Serum OxLDL Levels Are Positively Associated with the Number of Ischemic Events and Damaged Blood Vessels in Patients with Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
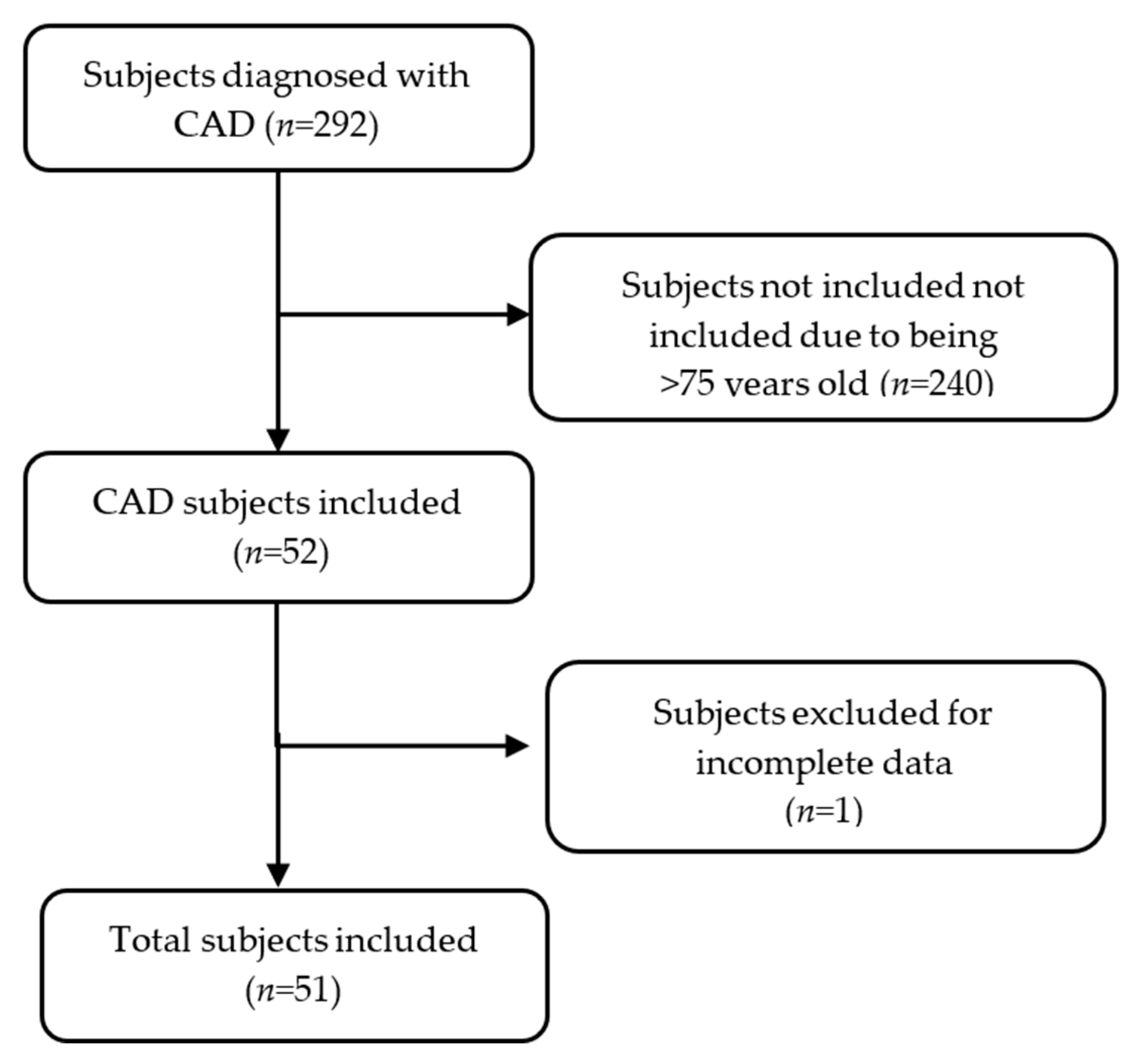
2.1. Study Subjects
2.2. CAD Diagnosis and Cardiological Evaluations
2.3. Blood Pressure Evaluation
2.4. Pharmacological and Interventional Treatment
2.5. Biochemical Analysis
2.6. Metabolic Comorbidities and Dyslipidemias
2.7. Quantification of Serum oxLDL and oxHDL
2.8. Anthropometric Measurements
2.9. Alcohol Consumption
2.10. Tobacco Index and Risk Exposure
2.11. Statistical Analysis
3. Results
3.1. Characteristics of the Study Subjects
3.2. Cardiological, Anthropometric, and Metabolic Variables
3.3. Comparative Analysis in Subjects Classified by Cardiological Variables
3.4. Association of oxLDL Levels with Cardiological Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ADA | American Diabetes Association |
| AHA | American Heart Association |
| AUDIT | Alcohol Use Disorders Identification Test |
| BF% | body fat percentage |
| BMI | body mass index |
| CAD | coronary artery disease |
| DBV | damaged blood vessel |
| ELISA | enzyme-linked immunosorbent assay |
| ETAP | Escala de Tabaquismo para Atención Primaria (Tobacco Scale for Primary Care) |
| HDL | high-density lipoprotein |
| IE | ischemic event |
| LDL | low-density lipoprotein |
| MDA | malondialdehyde |
| NSTEMI | non-ST-segment-elevation myocardial infarction |
| oxLDL | oxidized low-density lipoprotein |
| oxHDL | oxidized high-density lipoprotein |
| PON1 | paraoxonase 1 |
| PUFAs | polyunsaturated fatty acids |
| STEMI | ST-segment-elevation myocardial infarction |
| T2DM | type 2 diabetes mellitus |
| TC | total cholesterol |
| TG | triglycerides |
| WC | waist circumference |
Appendix A
| Variables | Values %, (n) |
|---|---|
| Metabolic comorbidities | |
| Hypertension | 73.0 (37) |
| T2DM | 49.0 (25) |
| Dyslipidemia | |
| Hypoalphalipoproteinemia | 70.6 (36) |
| Hypertriglyceridemia | 53 (27) |
| Elevated LDL | 51 (26) |
| Hypercholesterolemia | 12 (6) |
| Lipid ratios | |
