Maternal Nutrition and Gestational Weight Gain Among Saudi Women: Riyadh Mother and Baby Follow Up Study (RAHMA Explore)
Abstract
1. Introduction
- To investigate the nutritional status and the nutritional risks of Saudi pregnant women during the three trimesters of pregnancy using the FIGO nutritional checklist.
- To investigate the effect of the nutritional risks on GWG.
2. Methods
2.1. Study Design and Setting
2.2. Study Participants
2.3. Sampling Technique and Sample Size
2.4. Study Tool
2.5. Definitions
- -
- Fasting plasma glucose 5.1–6.9 mmol/L (92–125 mg/dL);
- -
- 1 h plasma glucose ≥10.0 mmol/L (180 mg/dL) following a 75 g oral glucose load;
- -
- 2 h plasma glucose 8.5–11.0 mmol/L (153–199 mg/dL) following a 75 g oral glucose load.
2.6. Statistical Analysis
2.7. Ethical Consideration
- Informed Consent Statement
3. Results
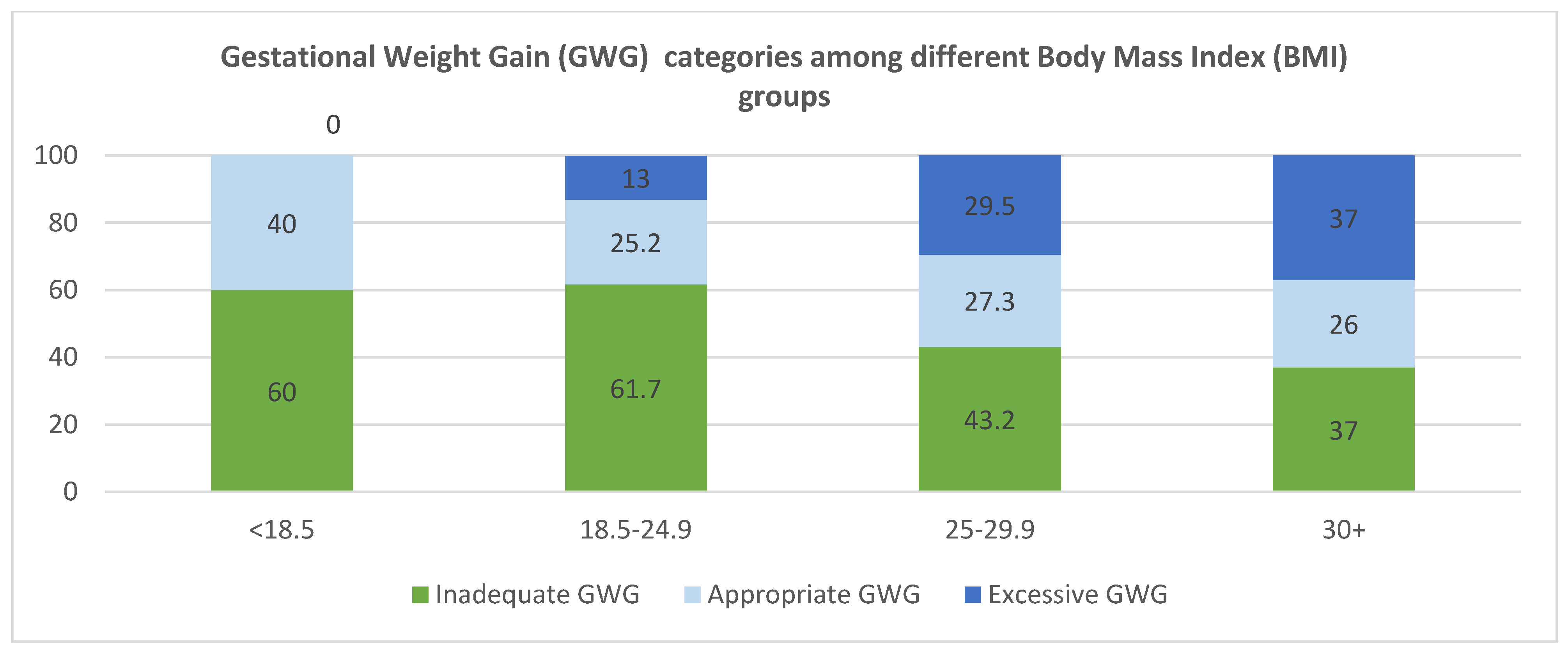
Gestational Weight Gain Among Participants at Term
4. Discussion
4.1. Implication to Practice and Research
The Results of This Study Suggest
- There is an urgent need for the implementation of nutritional assessment and counselling program for pregnant Saudi mothers, preferably to cover preconception, antenatal and postnatal periods, considering that 96% of the study cohort had at least one nutritional deficiency risk.
