Global Trends in Extracorporeal Membrane Oxygenation Support for Circulatory Failure: A Bibliometric Analysis
Abstract
1. Introduction
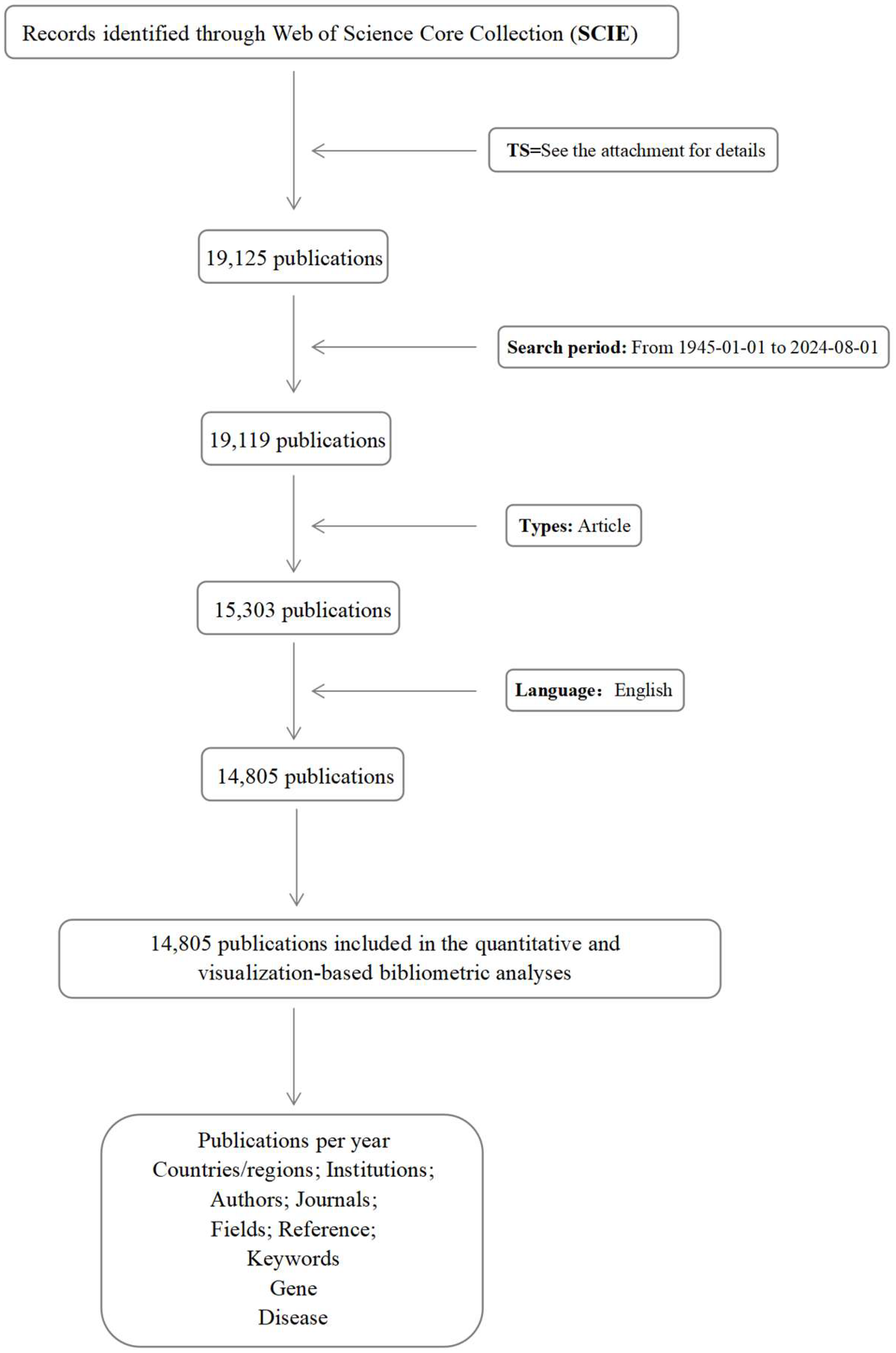
2. Methods
2.1. Data Sources
2.2. Data Analysis
3. Results
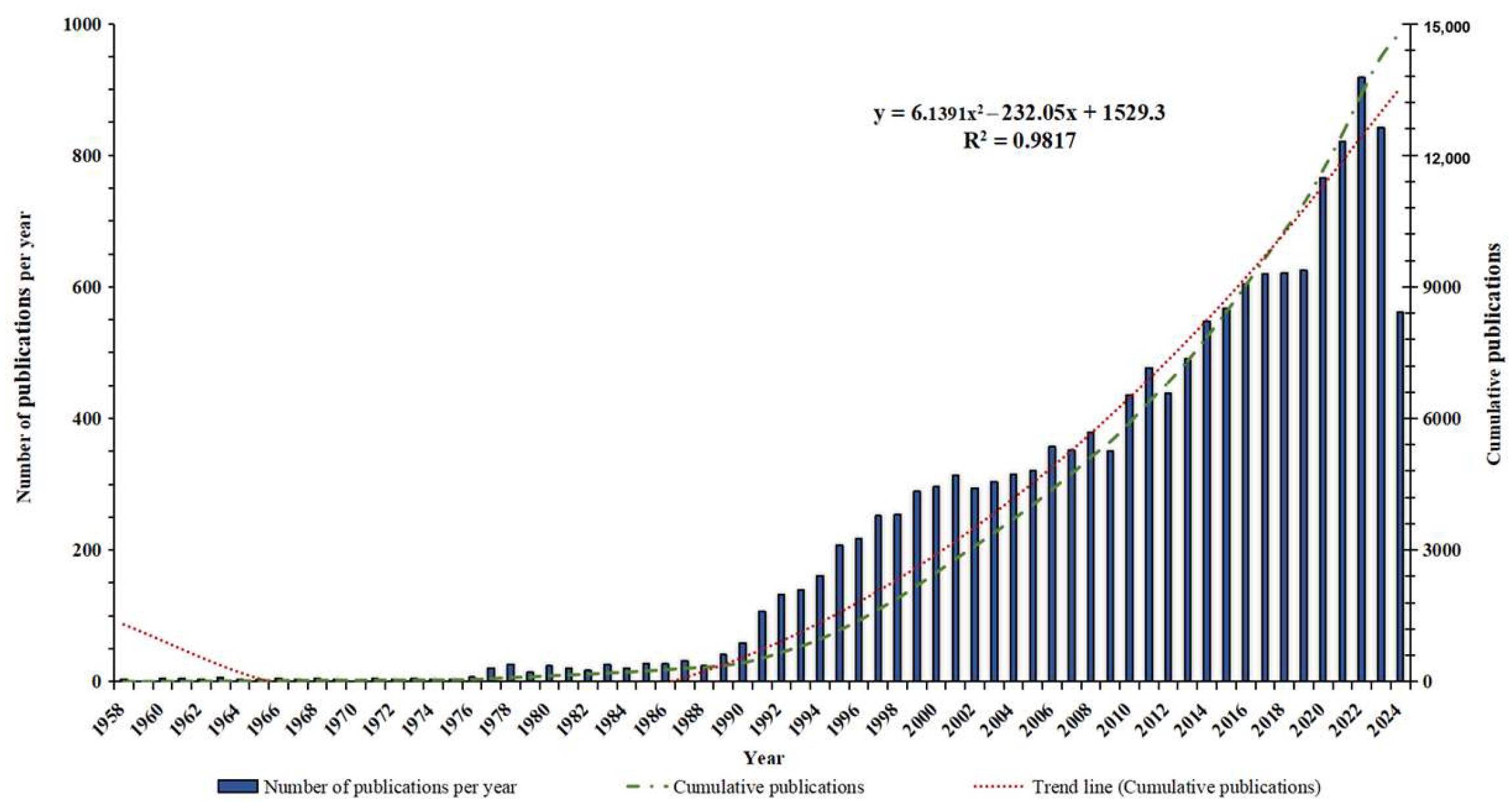
3.1. Global Publication Trend Analysis
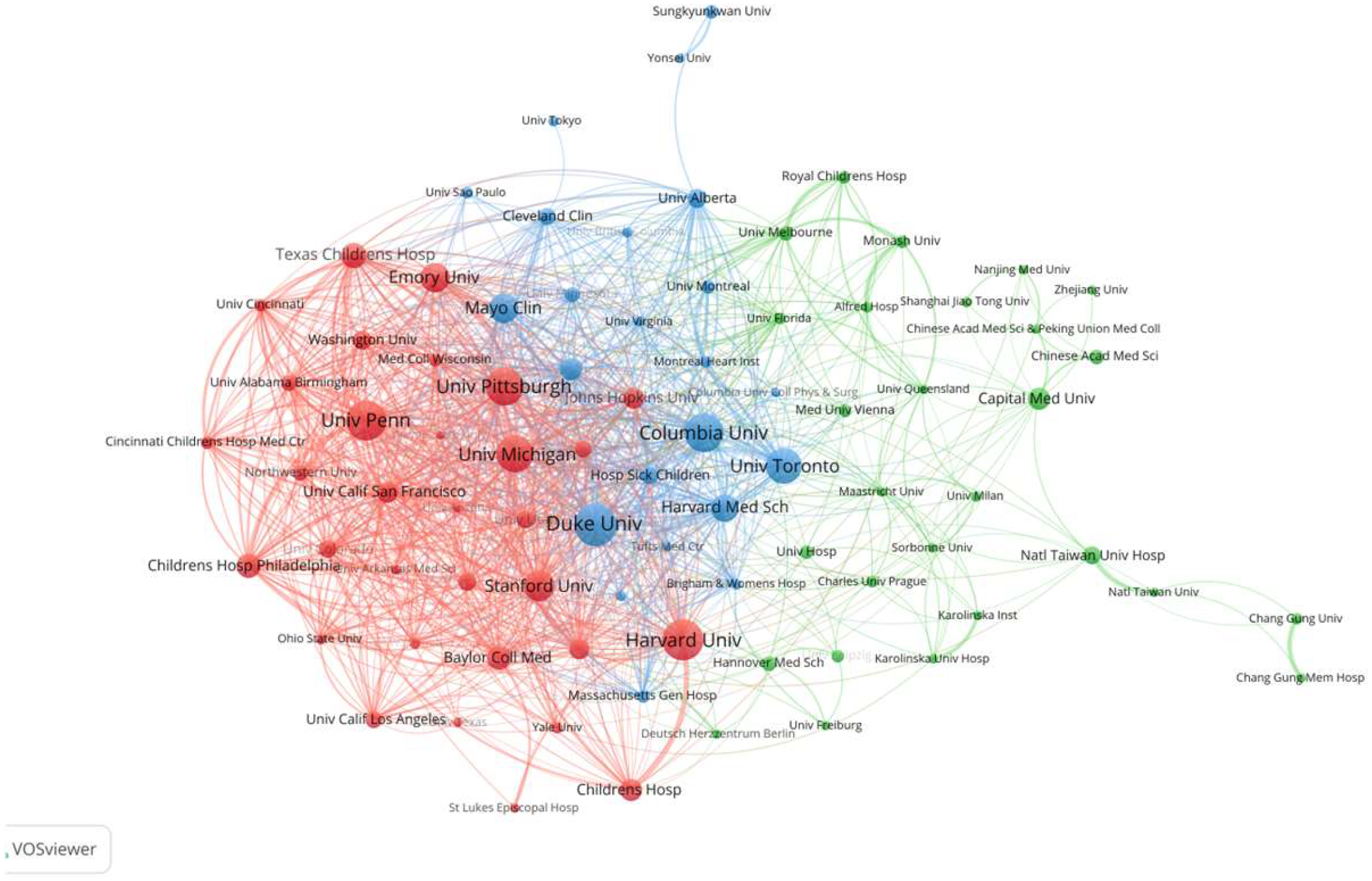
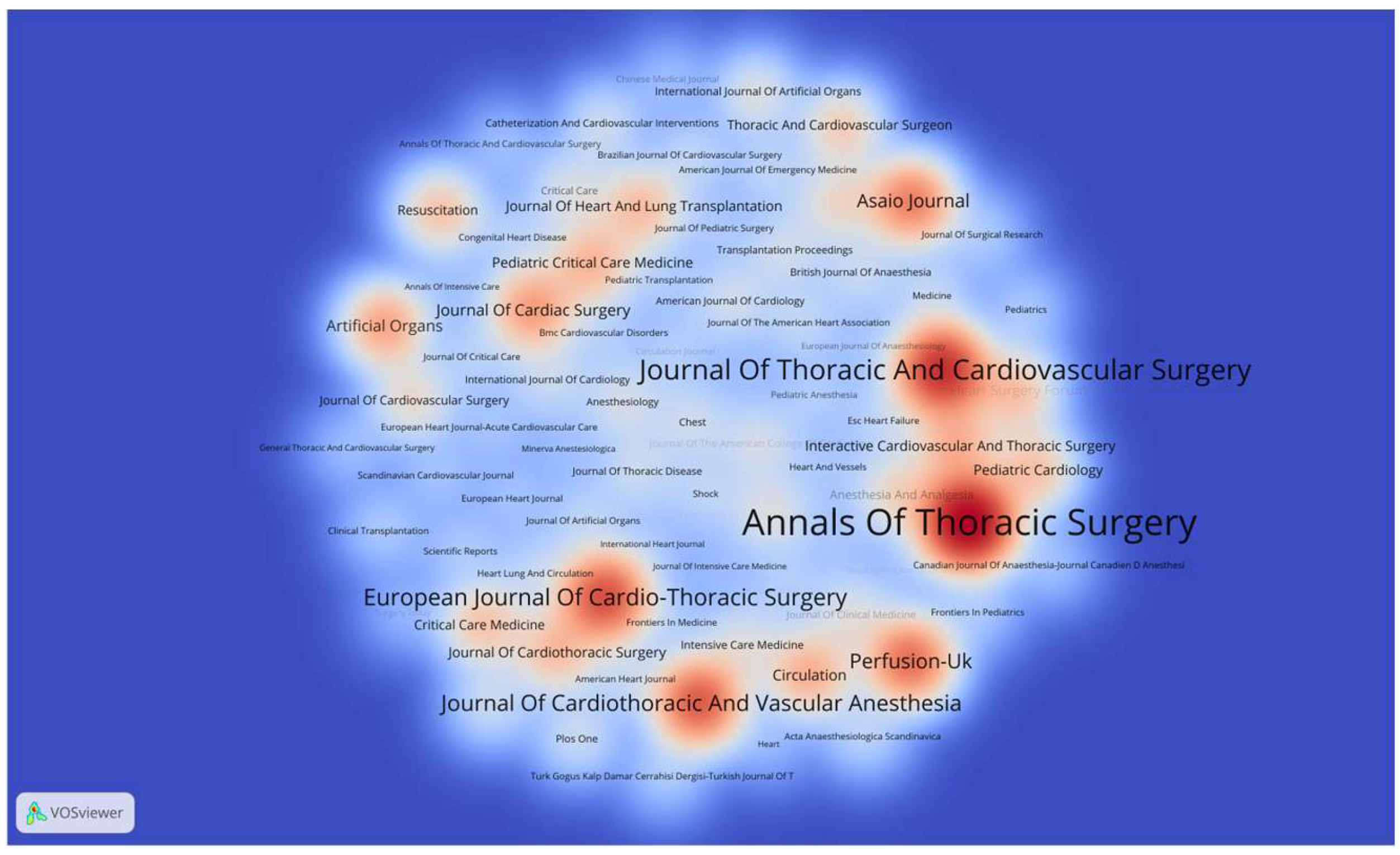
3.2. Analysis of Publications by Country, Institution, and Journal
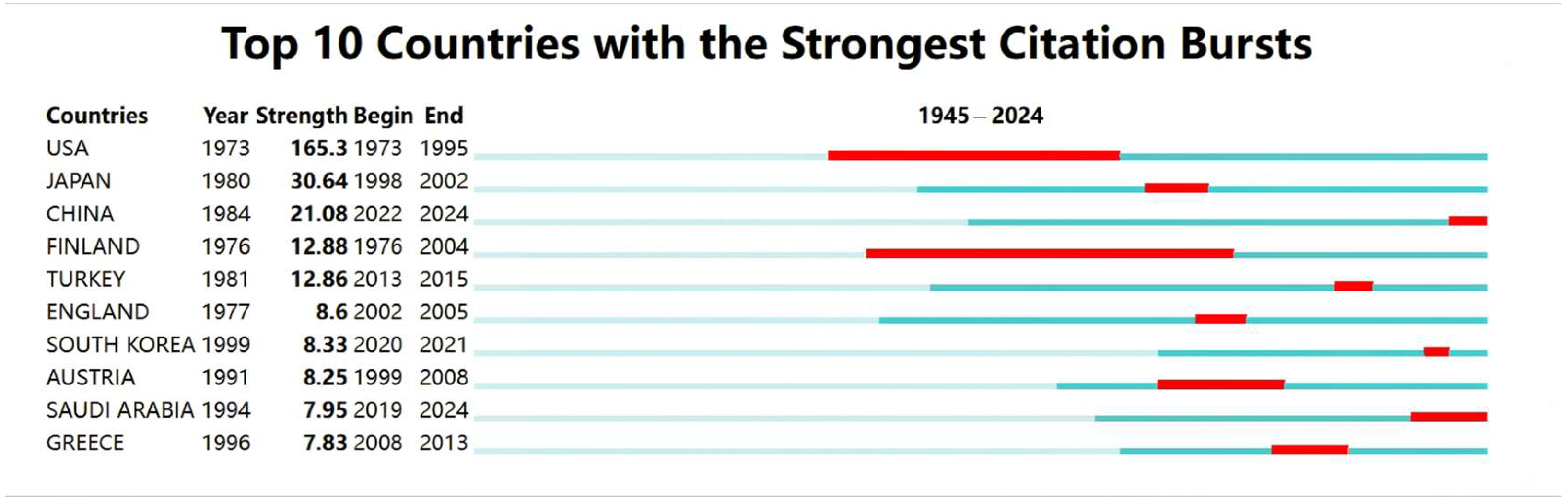
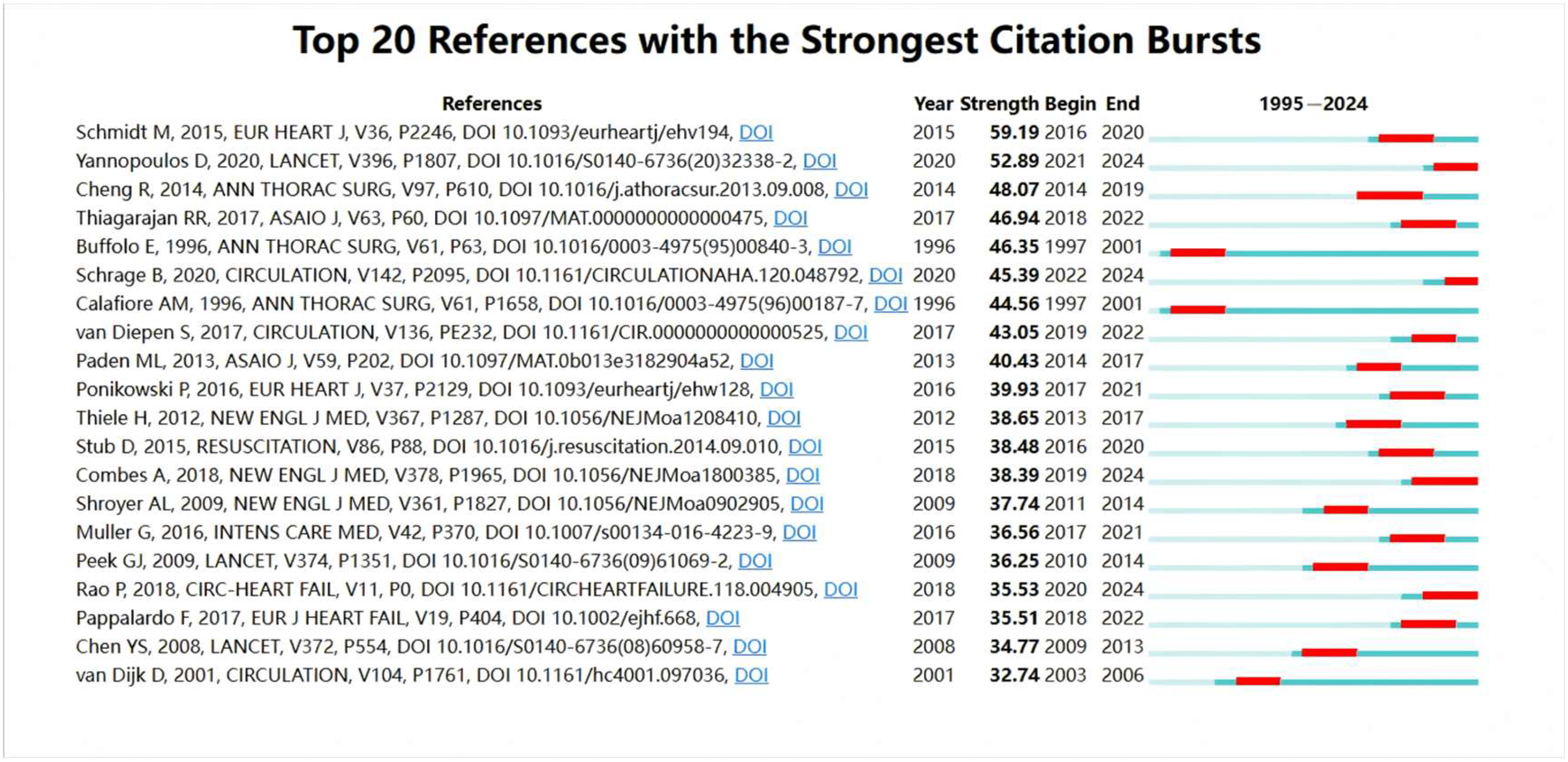
3.3. Citation Burst Analysis
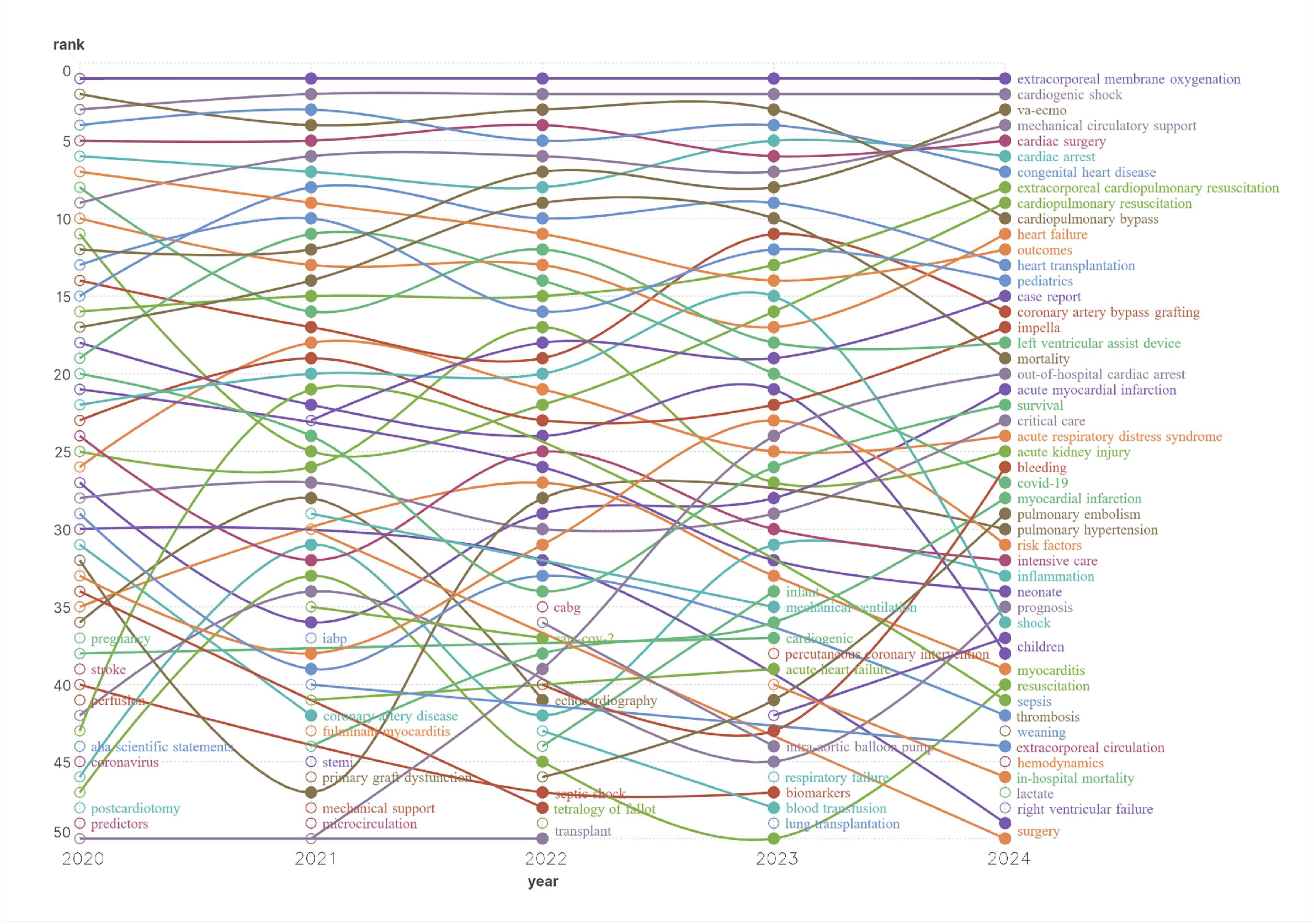
3.4. Hot Keyword Analysis
