Artificial Intelligence Applications to Personalized Dietary Recommendations: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Study Selection Criteria
2.3. Search Strategies
- AI-related terms (“artificial intelligence,” “AI,” “computational intelligence,” “machine intelligence,” “computer reasoning,” “machine learning,” “deep learning,” “neural network/s,” “reinforcement learning,”)
- Personalization-related terms (personal*, individual*, tailor*, customize*, customize*).
- Diet-related terms (diet*, nutrition*, “meal plan*”)
- Recommendation-related terms (recommend*, advice, plan*, generate*)
2.4. Data Extraction and Synthesis
2.5. Study Quality Assessment
3. Results
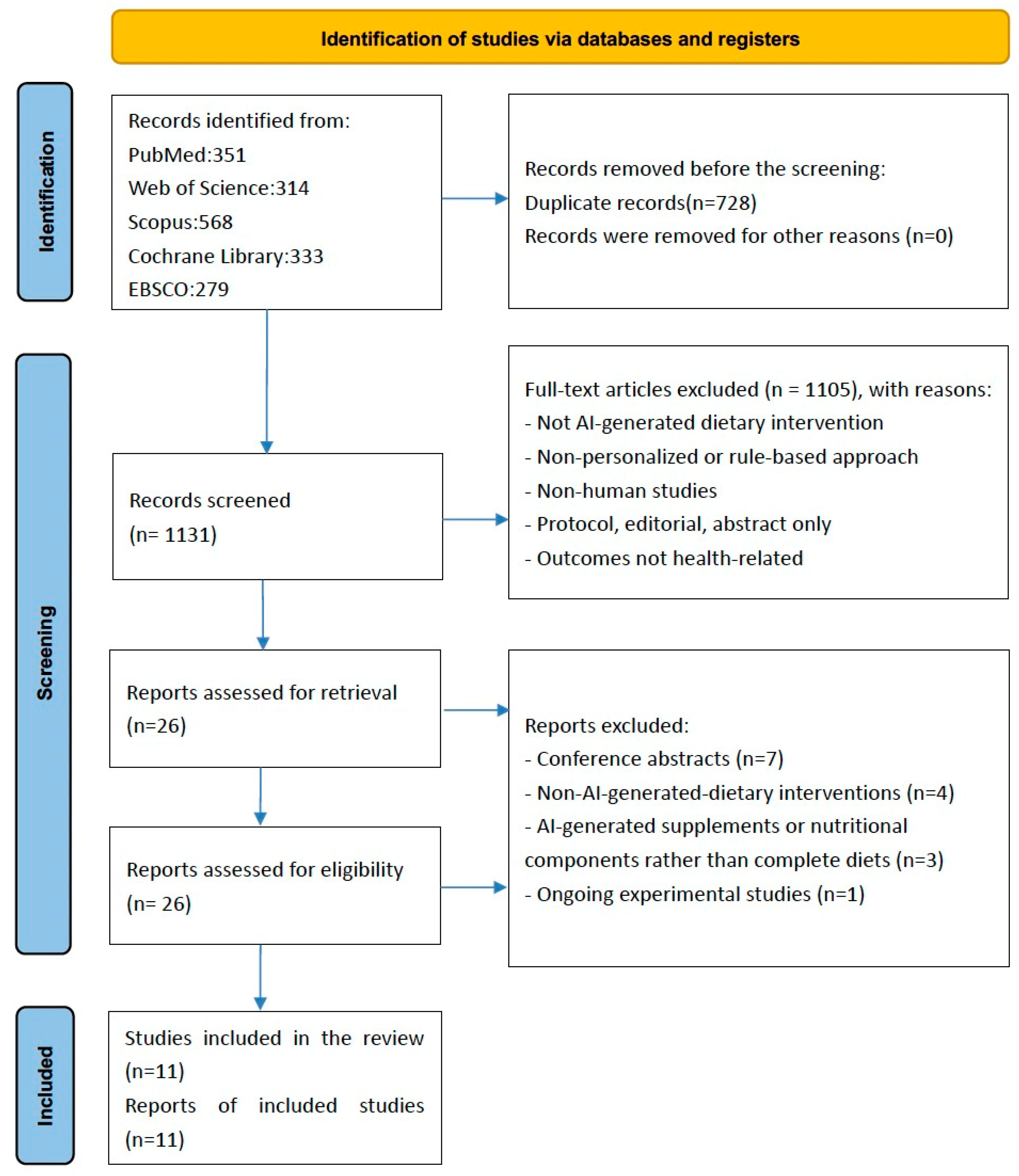
3.1. Identification of Studies
3.2. Study Characteristics
| Study ID | Author, Year | Country /Region | Study Design | Intervention Design | Sample Size | Age | Female (%) | Grade |
|---|---|---|---|---|---|---|---|---|
| 1 | Zeevi, 2015 [11] | Israel | RCT c | Healthy individuals were randomly assigned to the prediction arm (n = 12) and the expert arm (n = 14). Every participant followed a personalized “good” diet and “bad” diet (categorized by AI e or experts) for a full week in total 2 weeks. | 26 | N/A h | N/A | Moderate |
| 2 | Ben-Yacov, 2021 [12] | Israel | RCT | Adults with prediabetes were randomly assigned to follow a MED f diet (n = 112) or a PPT g diet (n = 113) for 6 months. | 225 (200 completed 6-month intervention; 177 completed 12-month follow-up) | 43–57 | 132 (58.7) | High |
| 3 | Rein, 2022 [13] | Israel | Pre-post | Short-term Crossover Intervention: 23 newly diagnosed T2D d patients received both diets, with the order randomized, each diet for 2 week, total 4 week. Long-term Intervention: 16 out of the original 23 participants continued PPT diet for 6 months. | 23 | 45–62 | 12 (52) | Moderate |
| 4 | Arslan, 2022 [14] | Turkey | RCT | Patients with FC n were randomly assigned to follow a conventional treatments group or an AI diet group for 10 weeks, with 6 weeks for AI diet intervention. | 45 | 21–42 | 40 (88.9) | High |
| 5 | Connell, 2023 [15] | USA | Pre-post | Four interventional trials (single-arm for IBS i, depression, and anxiety; two-arm for T2D) to evaluate the efficacy of a VPNP j, with the T2D trial lasting 8–9 months. | IBS-SSS k study, n = 105 (severe = 52, moderate = 41, mild = 12); Depression study, n = 410 (severe = 108, moderate = 129, mild = 173); Anxiety study, n = 490 (severe = 54, moderate = 124, mild = 312); T2D Study, n = 2912 (low adherence group = 1456, high adherence group = 1456) | 31–66 (for the T2D trial) | T2D trial: 1808 (62) | Moderate |
| 6 | Joshi, 2023 [16] | India | RCT | Adult participants with diabetes were randomly assigned to a DT l group or SC m group for 1 year. | n = 319 (DT = 233, SC = 86) | 18–70 | N/A | High |
| 7 | Ben-Yacov, 2023 [17] | Israel | RCT | Adults with prediabetes were randomly assigned to follow a MED diet or a PPT diet for 6 months. | 200 | 18–65 | N/A | High |
| 8 | Bul, 2023 [18] | UK | Pre-post | Adult participants with prediabetes or diabetes or their carers use the AI-driven web-based nutrition platform for 8 weeks. | Pre: 73 Post: 23 | 27–79 | Pre: 58 (79) | Moderate |