| Elevated TC/HDL | 62.7 (32) |
| Elevated TG/HDL | 45.1 (23) |
| Variables | Values %, (n) |
|---|---|
| Alcohol consumption | |
| Alcohol consumption | 24.0 (11) |
| High-risk alcohol consumption | 7.8 (4) |
| Smoking | |
| Light smoking | 23.0 (6) |
| Moderate smoking | 38.5 (10) |
| Severe smoking | 38.5 (10) |
| Suspended smoking | 33.3 (17) |
| High-risk passive smoking | 86.2 (25) |
| Medication Type | Medication | Values %, (n) |
|---|---|---|
| Anticoagulants and antiplatelets | Acetylsalicylic acid | 60.8 (31) |
| Clopidogrel | 31.4 (16) | |
| Vasodilators | Isosorbide | 13.7 (7) |
| Nifedipine | 3.9 (2) | |
| Antihypertensives | Metoprolol | 47.1 (24) |
| Losartan | 29.4 (15) | |
| Enalapril | 21.6 (11) | |
| Lipid-lowering | Atorvastatin | 58.8 (30) |
| Bezafibrate | 7.8 (4) | |
| Pravastatin | 3.9 (2) | |
| Ciprofibrate | 2 (1) | |
| Glucose-control | Metformin | 35.3 (18) |
References
- Longo, D.L.; Fauci, A.S.; Kasper, D.L.; Hauser, S.L.; Jameson, J.L.; Loscalzo, J. (Eds.) Harrison. In Principios de Medicina Interna, 18th ed.; McGraw Hill: New York, NY, USA, 2013; pp. 1–3000. [Google Scholar]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk factors for coronary artery disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554410/ (accessed on 14 December 2024).
- Mensah, G.A.; Fuster, V.; Murray, C.J.; Roth, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; Abdulah, D.M.; et al. Global burden of cardiovascular diseases and risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Vickrey, B.G.; Rector, T.S.; Wickstrom, S.L.; Guzy, P.M.; Sloss, E.M.; Gorelick, P.B.; Garber, S.; McCaffrey, D.F.; Dake, M.D.; Levin, R.A. Occurrence of secondary ischemic events among persons with atherosclerotic vascular disease. Stroke 2002, 33, 901–906. [Google Scholar] [CrossRef]
- Uzuner, N.; Uzuner, G.T. Risk factors for multiple recurrent ischemic strokes. Brain Circ. 2023, 9, 21–24. [Google Scholar] [CrossRef]
- Shahjehan, R.D.; Sharma, S.; Bhutta, B.S. Coronary artery disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564304/ (accessed on 14 May 2025).