4. Discussion
4.1. General Information
4.2. Analysis of the High-Impact Literature and Hot Keywords
4.2.1. Circulatory Failure After Cardiac Arrest
4.2.2. Left Ventricular Unloading
4.2.3. Cardiac Surgery-Related Circulatory Failure
4.2.4. Prognostic Factors and Patient Selection
4.2.5. Application of ECMO in Ventricular Tachycardia Ablation
4.2.6. Underrepresentation of Complications in Keyword Analysis
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| CPB | Cardiopulmonary Bypass |
| CITs | Conditional Inference Trees |
| CPC | Cerebral Performance Category |
| ECPR | Extracorporeal Cardiopulmonary Resuscitation |
| ECMO | Extracorporeal Membrane Oxygenation |
| ELSO | Extracorporeal Life Support Organization |
| SCIE | Science Citation Index Expanded |
| SAVE | Survival After Veno-Arterial ECMO (score) |
| PAL | Prediction and Analysis of Long-term outcomes |
| VOS | Visualization of Similarities (used in VOSviewer) |
| VA-ECMO | Veno-Arterial Extracorporeal Membrane Oxygenation |
| VAP | Ventilator-Associated Pneumonia |
References
- Conrad, S.A.; Broman, L.M.; Taccone, F.S.; Lorusso, R.; Malfertheiner, M.; Pappalardo, F.; Di Nardo, M.; Belliato, M.; Grazioli, L.; Barbaro, R.P.; et al. The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support: A