| 9 | Tunali, 2024 [19] | Turkey | Pre-post | IBS patients were randomly assigned to PD a and FODMAP b diets for 6 weeks supervised by a dietitian who was blinded to the diets. | 121 (PD = 70, FODMAP = 51) | 18–65 | 42 (60) | Moderate |
| 10 | Jin, 2024 [20] | China | Pre-post | In Phase 1, HD o patients receive standard dietary education regarding potassium management in HD. In Phase 2, they received GPT-based dietary guidance for 1 week. | 88 | 48–73 | N/A | Moderate |
| 11 | Buchan, 2024 [10] | USA | Cross-sectional | N/A | 2420 | 18–91 | 1765 (72.9) | Low |
| Study ID | Author, Year | AI-Recommended Diet | Health Outcome Measures | Statistical Tests | Estimated Effect of AI-Recommended Diet on Health Outcomes |
|---|---|---|---|---|---|
| 1 | Zeevi, 2015 [11] | In the prediction arm, the “good” diet was designed using machine learning to predict individual postprandial glycemic responses (PPGR k) based on each participant’s personal data, selecting meals from their prior dietary records that were expected to minimize PPGRs. | Postprandial Responses:
| Wilcoxon signed-rank test | Based on the Wilcoxon signed-rank test, across all participants, the “good” diet exhibited significantly lower PPGRs than the “bad” diet (p < 0.05); lower fluctuations in glucose levels across the CGM al connection week (p < 0.05), and a lower maximal PPGR (p < 0.05) in the “good” diet. In the prediction arm, the “good” diet is negatively associated with PPGR, M (good diet) vs. M (bad diet): 19 vs. 30 mg/dL.h, p < 0.001; In expert arm, “good” diet is negatively associated with PPGR, M (good diet) vs. M (bad diet): 18 vs. 39 mg/dL.h, p < 0.001. In the prediction arm, good diet is negatively associated with Glucose fluctuations, M (good diet) vs. M (bad diet): 0.12 vs. 0.14 σ/μ, p < 0.01; In the expert arm, good diet is negatively associated with PPGR, M (good diet) vs. M (bad diet): 0115 vs. 0.155 σ/μ, p < 0.01. In the prediction arm, good diet is negatively associated with Glucose fluctuations, M (good diet) vs. M (bad diet): 55 vs. 98 mg/dL.h, p < 0.01; In the expert arm, good diet is negatively associated with PPGR, M (good diet) vs. M (bad diet): 40 vs. 85 mg/dL.h, p < 0.01. The success of the prediction arm was comparable to that of the expert-based arm: In the predictor arm, 10 out of 12 participants showed significantly lower PPGRs (p < 0.05); in the expert arm, 8 out of 14 participants showed significantly lower PPGRs (p < 0.05). |
| 2 | Ben-Yacov, 2021 [12] | PPT diet (Dietary recommendations were personalized based on PPGR using a machine-learning algorithm that integrates clinical and microbiome data, selecting meals individually scored for each participant from a meal bank.) | Primary outcomes:
| Two-sample t-test, Mann-Whitney nonparametric test | Based on the two-sample t-test, the difference was significantly more significant in the PPT group than in the MED group after 6-month intervention: Daily hours with glucose levels > 140 mg/dL: M(SD) change: −0.3 (0.8) h/day for MED diet and −1.3 (1.5) h/day for PPT diet, with 95% CI between-group difference −1.29 to −0.66 h/day, p < 0.001. HbA1c: M(SD) change: −0.9 (2.1) mmol/mol for the MED diet and −1.7 (2.6) mmol/mol for the PPT diet, with 95% CI between-group difference −0.14 to −0.02% (1.5 to 0.2 mmol/mol), p = 0.007. Triglycerides: M(SD) change: −0.22 (0.51) mmol/L [−19 (45) mg/dL] for MED diet, and −0.43 (0.58) mmol/L [−38 (51) mg/dL] for PPT diet. 95% CI between-group difference −0.36 to −0.07 mmol/L [−31.51 to −6.11 mg/dL], p = 0.003. HDL cholesterol: M(SD) change: 0.02 (0.18) [0.8 (6.7) mg/dL] for MED diet and 0.09 (0.22) mmol/L [3.6 (8.5) mg/dL] in for PPT diet; 95% CI between-group difference 0.02–0.13 mmol/L [0.77–4.9 mg/dL] p = 0.003. Total cholesterol-to-HDL cholesterol ratio (Based on Mann-Whitney nonparametric test): M(SD) change: −0.29 (0.73) for MED diet, and −0.37 (0.71) for PPT diet; 95% CI between-group difference −0.3 to −0.00, p = 0.025. FLI: M(SD) change: 7.4 (3.7) for MED diet and 13.1 (14.3) for PPT diet; 95% CI between-group difference 8.34 to 1.41, p = 0.005. The significant between-group differences were maintained at the 12-month follow-up. |
| 3 | Rein, 2022 [13] | In the crossover phase, participants in PPT diet had limited (4–5) meal options, while in the long-term phase, they had hundreds of choices, selected based on a scoring system using their machine-learning-predicted PPGR | Crossover Intervention:
| Two-sample t-test, linear mixed models, Wilcoxon signed-rank test | In the crossover intervention: PPT diet is negatively associated with
PPT diet is negatively associated with
|
| 4 | Arslan, 2022 [14] | The AI-assisted (Enbiosis Biotechnology, Sariyer, Istanbul) diet was created by analyzing each patient’s gut microbiome, using machine learning to generate personalized nutritional recommendations focused on fiber-rich foods, and adjusting weekly based on patient feedback and progress. |
| Paired t-test | Based on the paired-sample t-test, AI diet is positively associated with CBMpW, M(SD): post: 4.3 (1.8) vs. pre: 1.7 (1.6), p < 0.001. Based on the paired t-test, AI diet is negatively associated with total PAC-QoL score, M(SD): post: 15.9 (16) vs. pre: 52.1 (16.9), p < 0.001. Customization of a diet based on individual microbiome tests provides better outcomes both clinically and socially in FC patients compare to conventional treatment group. |
| 5 | Connell, 2023 [15] | Precision food and personalized supplement recommendations computed by the VPNP l (based on each person’s stool or blood samples, Viome AI Recommendation Engine generates functional scores to determine final food and supplement recommendations based on machine learning.) |
| N/A | Using VPNP is negatively associated with IBS in all kinds of categories. For the severe group, the post-mean score of IBS-SSS is 39% lower than pre, p = 0.0058. Using VPNP is negatively associated with depression in all kinds of categories. For the severe group, the post-mean score of PHQ9 is 31% lower than pre, p < 0.0001. Using VPNP is negatively associated with anxiety in all kinds of categories. For the severe group, the post-mean score of GAD7 is 31% lower than pre, p < 0.0001. Using VPNP is negatively associated with T2D risk score in the high adherence group, M(SD): post: 41.59 (23.53) vs. Pre: 71.84 (25.19), p < 0.0001. Besides, comparing the low adherence group to the high adherence group, Participants who adhered to precision recommendations reduced their T2D risk score more than participants who did not, p < 0.001. |
| 6 | Joshi, 2023 [16] | Digital twin (DT) technology is used to predict PPGRs to specific foods (machine learning algorithms and Internet of Things (IoT)-integrated systems) and provide real-time dietary recommendations via a mobile app. | Primary outcomes:
Secondary outcomes:
| Paired t-test | For diabetes remission rates, at 1 year, T2D remission was achieved in 152 (72.7%) of 209 DT patients based on A1C compared with none in the SC group. Basing on paired t-test, using a novel nudge-touch DT-enabled personalized nutrition technology is negatively associated with many indexes:
|
| 7 | Ben-Yacov, 2023 [17] | PPT diet (Dietary recommendations were personalized based on PPGR using a machine-learning algorithm that integrates clinical and microbiome data, selecting meals individually scored for each participant from a meal bank) and PPT-adherence scores ranging from 0 to 100. |
| Causal mediation analysis | Basing on the causal mediation analysis, PPT diet adherence is negatively associated with HbA1C_Blood (coefficient = −0.0015; p = 0.0048) and Triglycerides (coefficient = −0.0053; p = 0.0007); Basing on the causal mediation analysis, PPT diet adherence is positively associated with HDL (coefficient = 0.0019; p = 0.0014) The PPT diet group showed statistically significant improvements in multiple metabolic and lipid indicators, while the changes in the MED group were smaller or not significant. |
| 8 | Bul, 2023 [18] | The AI-driven nutrition platform uses deep learning techniques to analyze user data and context, generating personalized meal plans and recipe suggestions from personally viewed or saved recipes and popular trending recipes online. |
| Wilcoxon matched pairs signed-rank test | Basing on the Wilcoxon matched pairs signed-rank test, using the platform is not statistically associated with general health status (n = 23, MD −1.7, 95% CI −9.0–6.0; p = 0.61; Cliff δ = −0.05). Basing on the Wilcoxon matched pairs signed-rank test, using the platform is negatively associated with weight (MD 4.5 kg/m2, 95% CI 1.0–12.0; p = 0.009; Cliff δ = 0.33) and waist size (n = 23, mean difference 3.9 cm, 95% CI 2.0–6.5; p = 0.008; Cliff δ = 0.48). |
| 9 | Tunali, 2024 [19] | Following the microbiome analysis, PD a and FODMAP b diets were administered. The PD was planned with the foods recommended by AI (based on machine learning models) according to the results of the microbiome analysis. Approximately 300 foods were scored between 0 and 10 for microbiome modulation. |
| Paired t-test, Wilcoxon signed-rank test | Basing on the paired t-test or Wilcoxon signed-rank tests, PD diet is negatively associated with IBS-SSS. In total, IBS-SSS, M(SD) i: post: 210.64 (130.63) vs. pre: 314.42 (92.79), p < 0.001; For IBS-C, M(SD): post: 201.68(122.67) vs. pre: 327.91(97.74), p < 0.001; For IBS-D, M(SD): post: 221.13 (126.24) vs. pre: 306.06 (100.88), p = 0.010; For IBS-M, M(SD): post: 187.41 (148.24) vs. pre: 300.86 (80.01), p < 0.001. Basing on the paired t-test or Wilcoxon signed-rank test, PD diet is positively associated with IBS-QOL. In total, IBS-QOL, M(SD): post: 55.79 (21.85) vs. pre: 45.55 (22.06); For IBS-C, M(SD): post: 55.30 (22.90) vs. pre: 45.86 (22.25), p < 0.001; For IBS-D, M(SD): post: 58.41 (20.83) vs. pre: 45.82 (25.21), p < 0.01; For IBS-M, M(SD): post: 54.60 (21.85) vs. pre: 44.89 (20.35), p = 0.08. Basing on the paired t-test or Wilcoxon signed-rank tests, PD diet is negatively associated with Hospital Anxiety. M(SD): post: 8.15 (3.37) vs. Pre: 10.27 (4.22), p < 0.001. Basing on the paired t-test or Wilcoxon signed-rank test, PD diet is negatively associated with HADS. M(SD): post: 6.22 (4.10) vs. Pre: 7.57 (4.35). No significant difference between two groups in all the scores. |