- Given the unique nutritional deficiency profile of Saudi mothers, healthcare providers should have the knowledge to manage all variations of maternal nutritional deficiencies and to provide counselling and advice.
- Estimation of vitamin D level should be included in the antenatal screening tests in Saudi Arabia and guidelines for replacement therapy should be available, considering that exposure to sunlight is one of the least positive responses in this study.
- Regular monitoring of GWG during pregnancy and counselling mothers to achieve proper GWG based on their BMI should be enforced considering that 50% of the mothers in this study group had inadequate GWG.
- Further research should be directed towards the investigation of the effects of nutritional deficiencies on the maternal and fetal outcomes of pregnancy in Saudi Arabia, especially vitamin D deficiency.
- Investigation of the associations and the outcomes of inadequate GWG among Saudi women.
- It is important to explore the opinions of the mothers and their families about healthy nutrition during pregnancy, which will form a solid base for successful evidence-based interventions and will place the mother at the center of care.
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miele, M.J.; Souza, R.T.; Calderon, I.M.; Feitosa, F.E.; Leite, D.F.; Rocha Filho, E.A.; Vettorazzi, J.; Mayrink, J.; Fernandes, K.G.; Vieira, M.C.; et al. Maternal nutrition status associated with pregnancy-related adverse outcomes. Nutrients 2021, 13, 2398. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.; Wickramasinghe, K.; Demaio, A.R.; Roberts, N.; Perez-Blanco, K.-M.; Noonan, K.; Townsend, N. The impact of maternal nutrition on offspring’s risk of non-communicable diseases in adulthood: A systematic review. J. Glob. Health 2019, 9, 020405. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal nutrition and neurodevelopment: A scoping review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Desai, M.; Ross, M.G. Maternal-infant nutrition and development programming of offspring appetite and obesity. Nutr. Rev. 2020, 78 (Suppl. 2), 25–31. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Perumal, N.; Wang, D.; Darling, A.M.; Liu, E.; Wang, M.; Ahmed, T.; Christian, P.; Dewey, K.G.; Kac, G.; Kennedy, S.H.; et al. Suboptimal gestational weight gain and neonatal outcomes in low and middle income countries: Individual participant data meta-analysis. BMJ 2023, 382, e072249. [Google Scholar] [CrossRef]
- Carolan-Olah, M.; Duarte-Gardea, M.; Lechuga, J. A critical review: Early life nutrition and prenatal programming for adult disease. J. Clin. Nurs. 2015, 24, 3716–3729. [Google Scholar] [CrossRef]
- Rinaudo, P.; Wang, E. Fetal programming and metabolic syndrome. Annu. Rev. Physiol. 2012, 74, 107–130. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Young, M.F.; Ramakrishnan, U. Maternal undernutrition before and during pregnancy and offspring health and development. Ann. Nutr. Metab. 2021, 76 (Suppl. 3), 41–53. [Google Scholar] [CrossRef]
- Konje, E.T.; Ngaila, B.V.; Kihunrwa, A.; Mugassa, S.; Basinda, N.; Dewey, D. High prevalence of anemia and poor compliance with preventive strategies among pregnant women in Mwanza City, Northwest Tanzania: A hospital-based cross-sectional study. Nutrients 2022, 14, 3850. [Google Scholar] [CrossRef] [PubMed]
- Checklis IFoGaOFN. International Federation of Gynecology and Obstetrics. FIGO Nutrition Checklist. Available online: https://www.figo.org/resource/figo-nutrition-checklist-available-12-different-languages-pdf-downloadable-format (accessed on 10 June 2025).