Position Paper of the Extracorporeal Life Support Organization. Am. J. Respir. Crit. Care Med. 2018, 198, 447–451. [Google Scholar] [CrossRef]
- Bartlett, R.H.; Arachichilage, D.J.; Chitlur, M.; Hui, S.K.R.; Neunert, C.; Doyle, A.J.; Retter, A.; Hunt, B.J.; Lim, H.S.; Saini, A.; et al. The History of Extracorporeal Membrane Oxygenation and the Development of Extracorporeal Membrane Oxygenation Anticoagulation. Semin. Thromb. Hemost. 2024, 50, 81–90. [Google Scholar] [CrossRef]
- Le Gall, A.; Follin, A.; Cholley, B.; Mantz, J.; Aissaoui, N.; Pirracchio, R. Veno-arterial-ECMO in the intensive care unit: From technical aspects to clinical practice. Anaesth. Crit. Care Pain Med. 2018, 37, 259–268. [Google Scholar] [CrossRef]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef]
- Assmann, A.; Beckmann, A.; Schmid, Ç.; Werdan, K.; Michels, G.; Miera, O.; Schmidt, F.; Klotz, S.; Starck, C.; Pilarczyk, K.; et al. Use of extracorporeal circulation (ECLS/ECMO) for cardiac and circulatory failure—A clinical practice Guideline Level 3. ESC Heart Fail. 2022, 9, 506–518. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization. ELSO Live Registry Dashboard. 2024. Available online: https://www.elso.org/registry/elsoliveregistrydashboard.aspx/ (accessed on 16 August 2024).