| 10 | Jin, 2024 [20] | A custom GPT-based tool that analyzed uploaded food photographs, estimated potassium content, and provided real-time personalized dietary advice based on guidelines for hemodialysis patients. |
| Mixed-effects linear regression, Chi-square test | Basing on mixed-effects linear regression analysis, GPT diet is negatively associated with predialysis serum potassium levels, M(SD): post: 4.57 (0.76) mmol/L vs. pre: 4.84(0.94) mmol/L, p = 0.004. Basing on Chi-square test, GPT diet is negatively associated with Proportion of hyperkalemia, proportion: traditional diet: 39.8% vs. GPT diet: 25.0%, p = 0.036. Basing on mixed-effects linear regression analysis, GPT diet is not statistical significantly associated with predialysis pH levels, M(SD): post: 7.383 (0.040) vs. pre: 7.380 (0.035), p = 0.632. The GPT diet performs better than the standard diet in reducing serum potassium levels: Mean predialysis serum potassium level: M(SD): GPT: 4.57 (0.76) mmol/L vs. Standard: 4.84 (0.94) mmol/L, p = 0.004. |
| 11 | Buchan, 2024 [10] | A text-based virtual dietitian, Ina (based on machine learning algorithms), is designed to provide cancer patients with personalized nutritional support and guidance. |
| Descriptive statistics | 93.6% of users were satisfied with the platform; 83.9% reported actively using advice to guide their diets; 87.7% reported the platform helped them manage symptoms; 81.4% felt the program improved their quality of life. 32.4% out of 785 users had a reduction in total number of symptoms; and 34.0% had a reduction in total symptom severity score. 61.8% of respondents experienced nutrition-related side effects: fatigue (42.8%), constipation (17.9%), dry mouth (15.4%), etc. |
3.3. AI-Driven Diet Recommendation System
3.3.1. Analyzing Individual Physical Health Indicators
3.3.2. Dietary Recommendations
3.4. Measure of Health Status
3.4.1. Physiological Measurements
3.4.2. Psychological Assessments
3.5. Estimated Effect of AI-Recommended Diet
3.5.1. Enhanced Metabolic Health and Well-Being
| Study ID | Study (Author, Year) | AI Technique | Target Outcome(s) | Key Findings |
|---|---|---|---|---|
| 1. | Zeevi, 2015 [11] | ML a | Postprandial glycemic control | ↓ s PPGR b‚ ↓ Glucose fluctuation |
| 2. | Ben-Yacov, 2021 [12] | ML | Glycemic control, lipids, liver function | ↓ HbA1c c‚ ↓ Triglycerides‚ ↑ r HDL d |
| 3. | Rein, 2022 [13] | ML | Glycemic control, fructosamine | ↓ PPGR‚ ↓ Glucose‚ ↓ Fructosamine |
| 4. | Arslan, 2022 [14] | ML | Constipation, QoL e | ↑ CBMpW f‚ ↓ PAC-QoL g |
| 5. | Connell, 2023 [15] | ML | IBS h, Depression, Anxiety, T2D i risk | ↓ IBS-SSS j‚ ↓ PHQ-9 k‚ ↓ GAD-7 l‚ ↓ T2D score |
| 6. | Joshi, 2023 [16] | ML + IoT m (Hybrid) | T2D, Liver, Inflammation | 72.7% remission, ↓ HbA1c, ↓ FLI n‚ ↓ hs-CRP o |
| 7. | Ben-Yacov, 2023 [17] | ML | HbA1c, lipids | ↓ HbA1c‚ ↑ HDL‚ ↓ Triglycerides |
| 8. | Bul, 2023 [18] | DL p | Weight, Waist circumference | ↓ Weight‚ ↓ Waist size |
| 9. | Tunali, 2024 [19] | ML | IBS, Anxiety, Depression | ↓ IBS-SSS, ↑ IBS-QOL‚ ↓ HADS q |