- Wahabi, H.; Esmaeil, S.; Fayed, A. maternal prepregnancy weight and pregnancy outcomes in Saudi women: Subgroup analysis from Riyadh Mother and Baby Cohort Study (RAHMA). BioMed Res. Int. 2021, 2021, 6655942. [Google Scholar] [CrossRef] [PubMed]
- Fayed, A.; Wahabi, H.A.; Esmaeil, S.; Elkouny, R.; Elmorshedy, H.; Bakhsh, H.; Brownie, S.M. Independent effect of gestational weight gain and prepregnancy obesity on pregnancy outcomes among Saudi women: A sub-cohort analysis from Riyadh mother and baby cohort study (RAHMA). PLoS ONE 2022, 17, e0262437. [Google Scholar] [CrossRef] [PubMed]
- Wahabi, H.A.; Fayed, A.A.; Tharkar, S.; Esmaeil, S.A.; Bakhsh, H. Postpartum Weight Retention and Cardiometabolic Risk among Saudi Women: A Follow-Up Study of RAHMA Subcohort. BioMed Res. Int. 2019, 2019, 2957429. [Google Scholar] [CrossRef]
- Wahabi, H.; Fayed, A.; Esmaeil, S.; Alzeidan, R.; Elawad, M.; Tabassum, R.; Hansoti, S.; Magzoup, M.E.; Al-Kadri, H.; Elsherif, E.; et al. Riyadh mother and baby multicenter cohort study: The Cohort Profile. PLoS ONE 2016, 11, e0150297. [Google Scholar]
- Wahabi, H.; Elmorshedy, H.; Amer, Y.S.; Saeed, E.; Razak, A.; Hamama, I.A.; Hadid, A.; Ahmed, S.; Aleban, S.A.; Aldawish, R.A.; et al. Neonatal Birthweight Spectrum: Maternal Risk Factors and Pregnancy Outcomes in Saudi Arabia. Medicina 2024, 60, 193. [Google Scholar] [CrossRef]
- Obstetrics TIFoGa. TR_EN_ARABIC _FIGO Nutrition Checklist: FIGO, 2023. Available online: https://www.figo.org/sites/default/files/2024-05/TR_EN_ARABIC_20.09.23_FIGO%20nutrition%20checklist%202024.pdf (accessed on 10 June 2025).
- Gilmore, L.A.; Redman, L.M. Weight gain in pregnancy and application of the 2009 IOM guidelines: Toward a uniform approach. Obesity 2015, 23, 507–511. [Google Scholar] [CrossRef]
- López Stewart, G. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy: A World Health Organization Guideline; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Program NHBPE. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am. J. Obstet. Gynecol. 2000, 183, s1–s22. [Google Scholar] [CrossRef]
- Killeen, S.L.; Callaghan, S.L.; Jacob, C.M.; Hanson, M.A.; McAuliffe, F.M. Examining the use of the FIGO nutrition checklist in routine antenatal practice: Multistakeholder feedback to implementation. Int. J. Gynecol. Obstet. 2020, 151, 51–56. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Nigdelis, M.P.; Haidich, A.-B.; Kyrezi, M.; Ntine, H.; Papaioannou, M.; Mintziori, G.; Bogdanos, D.P.; Mavromatidis, G.; Goulis, D.G. Diet Quality and Nutritional Risk based on the FIGO Nutrition Checklist among Greek pregnant women: A cross-sectional routine Antenatal Care Study. Nutrients 2023, 15, 2019. [Google Scholar] [CrossRef]
