase of Male Polydrug Abusers. Healthcare. 2025; 13(12):1462. https://doi.org/10.3390/healthcare13121462
Chicago/Turabian StyleGuzmán-González, Jesua, Alma Galvez-Contreras, Israel Jimenez-Navarro, Iris Perez-Alcaraz, Oscar Gonzalez-Perez, and Rocio E. Gonzalez-Castañeda. 2025. "IL-6 Expression and the Confidence Interval-Based Estimation of Relevance (CIBER) Help Identify Persistent Inflammation and Cognitive Parameters of Executive Dysfunction in the Withdrawal Phase of Male Polydrug Abusers" Healthcare 13, no. 12: 1462. https://doi.org/10.3390/healthcare13121462
APA StyleGuzmán-González, J., Galvez-Contreras, A., Jimenez-Navarro, I., Perez-Alcaraz, I., Gonzalez-Perez, O., & Gonzalez-Castañeda, R. E. (2025). IL-6 Expression and the Confidence Interval-Based Estimation of Relevance (CIBER) Help Identify Persistent Inflammation and Cognitive Parameters of Executive Dysfunction in the Withdrawal Phase of Male Polydrug Abusers. Healthcare, 13(12), 1462. https://doi.org/10.3390/healthcare13121462








