Harnessing Extended Reality for Neurocognitive Training in Chronic Pain: State of the Art, Opportunities, and Future Directions
Abstract
1. Introduction
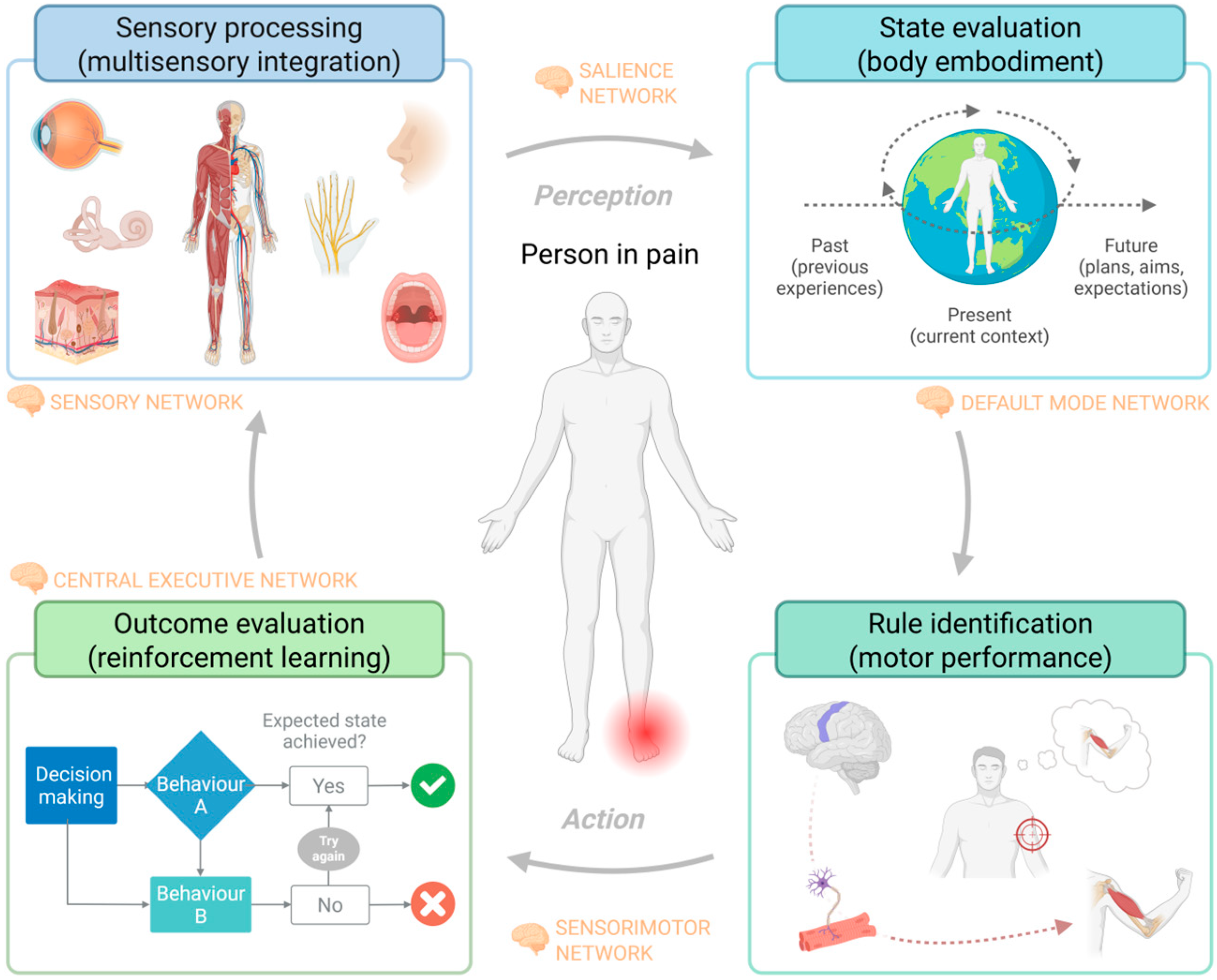
1.1. Mechanism of Neurocognitive Impairments in Chronic Pain Populations
1.2. Sensorimotor Influence of Neurocognitive Impairments in People with Pain
1.3. Chronic Pain Conditions Showing Neurocognitive Impairment
1.4. Neurocognitive Training in Chronic Pain
2. Materials and Methods
2.1. Research Question
2.2. Literature Sources
2.3. Search Parameters
2.4. Data Cleaning
3. Summary of Findings
XR-Based Neurocognitive Training: Benefits and Challenges
4. Future Directions
- Larger sample sizes and longer follow-up times (i.e., 6–12 months) to improve the findings’ trustworthiness.
- Evaluations of the additional value of XR-based neurocognitive training in chronic pain compared to usual care.