- Tsoi, K.Y.; Chan, R.S.; Li, L.S.; McAuliffe, F.M.; Hanson, M.A.; Tam, W.H.; Ma, R.C. Evaluation of dietary pattern in early pregnancy using the FIGO Nutrition Checklist compared to a food frequency questionnaire. Int. J. Gynecol. Obstet. 2020, 151, 37–44. [Google Scholar] [CrossRef]
- Killeen, S.L.; Donnellan, N.; O’Reilly, S.L.; Hanson, M.A.; Rosser, M.L.; Medina, V.P.; Jacob, C.M.; Divakar, H.; Hod, M.; Poon, L.C. Using FIGO Nutrition Checklist counselling in pregnancy: A review to support healthcare professionals. Int. J. Gynecol. Obstet. 2023, 160, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Stephanou, A.; Rayman, M.P. Dietary factors that affect the risk of pre-eclampsia. BMJ Nutr. Prev. Health 2022, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, P.; Frank, G.; Cianci, R.; Dominici, F.; Mappa, I.; Rizzo, G.; De Santis, G.L.; Bigioni, G.; Di Renzo, L. Fish Consumption and DHA Supplementation during Pregnancy: Study of Gestational and Neonatal Outcomes. Nutrients 2024, 16, 3051. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Brown, K.R.; Maslin, K.; Palmer, D.J. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr. Allergy Immunol. 2017, 28, 135–143. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Li, X.; Kang, T.; Cui, Z.; Bo, Y.; Liu, Y.; Ullah, A.; Suo, X.; Chen, H.; Lyu, Q. The association between dietary patterns before pregnancy and gestational diabetes mellitus: A matched case-control study in China. Asia Pac. J. Clin. Nutr. 2024, 33, 424. [Google Scholar]
- Xu, J.; Wang, H.; Bian, J.; Xu, M.; Jiang, N.; Luo, W.; Zu, P.; Yin, W.; Zhu, P. Association between the maternal mediterranean diet and perinatal outcomes: A systematic review and meta-analysis. Adv. Nutr. 2024, 15, 100159. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Obara, T.; Yamashita, T.; Sugawara, J.; Ishikuro, M.; Murakami, K.; Noda, A.; Ueno, F.; Suzuki, S.; Suganuma, H.; et al. Fruit and vegetable consumption before and during pregnancy and birth weight of new-borns in Japan: The Tohoku medical megabank project birth and three-generation cohort study. Nutr. J. 2020, 19, 80. [Google Scholar] [CrossRef]