- Hu, S.; Lu, A.; Pan, C.; Zhang, B.; Wa, Y.L.; Qu, W.; Bai, M. Limb Ischemia Complications of Veno-Arterial Extracorporeal Membrane Oxygenation. Front. Med. 2022, 9, 938634. [Google Scholar] [CrossRef]
- Patel, K.M.; Desai, R.G.; Krishnan, S. Outcomes for Patients on Peripheral Venoarterial Extracorporeal Membrane Oxygenation After 10-Year Analysis of the Extracorporeal Life Support Organization Registry-What Lessons Can Be Learned. J. Cardiothorac. Vasc. Anesth. 2024, 38, 12–15. [Google Scholar] [CrossRef]
- Mehta, A.; Vavilin, I.; Nguyen, A.H.; Batchelor, W.; Blumer, V.; Cilia, L.; Dewanjee, A.; Desai, M.; Desai, S.; Flanagan, M.C.; et al. Contemporary approach to cardiogenic shock care: A state-of-the-art review. Front. Cardiovasc. Med. 2024, 11, 1354158. [Google Scholar] [CrossRef]
- Lorusso, R.; Whitman, G.; Milojevic, M.; Raffa, G.M.; McMullan, D.M.; Boeken, U.; Haft, J.W.; Bermúdez, C.; Shah, A.S.; DʼAlessandro, D.A. 2020 EACTS/ELSO/STS/AATS Expert Consensus on Post-Cardiotomy Extracorporeal Life Support in Adult Patients. Ann. Thorac. Surg. 2021, 111, 327–369. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Akın, İ.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef]
- Biancari, F.; Kaserer, A.; Perrotti, A.; Ruggieri, V.G.; Cho, S.M.; Kang, J.K.; Dalén, M.; Welp, H.; Jónsson, K.; Ragnarsson, S.; et al. Central versus Peripheral Postcardiotomy Veno-Arterial Extracorporeal Membrane Oxygenation: Systematic Review and Individual Patient Data Meta-Analysis. J. Clin. Med. 2022, 11, 7406. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Diaz-Gomez, J.L.; Ghanta, R.K.; Bracey, A.W.; Chatterjee, S. Management of Extracorporeal Membrane Oxygenation for Postcardiotomy Cardiogenic Shock. Anesthesiology 2021, 135, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Swedzky, F.; Barbagelata, A.; Perrone, S.; Kaplinsky, E.; Ducharme, A. Emerging concepts in heart failure management and treatment: Circulatory support with extracorporeal membrane oxygenation (ECMO). Drugs Context 2023, 12, 2022-7-7. [Google Scholar] [CrossRef]
- Combes, A.; Price, S.; Levy, B. What’s new in VA-ECMO for acute myocardial infarction-related cardiogenic shock. Intensive Care Med. 2024, 50, 590–592. [Google Scholar] [CrossRef]
- Moral-Muñoz, J.A.; Liu, X.; Santisteban-Espejo, A.; Cobo, M.J. Software tools for conducting bibliometric analysis in science: An up-to-date review. Prof. Inf. 2020, 29, e290103. [Google Scholar] [CrossRef]
- Schmidt, M.; Burrell, A.; Roberts, L.A.; Bailey, M.; Sheldrake, J.; Rycus, P.; Hodgson, C.; Scheinkestel, C.; Cooper, J.; Thiagarajan, R.R.; et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur. Heart J. 2015, 36, 2246–2256. [Google Scholar] [CrossRef]
- Muller, G.; Flécher, E.; Lebreton, G.; Luyt, C.É.; Trouillet, J.L.; Bréchot, N.; Schmidt, M.; Mastroianni, C.; Chastre, J.; Leprince, P.; et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016, 42, 370–378. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.G.; Blankenberg, S.; Brunner, S.; Colson, P.; Cudemus Deseda, G.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated with Lower Mortality in Patients with Cardiogenic Shock Treated with Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.A.; Raveendran, G.; Walser, E.; Connett, J.E.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Conrad, S.A. ECMO: Past, present, and future. J. Card. Crit. Care TSS 2023, 7, 3–5. [Google Scholar] [CrossRef]
- Mosier, J.; Kelsey, M.; Raz, Y.; Gunnerson, K.J.; Meyer, R.J.; Hypes, C.; Malo, J.; Whitmore, S.P.; Spaite, D.W. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: History, current applications, and future directions. Crit. Care 2015, 19, 431. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.C. ECMO in the 90’S. In Cardiac Surgery; Springer: Berlin/Heidelberg, Germany, 1995; pp. 135–141. [Google Scholar] [CrossRef]
- Fagnoul, D.; Pasquier, P.; Bodson, L.; Ortiz, J.A.; Vincent, J.L.; De Backer, D. Myocardial dysfunction during H1N1 influenza infection. J. Crit. Care 2013, 28, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.; Peter, J.V.; Pichamuthu, K.; Ramakrishna, K.; Moorthy, M.; Karthik, R.; John, G. Cardiac manifestations in patients with pandemic (H1N1) 2009 virus infection needing intensive care. J. Crit. Care 2012, 27, 106.e1–106.e6. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Y.; Ullah, W.; Rauf, H.; Virk, H.U.H.; Yadav, S.; Chowdhury, M.; Connerney, M.; Mamtani, S.; Pahuja, M.; Patel, R.; et al. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. Int. J. Cardiol. Heart Vasc. 2020, 29, 100589. [Google Scholar] [CrossRef]
- Bartlett, R.H.; Gazzaniga, A.B.; Jefferies, M.R.; Huxtable, R.F.; Haiduc, N.J.; Fong, S.W. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans. Am. Soc. Artif. Intern. Organs 1976, 22, 80–93. [Google Scholar] [CrossRef]