- Because of the lack of and need for rigorous randomized controlled trials to examine the efficacy of XR compared to usual care or a wait-and-see approach, we encourage studies that would guide stakeholders in implementing XR-based neurocognitive training in daily practice. Future XR research would benefit from the development of standardized outcome measures and reporting frameworks. Initiatives such as the CONSORT extensions for non-pharmacologic interventions may serve as useful models.
- Standardized cognitive assessment tools to measure specific cognitive domains over time.
- Establishment of proper dosimetry and user experiences to guide evidence-based XR-based neurocognitive training in clinical practice.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| XR | Extended Reality |
| ADL | Activities of Daily Living |
| VR | Virtual Reality |
| MR | Mixed Reality |
| AR | Augmented Reality |
| ICD-11 | International Classification of Diseases |
| IASP | International Association for the Study of Pain |
| OUDs | Opioid-Related Use Disorders |
References
- Sunzini, F.; Schrepf, A.; Clauw, D.J.; Basu, N. The Biology of Pain: Through the Rheumatology Lens. Arthritis Rheumatol. 2023, 75, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Primary Pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Schmidt, J.; Michael, F.; Weisbrod, M. Relevance of Neurocognition in Chronic Pain Syndrome: A Systematic and Methodical Approach. J. Clin. Exp. Neuropsychol. 2023, 45, 874–889. [Google Scholar] [CrossRef]
- Berginström, N.; Wåhlin, S.; Österlund, L.; Holmqvist, A.; Löfgren, M.; Stålnacke, B.-M.; Möller, M.C. Executive Functioning Is Associated to Everyday Interference of Pain in Patients with Chronic Pain. PLoS ONE 2024, 19, e0313187. [Google Scholar] [CrossRef]
- Nahin, R.L.; DeKosky, S.T. Comorbid Pain and Cognitive Impairment in a Nationally Representative Adult Population: Prevalence and Associations with Health Status, Health Care Utilization, and Satisfaction with Care. Clin. J. Pain 2020, 36, 725–739. [Google Scholar] [CrossRef]
- Rodríguez-Andreu, J.; Ibáñez-Bosch, R.; Portero-Vázquez, A.; Masramon, X.; Rejas, J.; Gálvez, R. Cognitive Impairment in Patients with Fibromyalgia Syndrome as Assessed by the Mini-Mental State Examination. BMC Musculoskelet. Disord. 2009, 10, 162. [Google Scholar] [CrossRef]
- Berryman, C.; Stanton, T.R.; Bowering, K.J.; Tabor, A.; McFarlane, A.; Moseley, G.L. Do People with Chronic Pain Have Impaired Executive Function? A Meta-Analytical Review. Clin. Psychol. Rev. 2014, 34, 563–579. [Google Scholar] [CrossRef]
- Moriarty, O.; Finn, D.P. Cognition and Pain. Curr. Opin. Support. Palliat. Care 2014, 8, 130–136. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying Neurocognitive Disorders: The DSM-5 Approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.; Mickle, A.M.; Wiggins, M.E.; Cardoso, J.; Lai, S.; Tanner, J.J.; Staud, R.; Fillingim, R.B.; Price, C.C.; Sibille, K.T. Relationships Between Cognitive Screening Composite Scores and Pain Intensity and Pain Disability in Adults with/at Risk for Knee Osteoarthritis. Clin. J. Pain 2022, 38, 470–475. [Google Scholar] [CrossRef]
- Khera, T.; Rangasamy, V. Cognition and Pain: A Review. Front. Psychol. 2021, 12, 673962. [Google Scholar] [CrossRef]
- Pereira Nery, E.C.H.; Rocha, N.P.; Cruz, V.T.; Silva, A.G. Systematic Review and Meta-Analysis on the Association between Chronic Low Back Pain and Cognitive Function. Pain Pract. 2023, 23, 399–408. [Google Scholar] [CrossRef]
- Higgins, D.M.; Martin, A.M.; Baker, D.G.; Vasterling, J.J.; Risbrough, V. The Relationship between Chronic Pain and Neurocognitive Function: A Systematic Review. Clin. J. Pain 2018, 34, 262–275. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The Effect of Pain on Cognitive Function: A Review of Clinical and Preclinical Research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef]
- Baker, K.S.; Georgiou-Karistianis, N.; Lampit, A.; Valenzuela, M.; Gibson, S.J.; Giummarra, M.J. Computerised Training Improves Cognitive Performance in Chronic Pain: A Participant-Blinded Randomised Active-Controlled Trial with Remote Supervision. Pain 2018, 159, 644–655. [Google Scholar] [CrossRef]