- Wang, A.; Dai, L.; Zhang, N.; Lin, J.; Chen, G.; Zuo, Y.; Li, H.; Wang, Y.; Meng, X.; Wang, Y. Oxidized low-density lipoprotein (LDL) and LDL cholesterol are associated with outcomes of minor stroke and TIA. Atherosclerosis 2020, 297, 74–80. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, X.; Li, S.; Zhao, X.; Liu, L.; Johnston, S.C.; Meng, X.; Lin, J.; Zuo, Y.; Li, H.; et al. Oxidative lipoprotein markers predict poor functional outcome in patients with minor stroke or transient ischaemic attack. Eur. J. Neurol. 2019, 26, 1082–1090. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Zheng, L.; Hazen, S.L. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc. Med. 2005, 15, 212–219. [Google Scholar] [CrossRef]
- Ansell, B.J.; Fonarow, G.C.; Fogelman, A.M. The paradox of dysfunctional high-density lipoprotein. Curr. Opin. Lipidol. 2007, 18, 427–434. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Z.; Tang, Y.; Hu, G.; Lu, J.; He, D.; Wang, S. TLR4/NF-κB signaling pathway-mediated and oxLDL-induced up-regulation of LOX-1, MCP-1, and VCAM-1 expressions in human umbilical vein endothelial cells. Genet. Mol. Res. 2014, 13, 680–695. [Google Scholar] [CrossRef]
- Basit, H.; Huecker, M.R. Myocardial infarction serum markers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532966/ (accessed on 16 October 2024).
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and diagnosis of diabetes: Standards of care in diabetes—2023. Diabetes Care 2022, 46 (Suppl. S1), S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Mcbride, P.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63 Pt B, 2889–2934. [Google Scholar] [CrossRef]
- García Muñoz, A.I.; Melo Buitrago, P.J.; Rodríguez Arcila, M.A.; Silva Zambrano, D.A. Índices aterogénicos y composición corporal en cadetes de una escuela de formación militar colombiana. Sanid. Mil. 2020, 76, 13–18. [Google Scholar]
- Castelli, W.P. Epidemiology of coronary heart disease: The Framingham study. Am. J. Med. 1984, 76, 4–12. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1995; Available online: http://whqlibdoc.who.int/trs/WHO_TRS_854.pdf (accessed on 25 November 2024).
- Centers for Disease Control and Prevention (CDC). Índice de Masa Corporal|Peso Saludable|DNPAO|CDC. 2022. Available online: https://www.cdc.gov/healthyweight/spanish/assessing/bmi/index.html (accessed on 4 December 2022).
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Government Publishing Office: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (accessed on 4 March 2025).
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). National Institute on Alcohol Abuse and Alcoholism (NIAAA). Available online: https://www.niaaa.nih.gov/ (accessed on 4 March 2025).
- Saunders, J.B.; Aasland, O.G. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Alcohol Health Res. World 1993. [Google Scholar] [CrossRef]
- Gobierno Federal. Guía de Práctica Clínica Para la Prevención, Diagnóstico y Tratamiento del Consumo del Tabaco; Centro Nacional de Excelencia Tecnológica en Salud: México City, México, 2009. Available online: https://www.cenetec.salud.gob.mx/interior/gpc.html (accessed on 4 March 2025).
- González Romero, M.P.; Cuevas-Fernández, F.J.; Marcelino-Rodríguez, I.; Covas, V.J.; Rodríguez Pérez, M.C.; Cabrera de León, A.; Aguirre-Jaime, A. Aplicación de la Escala de Tabaquismo para Atención Primaria (ETAP) en la práctica clínica. Aten. Primaria 2018, 50, 414–421. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Velázquez Monroy, Ó.; Aldatz, B.; S, F.; Guerra, R.; Francisco, A.; Verdejo, J.; Bello, M.Á.M.; Violante, R.; Esqueda, A.L. Morbilidad y mortalidad de la enfermedad isquémica del corazón y cerebrovascular en México, 2005. Arch. Cardiol. Méx. 2007, 77, 31–39. [Google Scholar]
- Solorio, S.; Hernández-González, M.A.; Rangel Abundis, A.; Murillo-Ortiz, B. Cardiopatía isquémica en mujeres mexicanas. Arch. Cardiol. Méx. 2007, 77, 226–231. [Google Scholar] [PubMed]
- Franco, Y.; Mendoza-Fernández, V.; Lemini, C. Mecanismos de acción de los efectos protectores de los estrógenos sobre el sistema cardiovascular. Rev. Fac. Med. UNAM 2003, 46, 101–108. [Google Scholar]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Kawakami, R.; Finn, A.V.; Virmani, R. Differences in stable and unstable atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1474–1484. [Google Scholar] [CrossRef]
- Martinez-Sanchez, C.; Borrayo, G.; Carrillo, J.; Juarez, U.; Quintanilla, J.; Jerjes-Sanchez, C. Clinical management and hospital outcomes of acute coronary syndrome patients in Mexico: The Third National Registry of Acute Coronary Syndromes (RENASICA III). Arch. Cardiol. Méx. 2016, 86, 221–232. [Google Scholar] [CrossRef]
- Volpe, M.; Gallo, G. Hypertension, coronary artery disease and myocardial ischemic syndromes. Vasc. Pharmacol. 2023, 153, 107230. [Google Scholar] [CrossRef]
- Gupta, N.; Elnour, A.A.; Sadeq, A.; Gupta, R. Diabetes and the Heart: Coronary Artery Disease. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-22/diabetes-and-the-heart-coronary-artery-disease (accessed on 4 March 2025).