- Ramόn, R.; Ballester, F.; Iñiguez, C.; Rebagliato, M.; Murcia, M.; Esplugues, A.; Marco, A.; de la Hera, M.G.; Vioque, J. Vegetable but not fruit intake during pregnancy is associated with newborn anthropometric measures. J. Nutr. 2009, 139, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Al-Musharaf, S.; Fouda, M.A.; Turkestani, I.Z.; Al-Ajlan, A.; Sabico, S.; Alnaami, A.M.; Wani, K.; Hussain, S.D.; Alraqebah, B.; Al-Serehi, A.; et al. Vitamin D deficiency prevalence and predictors in early pregnancy among Arab women. Nutrients 2018, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.A.; Turkestani, I.Z.; Almusharraf, S.; Al-Ajlan, A.; Angkaya-Bagayawa, F.F.; Sabico, S.; Mohammed, A.G.; Hassanato, R.; Al-Serehi, A.; Alshingetti, N.M.; et al. Extremely High Prevalence of Maternal and Neonatal Vitamin D Deficiency in the Arab Population. Neonatology 2017, 112, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajlan, A.; Al-Musharaf, S.; Fouda, M.A.; Krishnaswamy, S.; Wani, K.; Aljohani, N.J.; Al-Serehi, A.; Sheshah, E.; Alshingetti, N.M.; Turkistani, I.Z.; et al. Lower vitamin D levels in Saudi pregnant women are associated with higher risk of developing GDM. BMC Pregnancy Childbirth 2018, 18, 86. [Google Scholar] [CrossRef]
- Al-Shaikh, G.K.; Ibrahim, G.H.; Fayed, A.A.; Al-Mandeel, H. Impact of vitamin D deficiency on maternal and birth outcomes in the Saudi population: A cross-sectional study. BMC Pregnancy Childbirth 2016, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Cliffer, I.; Darling, A.M.; Madzorera, I.; Wang, D.; Perumal, N.; Wang, M.; Liu, E.; Pembe, A.B.; Urassa, W.; Fawzi, W.W. Associations of diet quality, socioeconomic factors, and nutritional status with gestational weight gain among pregnant women in Dar es Salaam, Tanzania. Curr. Dev. Nutr. 2023, 7, 100041. [Google Scholar] [CrossRef]
- Diemert, A.; Lezius, S.; Pagenkemper, M.; Hansen, G.; Drozdowska, A.; Hecher, K.; Arck, P.; Zyriax, B.C. Maternal nutrition, inadequate gestational weight gain and birth weight: Results from a prospective birth cohort. BMC Pregnancy Childbirth 2016, 16, 224. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. J. Am. Med. Assoc. 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Kapadia, M.Z.; Gaston, A.; Van Blyderveen, S.; Schmidt, L.; Beyene, J.; McDonald, H.; McDonald, S. Psychological factors and trimester-specific gestational weight gain: A systematic review. J. Psychosom. Obstet. Gynaecol. 2015, 36, 15–22. [Google Scholar] [CrossRef]
- Catov, J.M.; Sun, B.; Lewis, C.E.; Bertolet, M.; Gunderson, E.P. Prepregnancy weight change associated with high gestational weight gain. Obesity 2022, 30, 524–534. [Google Scholar] [CrossRef]
- Widen, E.M.; Whyatt, R.M.; Hoepner, L.A.; Ramirez-Carvey, J.; Oberfield, S.E.; Hassoun, A.; Perera, F.P.; Gallagher, D.; Rundle, A.G. Excessive gestational weight gain is associated with long-term body fat and weight retention at 7 y postpartum in African American and Dominican mothers with underweight, normal, and overweight prepregnancy BMI. Am. J. Clin. Nutr. 2015, 102, 1460–1467. [Google Scholar] [CrossRef]