| 10. | Jin, 2024 [20] | DL | Potassium level (HD patients) | ↓ Serum potassium‚ ↓ Hyperkalemia |
| 11. | Buchan, 2024 [10] | ML | QoL, Symptom management | ↑ QoL‚ ↓ Symptoms, side effects reported |
3.5.2. AI Diets Surpass Traditional Plans
3.6. Study Quality Assessment
4. Discussion
4.1. Overview of AI-Driven Dietary Interventions
4.2. Generalizability and Algorithmic Transparency
4.3. Cultural Relevance and Model Bias
4.4. Feasibility and Long-Term Sustainability
4.5. Limitations of the Review
4.6. Critical Gaps and Future Directions
4.7. When Does AI Work Best?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lara-Castor, L.; O’Hearn, M.; Cudhea, F.; Miller, V.; Shi, P.; Zhang, J.; Sharib, J.R.; Cash, S.B.; Barquera, S.; Micha, R.; et al. Burdens of type 2 diabetes and cardiovascular disease attributable to sugar-sweetened beverages in 184 countries. Nat. Med. 2025, 31, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Kviatcovsky, D.; Zheng, D.; Elinav, E. Gut microbiome and its potential link to personalized nutrition. Curr. Opin. Physiol. 2021, 22, 100439. [Google Scholar] [CrossRef]
- Papastratis, I.; Konstantinidis, D.; Daras, P.; Dimitropoulos, K. AI nutrition recommendation using a deep generative model and ChatGPT. Sci. Rep. 2024, 14, 14620. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The emergence of AI-based wearable sensors for digital health technology: A review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef]
- Detopoulou, P.; Voulgaridou, G.; Moschos, P.; Levidi, D.; Anastasiou, T.; Dedes, V.; Diplari, E.-M.; Fourfouri, N.; Giaginis, C.; Panoutsopoulos, G.I.; et al. Artificial intelligence, nutrition, and ethical issues: A mini-review. Clin. Nutr. Open Sci. 2023, 50, 46–56. [Google Scholar] [CrossRef]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The role of AI in hospitals and clinics: Transforming healthcare in the 21st century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef]
- Kirk, D.; Kok, E.; Tufano, M.; Tekinerdogan, B.; Feskens, E.J.M.; Camps, G. Machine learning in nutrition research. Adv. Nutr. 2022, 13, 2573–2589. [Google Scholar] [CrossRef]
- Patil, A.; Singh, N.; Patwekar, M.; Patwekar, F.; Patil, A.; Gupta, J.K.; Elumalai, S.; Priya, N.S.; Sahithi, A. AI-driven insights into the microbiota: Figuring out the mysterious world of the gut. Intell. Pharm. 2025, 3, 46–52. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for scoping reviews (prisma-scr): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Buchan, M.L.; Goel, K.; Schneider, C.K.; Steullet, V.; Bratton, S.; Basch, E. National implementation of an artificial intelligence-based virtual dietitian for patients with cancer. JCO Clin. Cancer Inform. 2024, 8, e2400085. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Kolobkov, D.; Koren, N.; Cohen Dolev, N.; Travinsky Shmul, T.; Wolf, B.C.; Kosower, N.; et al. Personalized postprandial glucose response-targeting diet versus mediterranean diet for glycemic control in prediabetes. Diabetes Care 2021, 44, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.; Ben-Yacov, O.; Godneva, A.; Shilo, S.; Zmora, N.; Kolobkov, D.; Cohen-Dolev, N.; Wolf, B.-C.; Kosower, N.; Lotan-Pompan, M.; et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: A randomized dietary intervention pilot trial. BMC Med. 2022, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N.; Gundogdu, A.; Tunali, V.; Topgul, O.; Beyazgul, D.; Nalbantoglu, O. Efficacy of AI-assisted personalized microbiome modulation by diet in functional constipation: A randomized controlled trial. J. Clin. Med. 2022, 11, 6612. [Google Scholar] [CrossRef]