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong, M.E.H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.; Alonso, Á.; Beaton, A.; Bittencourt, M.; Boehme, A.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Taccone, F.S.; Minini, A.; Avalli, L.; Alm-Kruse, K.; Annoni, F.; Bougouin, W.; Burrell, A.; Cariou, A.; Coppalini, G.; Grunau, B.; et al. Impact of extracorporeal cardiopulmonary resuscitation on neurological prognosis and survival in adult patients after cardiac arrest: An individual pooled patient data meta-analysis. Resuscitation 2024, 202, 110357. [Google Scholar] [CrossRef]
- Bertić, M.; Worme, M.; Foroutan, F.; Rao, V.; Ross, H.J.; Billia, F.; Alba, A.C. Predictors of Survival and Favorable Neurologic Outcome in Patients Treated with eCPR: A Systematic Review and Meta-analysis. J. Cardiovasc. Transl. Res. 2022, 15, 279–290. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, J.W.; Yu, H.Y.; Ko, W.J.; Jerng, J.S.; Chang, W.T.; Chen, W.J.; Huang, S.C.; Chi, N.H.; Wang, C.H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Stub, D.; Bernard, S.; Pellegrino, V.; Smith, K.; Walker, T.; Sheldrake, J.; Hockings, L.E.; Shaw, J.A.; Duffy, S.J.; Burrell, A.; et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 2015, 86, 88–94. [Google Scholar] [CrossRef]
- Granfeldt, A.; Holmberg, M.J.; Andersen, L.W. Extracorporeal Cardiopulmonary Resuscitation for Cardiac Arrest. JAMA 2023, 329, 1693–1694. [Google Scholar] [CrossRef] [PubMed]
- Resuscitation Group of the Emergency Medicine Branch of the Chinese Medical Association, Emergency Professional Committee of the China Medical Education Association. Expert Consensus on Adult Extracorporeal Cardiopulmonary Resuscitation (2023 Edition). Chin. J. Emerg. Med. 2023, 32, 298–304. [Google Scholar] [CrossRef]
- Hirsch, K.G.; Abella, B.S.; Amorim, E.; Bader, M.K.; Barletta, J.F.; Berg, K.M.; Callaway, C.W.; Friberg, H.; Gilmore, E.J.; Greer, D.M.; et al. Critical Care Management of Patients After Cardiac Arrest: A Scientific Statement From the American Heart Association and Neurocritical Care Society. Circulation 2024, 149, e168–e200. [Google Scholar] [CrossRef] [PubMed]
- Roumy, A.; Liaudet, L.; Rusca, M.; Marcucci, C.; Kirsch, M. Pulmonary complications associated with veno-arterial extra-corporeal membrane oxygenation: A comprehensive review. Crit. Care 2020, 24, 212. [Google Scholar] [CrossRef]
- Ezad, S.; Ryan, M.J.; Donker, D.W.; Pappalardo, F.; Barrett, N.A.; Camporota, L.; Price, S.; Kapur, N.K.; Perera, D. Unloading the Left Ventricle in Venoarterial ECMO: In Whom, When, and How. Circulation 2023, 147, 1237–1250. [Google Scholar] [CrossRef]
- Kim, M.C.; Lim, Y.; Lee, S.H.; Shin, Y.; Ahn, J.H.; Hyun, D.Y.; Cho, K.H.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; et al. Early Left Ventricular Unloading or Conventional Approach After Venoarterial Extracorporeal Membrane Oxygenation: The EARLY-UNLOAD Randomized Clinical Trial. Circulation 2023, 148, 1570–1581. [Google Scholar] [CrossRef]
- Baldetti, L.; Gallone, G. Left ventricular unloading and venting in veno-arterial extracorporeal membrane oxygenation: The importance of cardiogenic shock aetiology in guiding treatment strategies. ESC Heart Fail. 2024, 11, 615–618. [Google Scholar] [CrossRef]
- Hessel, E.A., 2nd. History of cardiopulmonary bypass (CPB). Best. Pract. Res. Clin. Anaesthesiol. 2015, 29, 99–111. [Google Scholar] [CrossRef]
- Mariani, S.; Bari, G.; Ravaux, J.M.; van Bussel, B.C.T.; De Piero, M.E.; Schaefer, A.K.; Jawad, K.; Pozzi, M.; Loforte, A.; Kalampokas, N.; et al. Heterogeneity in Clinical Practices for Post-Cardiotomy Extracorporeal Life Support: A Pilot Survey from the PELS-1 Multicenter Study. Artif. Organs 2023, 47, 1641–1653. [Google Scholar] [CrossRef]
- Mariani, S.; Heuts, S.; van Bussel, B.C.T.; Di Mauro, M.; Wiedemann, D.; Saeed, D.; Pozzi, M.; Loforte, A.; Boeken, U.; Samalavičius, R.; et al. Patient and Management Variables Associated with Survival After Postcardiotomy Extracorporeal Membrane Oxygenation in Adults: The PELS-1 Multicenter Cohort Study. J. Am. Heart Assoc. 2023, 12, e029609. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Wang, I.-W.; van Bussel, B.C.T.; Heuts, S.; Wiedemann, D.; Saeed, D.; van der Horst, I.C.C.; Pozzi, M.; Loforte, A.; Boeken, U.; et al. The importance of timing in postcardiotomy venoarterial extracorporeal membrane oxygenation: A descriptive multicenter observational study. J. Thorac. Cardiovasc. Surg. 2023, 166, 1670–1682.e33. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Schaefer, A.-K.; van Bussel, B.C.T.; Di Mauro, M.; Conci, L.; Szalkiewicz, P.; De Piero, M.E.; Heuts, S.; Ravaux, J.M.; van der Horst, I.C.C.; et al. On-Support and Postweaning Mortality in Postcardiotomy Extracorporeal Membrane Oxygenation. Ann. Thorac. Surg. 2023, 116, 1079–1089. [Google Scholar] [CrossRef]
- Chiarini, G.; Mariani, S.; Schaefer, A.-K.; van Bussel, B.C.T.; Di Mauro, M.; Wiedemann, D.; Saeed, D.; Pozzi, M.; Botta, L.; Boeken, U.; et al. Neurologic complications in patients receiving aortic versus subclavian versus femoral arterial cannulation for post-cardiotomy extracorporeal life support: Results of the PELS observational multicenter study. Crit. Care 2024, 28, 265. [Google Scholar] [CrossRef]
- Winiszewski, H.; Piton, G.; Capellier, G. Early hyperoxia and 28-day mortality in patients on venoarterial ECMO support for refractory cardiogenic shock: Discussion about potential confounding factors. Crit. Care 2022, 26, 313. [Google Scholar] [CrossRef]