| Frequency (N) | Percentage (%) | ||
|---|---|---|---|
| Age (years) | less than 20 | 5 | 0.9 |
| 21–35 | 411 | 72.4 | |
| 36+ | 152 | 26.8 | |
| Education | School | 182 | 31.9 |
| University | 341 | 59.8 | |
| Postgraduate | 47 | 8.2 | |
| Job | Student | 22 | 3.9 |
| Housewife | 374 | 65.8 | |
| Employee | 172 | 30.3 | |
| Family Income | Enough and save | 125 | 21.9 |
| Enough | 336 | 58.9 | |
| Not enough | 104 | 18.2 | |
| In debt | 5 | 0.9 | |
| Trimester | 1st trimester | 78 | 13.7 |
| 2nd trimester | 190 | 33.3 | |
| 3rd trimester | 302 | 53.0 | |
| Pre-pregnancy BMI (kg/m2) | <18.5 | 20 | 3.7 |
| 18.5–24.9 | 210 | 38.4 | |
| 25–29.9 | 176 | 32.2 | |
| 30+ | 141 | 25.8 | |
| Diabetes Mellitus | no | 548 | 96.1 |
| yes | 22 | 3.9 | |
| Gestational Diabetes | no | 502 | 88.1 |
| yes | 68 | 11.9 | |
| Hypertension | no | 558 | 97.9 |
| yes | 12 | 2.1 | |
| Hyperemesis gravida | no | 563 | 98.8 |
| yes | 7 | 1.2 | |
| Data are presented as frequency and percentages | |||
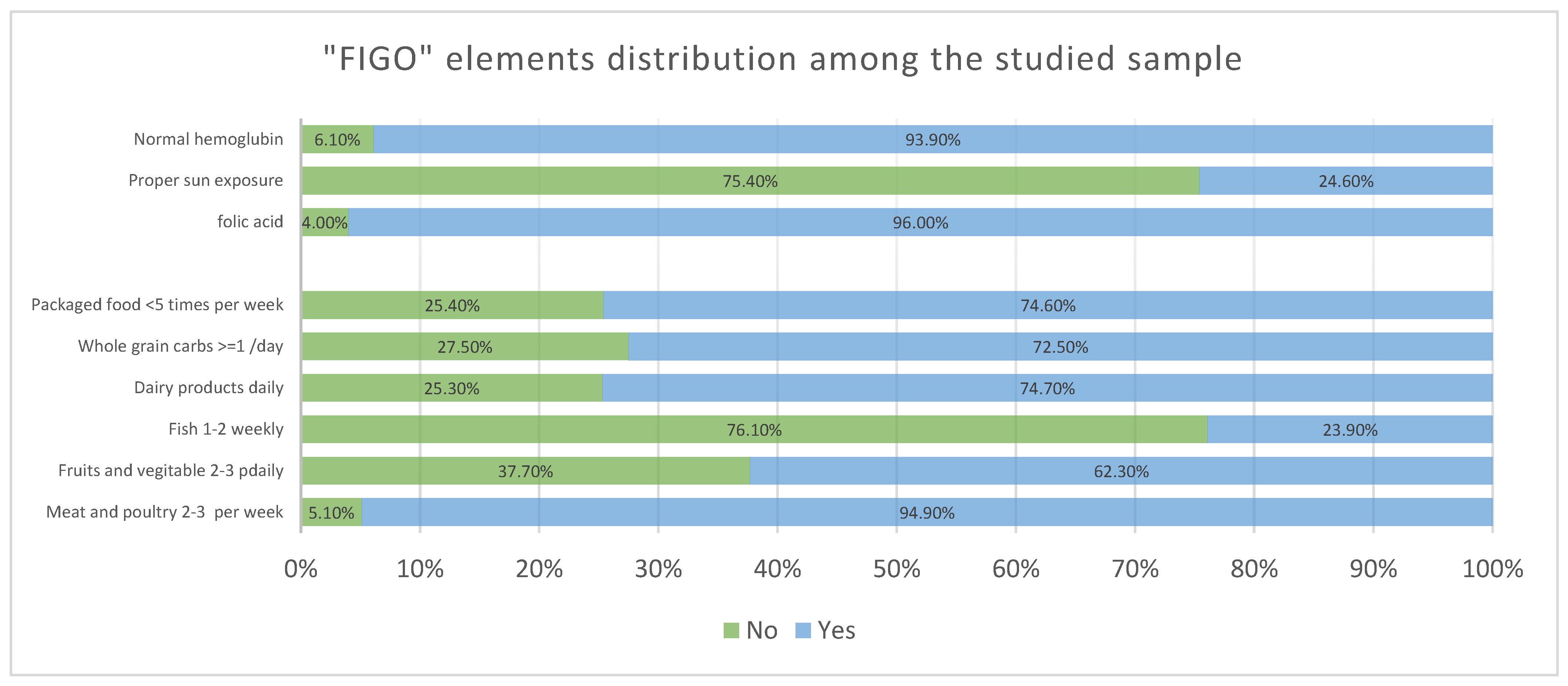
| Positive Answers | ||
|---|---|---|
| Frequency (N) | Percentage (%) | |
| Diet Quality Score (FFQ) | ||
| Meat and poultry 2–3 per week | 541 | 94.9 |
| Fruits and vegetable 2–3 daily | 355 | 62.3 |
| Fish 1–2 weekly | 136 | 23.9 |
| Dairy products daily | 426 | 74.7 |
| Whole grain carbs ≥ 1/day | 413 | 72.5 |
| Packaged food <5 times per week | 425 | 74.6 |
| No negative answers | 1 | 0.2 |
| One or more negative answers | 569 | 99.8 |
| Poor quality FFQ (≤3) | 173 | 30.4 |
| Good quality FFQ (>3) | 397 | 69.6 |
| Food Supplements | ||
| Proper intake of folic acid | 547 | 96.0 |
| Enough sun exposure | 140 | 24.6 |
| No anemia | 535 | 93.9 |
| No negative answers | 0 | 0.0 |
| One or more negative answers | 570 | 100.0 |
| FIGO—overall nutritional risk score (NRS) (Median, Interquartile Range) | (6, 5–7) | |
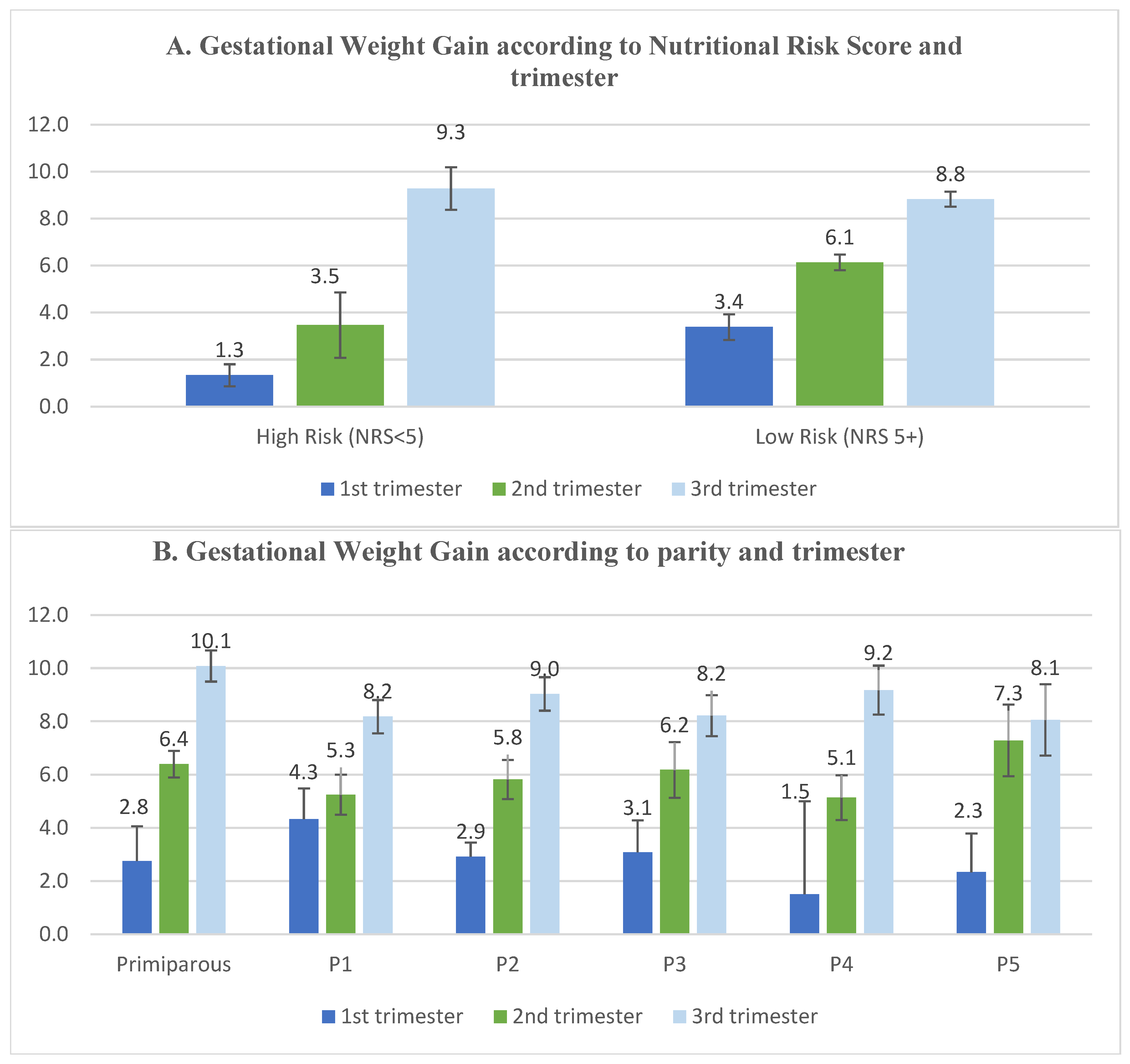
| High Risk (NRS < 5) | 62 | 10.9 |
| Low Risk (NRS ≥ 5) | 508 | 89.1 |
| Trimester | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| 1st Trimester | 2nd Trimester | 3rd Trimester | |||||
| N | % | N | % | N | % | ||
| Food Frequency items (FFQ) | |||||||
| Meat and poultry 2–3 per week | 69 | 88.5 | 180 | 94.7 | 292 | 96.7 | 0.01 |
| Fruits and vegetables 2–3 daily | 43 | 55.1 | 122 | 64.2 | 190 | 62.9 | 0.36 |
| Fish 1–2 weekly | 16 | 20.5 | 43 | 22.6 | 77 | 25.5 | 0.58 |
| dairy products daily | 59 | 75.6 | 141 | 74.2 | 226 | 74.8 | 0.97 |