- Instituto Nacional de Salud Pública (INSP). Encuesta Nacional de Salud y Nutrición Continua 2023; INSP: Cuernavaca, México, 2024; Available online: https://ensanut.insp.mx/encuestas/ensanutcontinua2023/index.php (accessed on 4 February 2025).
- Agarwala, A.; Kulkarni, S.; Maddox, T. The association of statin therapy with incident diabetes: Evidence, mechanisms, and recommendations. Curr. Cardiol. Rep. 2018, 20, 50. [Google Scholar] [CrossRef]
- Davignon, J.; Jacob, R.F.; Mason, R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004, 15, 251–258. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Ławiński, J.; Rysz, J. The impact of aerobic exercise on HDL quantity and quality: A narrative review. Int. J. Mol. Sci. 2023, 24, 4653. [Google Scholar] [CrossRef]
- Torres-Vanegas, J.; Rodríguez-Echevarría, R.; Campos-Pérez, W.; Rodríguez-Reyes, S.C.; Reyes-Pérez, S.D.; Pérez-Robles, M.; Martínez-López, E. Effect of a diet supplemented with marine omega-3 fatty acids on inflammatory markers in subjects with obesity: A randomized active placebo-controlled trial. Healthcare 2025, 13, 103. [Google Scholar] [CrossRef]
- Xue, R.; Li, Q.; Geng, Y.; Wang, H.; Wang, F.; Zhang, S. Abdominal obesity and risk of CVD: A dose–response meta-analysis of thirty-one prospective studies. Br. J. Nutr. 2021, 126, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599,912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Shirpoor, A.; Salami, S.; Khadem Ansari, M.H.; Ilkhanizadeh, B.; Abdollahzadeh, N. Ethanol promotes rat aortic vascular smooth muscle cell proliferation via increase of homocysteine and oxidized-low-density lipoprotein. J. Cardiol. 2013, 62, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Wells, A.J. Passive smoking as a cause of heart disease. J. Am. Coll. Cardiol. 1994, 24, 546–554. [Google Scholar] [CrossRef]
- Yazdandoust, S.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M.; Yaghmaei, P.; Sahebkar, A.H. High-density lipoprotein lipid peroxidation as a diagnostics biomarker in coronary artery disease. Biofactors 2022, 48, 634–642. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Tao, H.; Davies, S.S. HDL function and atherosclerosis: Reactive dicarbonyls as promising targets of therapy. Circ. Res. 2023, 132, 1521–1545. [Google Scholar] [CrossRef]
- Holvoet, P.; Mertens, A.; Verhamme, P.; Bogaerts, K.; Beyens, G.; Verhaeghe, R.; Collen, D.; Muls, E.; Van de Werf, F. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 844–848. [Google Scholar] [CrossRef]
- World Medical Association (WMA). Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects; WMA: Fortaleza, Brazil, 2013; Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 16 July 2020).