- Connell, J.; Toma, R.; Ho, C.H.-C.; Shen, N.; Moura, P.; Le, T.; Patridge, E.; Antoine, G.; Keiser, H.; Julian, C.; et al. Data-driven precision nutrition improves clinical outcomes and risk scores for IBS, depression, anxiety, and T2D. Am. J. Lifestyle Med. 2023. [Google Scholar] [CrossRef]
- Joshi, S.; Shamanna, P.; Dharmalingam, M.; Vadavi, A.; Keshavamurthy, A.; Shah, L.; Mechanick, J.I. Digital twin-enabled personalized nutrition improves metabolic dysfunction-associated fatty liver disease in type 2 diabetes: Results of a 1-year randomized controlled study. Endocr. Pract. 2023, 29, 960–970. [Google Scholar] [CrossRef]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Lotan-Pompan, M.; Weinberger, A.; Segal, E. Gut microbiome modulates the effects of a personalised postprandial-targeting (ppt) diet on cardiometabolic markers: A diet intervention in pre-diabetes. Gut 2023, 72, 1486–1496. [Google Scholar] [CrossRef]
- Bul, K.; Holliday, N.; Bhuiyan, M.R.A.; Clark, C.C.T.; Allen, J.; Wark, P.A. Usability and preliminary efficacy of an artificial intelligence-driven platform supporting dietary management in diabetes: Mixed methods study. JMIR Hum. Factors 2023, 10, e43959. [Google Scholar] [CrossRef]
- Tunali, V.; Arslan, N.; Ermis, B.; Hakim, G.; Gundogdu, A.; Hora, M.; Nalbantoglu, O. A multicenter randomized controlled trial of microbiome-based artifıcial intelligence-assisted personalized diet vs low fodmap diet: A novel approach for the management of irritable bowel syndrome. Am. J. Gastroenterol. 2024, 119, 1901–1912. [Google Scholar] [CrossRef]
- Jin, H.; Lin, Q.; Lu, J.; Hu, C.; Lu, B.; Jiang, N.; Wu, S.; Li, X. Evaluating the effectiveness of a generative pretrained transformer-based dietary recommendation system in managing potassium intake for hemodialysis patients. J. Ren. Nutr. 2024, 34, 539–545. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Spooner, B.; Isherwood, J.; Lane, M.; Orrock, E.; Dennison, A. A systematic review of the barriers to the implementation of artificial intelligence in healthcare. Cureus 2023, 15, e46454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sun, Z.; Xue, H.; An, R. Artificial Intelligence Applications to Personalized Dietary Recommendations: A Systematic Review. Healthcare 2025, 13, 1417. https://doi.org/10.3390/healthcare13121417
Wang X, Sun Z, Xue H, An R. Artificial Intelligence Applications to Personalized Dietary Recommendations: A Systematic Review. Healthcare. 2025; 13(12):1417. https://doi.org/10.3390/healthcare13121417
Chicago/Turabian StyleWang, Xi, Zhiyuan Sun, Hong Xue, and Ruopeng An. 2025. "Artificial Intelligence Applications to Personalized Dietary Recommendations: A Systematic Review" Healthcare 13, no. 12: 1417. https://doi.org/10.3390/healthcare13121417
APA StyleWang, X., Sun, Z., Xue, H., & An, R. (2025). Artificial Intelligence Applications to Personalized Dietary Recommendations: A Systematic Review. Healthcare, 13(12), 1417. https://doi.org/10.3390/healthcare13121417