| Whole grain carbs ≥ 1/day | 55 | 70.5 | 140 | 73.7 | 218 | 72.2 | 0.86 |
| packaged food <5 times per week | 60 | 76.9 | 137 | 72.1 | 228 | 75.5 | 0.62 |
| FFQ (>3 good quality) | 47 | 60.3 | 135 | 71.1 | 215 | 71.2 | 0.15 |
| FFQ (≤3 poor quality) | 31 | 39.7 | 55 | 28.9 | 87 | 28.8 | |
| Food Supplements | |||||||
| Folic acid intake | 71 | 91.0 | 185 | 97.4 | 291 | 96.4 | 0.05 |
| Good sun exposure | 18 | 23.1 | 57 | 30.0 | 65 | 21.5 | 0.09 |
| Normal hemoglobin | 75 | 96.2 | 177 | 93.2 | 283 | 93.7 | 0.64 |
| Total FIGO Score | |||||||
| High Risk NRS (<5) | 9 | 11.5 | 17 | 8.9 | 36 | 11.9 | 0.58 |
| Low Risk NRS (≥5) | 69 | 88.5 | 173 | 91.1 | 266 | 88.1 | |
| GWG (in Kg) (mean ± SD) | 3.14 ± 4.27 | 5.92 ± 4.43 | 8.88 ± 5.10 | <0.01 | |||
| FFQ | NRS | ||||
|---|---|---|---|---|---|
| Poor Quality (≤3) N (%) | Good Quality (>3) N % | High Risk (<5) N (%) | Low Risk (≥5) N (%) | ||
| Age (years) | less than 20 | 2 (40.0) | 3 (60.0) | 0 (0.0) | 5 (100) |
| 21–35 | 132 (32.1) | 279 (67.9) | 50 (12.2) | 361 (87.8) | |
| 36+ | 38 (25.0) | 114 (75.0) | 12 (7.9) | 140 (92.1) | |
| p-value | 0.23 | 0.26 | |||
| Education attainment | School | 52 (28.6) | 130 (71.4) | 20 (11.0) | 162 (89.0) |
| University | 108 (31.7) | 233 (68.3) | 38 (11.1) | 303 (88.9) | |
| Postgraduate | 13 (27.7) | 34 (72.3) | 4 (8.5) | 43 (91.5) | |
| p-value | 0.96 | 0.86 | |||
| Occupation | Student | 8 (36.4) | 14 (63.6) | 3 (13.6) | 19 (86.4) |
| Housewife | 111 (29.7) | 263 (70.3) | 41 (11.0) | 333 (89.0) | |
| Employee | 52 (30.2) | 120 (69.8) | 16 (9.3) | 156 (90.7) | |
| p-value | 0.81 | 0.75 | |||
| Family income | Enough and save | 39 (31.2) | 86 (68.8) | 17 (13.7) | 108 (86.4) |
| Enough | 100 (29.8) | 236 (70.2) | 28 (8.3) | 308 (91.7) | |
| Not enough | 33 (31.7) | 71 (68.3) | 17 (16.3) | 87 (83.7) | |
| In debt | 1 (20.0) | 4 (80.0) | 0 (0.0) | 5 (100.0) | |
| p-value | 0.93 | 0.07 | |||
| Pre-pregnancy BMI (kg/m2) | <18.5 | 6 (30.0) | 14 (70.0) | 2 (10.0) | 18 (90.0) |
| 18.5–24.9 | 64 (30.5) | 146 (69.5) | 22 (10.5) | 188 (89.5) | |
| 25–29.9 | 52 (29.5) | 124 (70.5) | 15 (8.5) | 161 (91.5) | |
| 30+ | 41 (29.1) | 100 (70.9) | 17 (12.1) | 124 (87.9) | |
| p-value | 0.99 | 0.78 | |||
| GWG | Adequate | 36 (27.9) | 93 (72.1) | 10 (7.8) | 119 (92.2) |
| Below | 102 (31.4) | 223 (68.6) | 39 (12.0) | 286 (88.0) | |
| Above | 25 (29.1) | 61 (70.9) | 7 (8.1) | 79 (91.9) | |
| p-value | 0.77 | 0.31 | |||
| Diabetes Mellitus | No | 166 (30.3) | 382 (69.7) | 60 (10.9) | 488 (89.1) |
| Yes | 7 (31.8) | 15 (68.2) | 3 (4.4) | 65 (95.6) | |