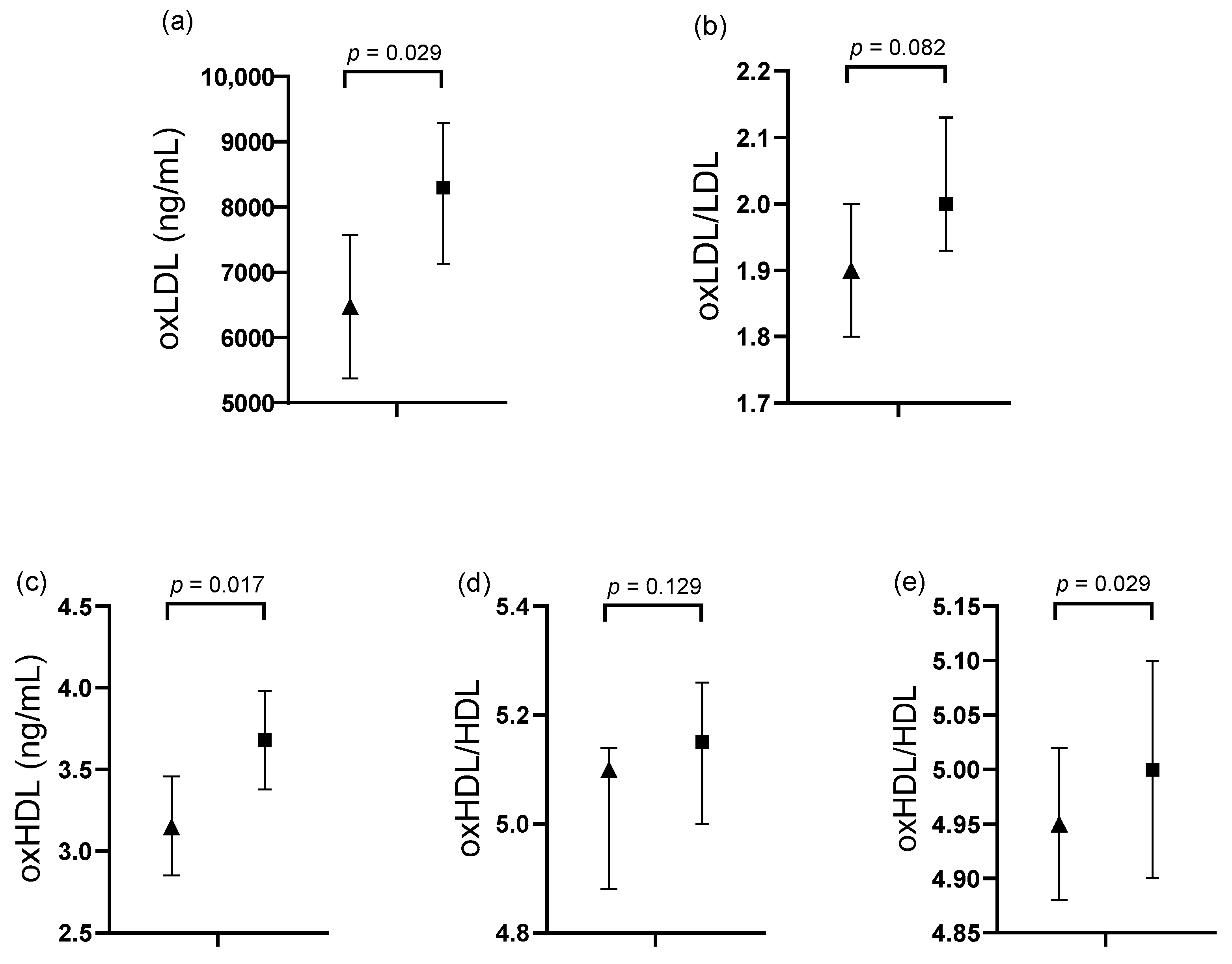
| Variables | Values |
|---|---|
| Clinical | |
| SBP (mmHg) | 129 (111–140) |
| DBP (mmHg) | 78 (71–86) |
| DBVs (number) | 2 (1–2) |
| IEs (number) | 2 (1–2) |
| Anthropometric | |
| Body weight (kg) | 74 (64.7–88) |
| BMI (kg/m2) | 28.4 (26.6–30.9) |
| Body fat (%) | |
| M (n = 34) | 28.1 (20.5–32.5) |
| W (n = 17) | 42.1 (37.9–47.25) |
| WC (cm) | |
| M (n = 34) | 98 (90–107) |
| W (n = 17) | 97.5 (93.2–102.7) |
| Biochemical Variables | Values |
|---|---|
| TC (mg/dL) | 139 (109–171) |
| LDL (mg/dL) | 72 (45–98) |
| VLDL (mg/dL) | 30 (21–41) |
| HDL (mg/dL) | |
| M (n = 34) | 33 (25.5–40) |
| W (n = 17) | 43 (33.5–51.5) |
| TG (mg/dL) | 157 (104–208) |
| TG/HDL-c ratio | |
| M (n = 34) | 4.67 (2.7–7.1) |
| W (n = 17) | 3.94 (2.4–6.2) |
| TC/HDL-c ratio | |
| M (n = 34) | 4 (3–5) |
| W (n = 17) | 4 (2–5) |
| oxLDL (ng/mL) | 6500 (5560–8320) |
| oxHDL (ng/mL) | |
| M (n = 34) | 3.2 (2.7–3.6) |
| W (n = 17) | 3.4 (3.3–4.3) |
| oxLDL/LDL | 5.04 (4.9–5.12) |
| oxHDL/HDL | |
| M (n = 34) | 5.04 (4.98–5.19) |
| W (n = 17) | 5.03 (4.87–5.09) |
| Variables | 1 IE and 1 DBV (n = 25) | ≥2 IEs and ≥2 DBVs (n = 26) | p Value |
|---|---|---|---|
| TC (mg/dL) | 148.15 (132.73–163.56) | 138.39 (123.27–153.51) | 0.371 |
| LDL (mg/dL) | 78.87 (64.84–92.90) | 70.08 (53.33–83.83) | 0.376 |
| VLDL (mg/dL) | 33.80 (27.08–40.52) | 32.14 (25.56–38.73) | 0.727 |
| HDL (mg/dL) | |||
| M (n = 34) | 34.03 (29.55–38.59) | 32.83 (28.82–36.85) | 0.679 |
| W (n = 17) | 49.81 (36–63.58) | 39.69 (23.10–56.27) | 0.333 |
| TG (mg/dL) | 187.88 (147.55–228.21) | 159.34 (119.80–198.87) | 0.318 |
| TG/HDL ratio | |||
| M (n = 34) | 5.99 (4.03–7.95) | 5.18 (3.44–6.92) | 0.932 |
| W (n = 17) | 4.23 (2.65–5.81) | 4.33 (2.43–6.24) | 0.534 |
| TC/HDL-c ratio | |||
| M (n = 34) | 4.20 (3.43–4.97) | 4.25 (3.57–4.94) | 0.416 |