- Montero, S.; Rivas-Lasarte, M.; Huang, F.; Chommeloux, J.; Demondion, P.; Bréchot, N.; Hékimian, G.; Franchineau, G.; Persichini, R.; Luyt, C.-É.; et al. Time course, factors related to, and prognostic impact of venoarterial extracorporeal membrane flow in cardiogenic shock. ESC Heart Fail. 2023, 10, 568–577. [Google Scholar] [CrossRef]
- Danial, P.; Olivier, M.-E.; Bréchot, N.; Ponnaiah, M.; Schoell, T.; D’Alessandro, C.; Demondion, P.; Clément, M.; Juvin, C.; Carillion, A.; et al. Association Between Shock Etiology and 5-Year Outcomes After Venoarterial Extracorporeal Membrane Oxygenation. J. Am. Coll. Cardiol. 2023, 81, 897–909. [Google Scholar] [CrossRef]
- Ammirati, E.; Vandenbriele, C.; Nascimbene, A. Key Predictors of Outcome in Patients with Fulminant Myocarditis Supported by Venoarterial Extracorporeal Membrane Oxygenation. Circ. Heart Fail. 2023, 16, e010670. [Google Scholar] [CrossRef]
- Giordano, L.; Francavilla, A.; Bottio, T.; Dell’Amore, A.; Gregori, D.; Navalesi, P.; Lorenzoni, G.; Baldi, I. Predictive models in extracorporeal membrane oxygenation (ECMO): A systematic review. Syst. Rev. 2023, 12, 44. [Google Scholar] [CrossRef]
- Stephens, A.F.; Šeman, M.; Diehl, A.; Pilcher, D.; Barbaro, R.P.; Brodie, D.; Pellegrino, V.; Kaye, D.M.; Gregory, S.D.; Hodgson, C. ECMO PAL: Using deep neural networks for survival prediction in venoarterial extracorporeal membrane oxygenation. Intensive Care Med. 2023, 49, 1090–1099. [Google Scholar] [CrossRef]
- Braun, J.; Sahli, S.D.; Spahn, D.R.; Röder, D.; Neb, H.; Lotz, G.; Aser, R.; Wilhelm, M.J.; Kaserer, A. Predicting Survival for Veno-Arterial ECMO Using Conditional Inference Trees-A Multicenter Study. J. Clin. Med. 2023, 12, 6243. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Pierucci, N.; Cipollone, P.; Vignaroli, W.; Piro, A.; Compagnucci, P.; Matteucci, A.; Chimenti, C.; Pandozi, C.; Dello Russo, A.; et al. Mechanical Circulatory Support Systems in the Management of Ventricular Arrhythmias: A Contemporary Overview. J. Clin. Med. 2024, 13, 1746. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Varrias, D.; Smoller, R.; Mokhtari, M.B.; Rangasamy, S.; Pierce, M.; Alvarez Villela, M. Effect of mechanical unloading in venoarterial extracorporeal membrane oxygenation on hemolysis, bleeding and renal function. J. Heart Lung Transplant. 2024, 43, S245. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Z.; Feng, W.; Li, C.-e.; Chen, W.; Zhang, Y.; Pan, S.-x.; Fan, Z.-k. Improved remote infusion catheter for prevention of acute limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation. Brief Commun. 2024, 1, 32. [Google Scholar] [CrossRef]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabía, F.A.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef]


| Key Word | 2020 | 2021 | 2022 | 2023 | 2024 | Total |
|---|---|---|---|---|---|---|
| Extracorporeal membrane oxygenation | 229 | 265 | 267 | 259 | 182 | 1202 |
| Cardiogenic shock | 76 | 99 | 121 | 84 | 76 | 456 |
| Cardiopulmonary bypass | 78 | 68 | 83 | 68 | 27 | 324 |
| Congenital heart disease | 63 | 69 | 65 | 67 | 31 | 295 |
| Cardiac surgery | 57 | 58 | 68 | 55 | 35 | 273 |
| Mechanical circulatory support | 34 | 57 | 63 | 51 | 42 | 247 |
| Cardiac arrest | 49 | 51 | 53 | 60 | 34 | 247 |
| VA-ECMO | 32 | 38 | 61 | 45 | 47 | 223 |
| Outcomes | 44 | 44 | 40 | 32 | 26 | 186 |
| Heart transplantation | 28 | 44 | 40 | 39 | 25 | 176 |
| Mortality | 25 | 37 | 43 | 39 | 16 | 160 |
| Heart failure | 33 | 37 | 35 | 28 | 26 | 159 |
| Pediatrics | 31 | 41 | 28 | 35 | 24 | 159 |
| Extracorporeal cardiopulmonary resuscitation | 25 | 35 | 32 | 32 | 31 | 155 |
| Left ventricular assist device | 37 | 30 | 38 | 26 | 16 | 147 |
| Coronary artery bypass grafting | 28 | 29 | 25 | 37 | 20 | 139 |
| Cardiopulmonary resuscitation | 32 | 16 | 23 | 28 | 28 | 127 |
| COVID-19 | 20 | 38 | 34 | 21 | 9 | 122 |
| Shock | 18 | 22 | 25 | 32 | 7 | 104 |
| Impella | 16 | 22 | 22 | 20 | 18 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhou, K.; Chen, Y.; Ling, Y.; Qin, Q.; Lu, J. Global Trends in Extracorporeal Membrane Oxygenation Support for Circulatory Failure: A Bibliometric Analysis. Healthcare 2025, 13, 1365. https://doi.org/10.3390/healthcare13121365
Gao H, Zhou K, Chen Y, Ling Y, Qin Q, Lu J. Global Trends in Extracorporeal Membrane Oxygenation Support for Circulatory Failure: A Bibliometric Analysis. Healthcare. 2025; 13(12):1365. https://doi.org/10.3390/healthcare13121365
Chicago/Turabian StyleGao, Hanming, Kaihuan Zhou, Yin Chen, Yicong Ling, Qianqian Qin, and Junyu Lu. 2025. "Global Trends in Extracorporeal Membrane Oxygenation Support for Circulatory Failure: A Bibliometric Analysis" Healthcare 13, no. 12: 1365. https://doi.org/10.3390/healthcare13121365
APA StyleGao, H., Zhou, K., Chen, Y., Ling, Y., Qin, Q., & Lu, J. (2025). Global Trends in Extracorporeal Membrane Oxygenation Support for Circulatory Failure: A Bibliometric Analysis. Healthcare, 13(12), 1365. https://doi.org/10.3390/healthcare13121365