| p-value | 0.89 | 0.78 | |||
| Gestational Diabetes | No | 163 (32.5) | 339 (67.5) | 59 (11.8) | 443 (88.2) |
| Yes | 10 (14.7) | 58 (85.3) | 3 (4.4) | 65 (95.6) | |
| p-value | <0.01 | 0.06 | |||
| Hypertension | No | 168 (31.0) | 390 (69.9) | 59 (10.6) | 499 (89.4) |
| Yes | 5 (41.7) | 7 (58.3) | 3 (25.0) | 9 (75.0) | |
| p-value | 0.39 | 0.11 | |||
| Gestational Weight Gain in Kg Median (IQR) | p-Value | ||
|---|---|---|---|
| Trimester | First trimester | 2 (0–5) | <0.01 |
| Second trimester | 6 (3–9) | ||
| Third trimester | 9 (5–12) | ||
| Pre-pregnancy BMI (kg/m2) | <18.5 | 5 (3–11) | <0.01 |
| 18.5–24.9 | 8 (5–12) | ||
| 25–29.9 | 6 (3–10) | ||
| 30+ | 5 (2–10) | ||
| FIGO-NRS | High risk | 5.5 (2–11) | 0.34 |
| Low risk | 6 (4–10) | ||
| Model | Unstandardized Coefficients | Wald Chi-Square | p-Value | |
|---|---|---|---|---|
| B | Std. Error | |||
| (Intercept) | 1.79 | 0.21 | 73.5 | <0.01 |
| Parity | 0.15 | 0.01 | 1.17 | 0.28 |
| Age | −0.01 | 0.005 | 4.01 | 0.05 |
| Trimester | ||||
| First # | - | - | - | <0.01 |
| Second | 0.35 | 0.09 | 14.76 | |
| Third | 0.67 | 0.09 | 59.4 | |
| Total FIGO (NRS) | 0.02 | 0.02 | 0.69 | 0.49 |
| GDM | 0.03 | 0.08 | 0.11 | 0.74 |
| HTN | 0.08 | 0.19 | 0.17 | 0.68 |
| Estimated Marginal means of Gestational Weight Gain in different trimesters | ||||
| Mean GWG | Standard Error | 95% Wald Confidence Interval | ||
| Third Trimester | 9.77 | 1.01 | 7.98–11.98 | |
| Second Trimester | 7.13 | 0.75 | 5.80–8.76 | |
| First Trimester | 5.03 | 0.64 | 3.91–6.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahabi, H.; Fayed, A.; Esmaeil, S.; Almadhun, A.A. Maternal Nutrition and Gestational Weight Gain Among Saudi Women: Riyadh Mother and Baby Follow Up Study (RAHMA Explore). Healthcare 2025, 13, 1446. https://doi.org/10.3390/healthcare13121446
Wahabi H, Fayed A, Esmaeil S, Almadhun AA. Maternal Nutrition and Gestational Weight Gain Among Saudi Women: Riyadh Mother and Baby Follow Up Study (RAHMA Explore). Healthcare. 2025; 13(12):1446. https://doi.org/10.3390/healthcare13121446
Chicago/Turabian StyleWahabi, Hayfaa, Amel Fayed, Samia Esmaeil, and Ansam Ayman Almadhun. 2025. "Maternal Nutrition and Gestational Weight Gain Among Saudi Women: Riyadh Mother and Baby Follow Up Study (RAHMA Explore)" Healthcare 13, no. 12: 1446. https://doi.org/10.3390/healthcare13121446
APA StyleWahabi, H., Fayed, A., Esmaeil, S., & Almadhun, A. A. (2025). Maternal Nutrition and Gestational Weight Gain Among Saudi Women: Riyadh Mother and Baby Follow Up Study (RAHMA Explore). Healthcare, 13(12), 1446. https://doi.org/10.3390/healthcare13121446