| W (n = 17) | 3.52 (2.59–4.46) | 4.10 (2.97–5.22) | 0.914 |
| Model | Dependent Variable | R2 | B (95% CI) | p Value |
|---|---|---|---|---|
| 1 | Number of ischemic events | 0.152 | 0.142 (0.046–0.239) | 0.005 |
| 2 | Number of damaged blood vessels | 0.192 | 0.196 (0.018–0.374) | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Robles, M.; Campos-Perez, W.; Godinez-Mora, S.; Lopez-Alvarez, V.; Ramos-Villalobos, L.E.; Corona-Ruiz, F.; Armendariz-Borunda, J.; Martinez-Lopez, E. Serum OxLDL Levels Are Positively Associated with the Number of Ischemic Events and Damaged Blood Vessels in Patients with Coronary Artery Disease. Healthcare 2025, 13, 1426. https://doi.org/10.3390/healthcare13121426
Perez-Robles M, Campos-Perez W, Godinez-Mora S, Lopez-Alvarez V, Ramos-Villalobos LE, Corona-Ruiz F, Armendariz-Borunda J, Martinez-Lopez E. Serum OxLDL Levels Are Positively Associated with the Number of Ischemic Events and Damaged Blood Vessels in Patients with Coronary Artery Disease. Healthcare. 2025; 13(12):1426. https://doi.org/10.3390/healthcare13121426
Chicago/Turabian StylePerez-Robles, Mariana, Wendy Campos-Perez, Sissi Godinez-Mora, Victor Lopez-Alvarez, Liliana Estefanía Ramos-Villalobos, Felisardo Corona-Ruiz, Juan Armendariz-Borunda, and Erika Martinez-Lopez. 2025. "Serum OxLDL Levels Are Positively Associated with the Number of Ischemic Events and Damaged Blood Vessels in Patients with Coronary Artery Disease" Healthcare 13, no. 12: 1426. https://doi.org/10.3390/healthcare13121426
APA StylePerez-Robles, M., Campos-Perez, W., Godinez-Mora, S., Lopez-Alvarez, V., Ramos-Villalobos, L. E., Corona-Ruiz, F., Armendariz-Borunda, J., & Martinez-Lopez, E. (2025). Serum OxLDL Levels Are Positively Associated with the Number of Ischemic Events and Damaged Blood Vessels in Patients with Coronary Artery Disease. Healthcare, 13(12), 1426. https://doi.org/10.3390/healthcare13121426