- Baker, K.S.; Georgiou-Karistianis, N.; Gibson, S.J.; Giummarra, M.J. Optimizing Cognitive Function in Persons With Chronic Pain. Clin. J. Pain 2017, 33, 462–472. [Google Scholar] [CrossRef]
- McGuire, B.E. Chronic Pain and Cognitive Function. Pain 2013, 154, 964–965. [Google Scholar] [CrossRef]
- Fleck, D.E.; Marian, W.; Daniel, L.; Jeffrey, A.W.; Grace, A.; Anoop, S.; Kelly, C.; Winhusen, T.J. Neurocognitive Predictors of Adherence to an Online Pain Self-Management Program Adjunct to Long-Term Opioid Therapy. J. Clin. Exp. Neuropsychol. 2023, 45, 242–254. [Google Scholar] [CrossRef]
- Rusu, A.C.; Gajsar, H.; Schlüter, M.-C.; Bremer, Y.-I. Cognitive Biases Toward Pain: Implications for a Neurocognitive Processing Perspective in Chronic Pain and Its Interaction With Depression. Clin. J. Pain 2019, 35, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K.; Ploner, M.; Tracey, I. Neurocognitive Aspects of Pain Perception. Trends Cogn. Sci. 2008, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Legrain, V.; Van Damme, S.; Eccleston, C.; Davis, K.D.; Seminowicz, D.A.; Crombez, G. A Neurocognitive Model of Attention to Pain: Behavioral and Neuroimaging Evidence. Pain 2009, 144, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, L.; Tang, F.; Wu, S.; Hu, Y. Memory Impairment in Chronic Pain Patients and the Related Neuropsychological Mechanisms: A Review. Acta Neuropsychiatr. 2014, 26, 195–201. [Google Scholar] [CrossRef]
- Han, S.; Wang, J.; Zhang, W.; Tian, X. Chronic Pain–Related Cognitive Deficits: Preclinical Insights into Molecular, Cellular, and Circuit Mechanisms. Mol. Neurobiol. 2024, 61, 8123–8143. [Google Scholar] [CrossRef]
- Torta, D.M.; Legrain, V.; Mouraux, A.; Valentini, E. Attention to Pain! A Neurocognitive Perspective on Attentional Modulation of Pain in Neuroimaging Studies. Cortex 2017, 89, 120–134. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Smith, M.; Adhia, D. Pain and the Triple Network Model. Front. Neurol. 2022, 13, 757241. [Google Scholar] [CrossRef]
- Sabet, T.S.; Anderson, D.B.; Stubbs, P.W.; Buchbinder, R.; Terwee, C.B.; Chiarotto, A.; Gagnier, J.; Verhagen, A.P. Pain and Physical Function Are Common Core Domains across 40 Core Outcome Sets of Musculoskeletal Conditions: A Systematic Review. J. Clin. Epidemiol. 2025, 180, 111687. [Google Scholar] [CrossRef]
- Vittersø, A.D.; Halicka, M.; Buckingham, G.; Proulx, M.J.; Bultitude, J.H. The Sensorimotor Theory of Pathological Pain Revisited. Neurosci. Biobehav. Rev. 2022, 139, 104735. [Google Scholar] [CrossRef]
- Murray, G.M.; Sessle, B.J. Pain-Sensorimotor Interactions: New Perspectives and a New Model. Neurobiol. Pain 2024, 15, 100150. [Google Scholar] [CrossRef]
- Ahissar, E.; Assa, E. Perception as a Closed-Loop Convergence Process. eLife 2016, 5, e12830. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.; Crook, R.J.; Chen, Z.S. Post-Injury Pain and Behaviour: A Control Theory Perspective. Nat. Rev. Neurosci. 2023, 24, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Armas, J.; Flores-Cortes, M.; Pineda-Galan, C.; Luque-Suarez, A.; La Touche, R. Role of Immersive Virtual Reality in Motor Behaviour Decision-Making in Chronic Pain Patients. Brain Sci. 2023, 13, 617. [Google Scholar] [CrossRef]
- Chen, Z.S.; Wang, J. Pain, from Perception to Action: A Computational Perspective. iScience 2023, 26, 105707. [Google Scholar] [CrossRef]
- Maixner, W.; Fillingim, R.B.; Williams, D.A.; Smith, S.B.; Slade, G.D. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J. Pain 2016, 17, T93–T107. [Google Scholar] [CrossRef]
- Eilenberg, J.S.; Otis, J.D. A Unified, Transdiagnostic Approach to Pain Management. In Applications of the Unified Protocol in Health Conditions; Oxford Academic: Oxford, UK, 2023; pp. 131–154. [Google Scholar] [CrossRef]
- Krueger, R.F.; Eaton, N.R. Transdiagnostic Factors of Mental Disorders. World Psychiatry 2015, 14, 27. [Google Scholar] [CrossRef]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic Pain: Towards an Understanding of Prevalent Pain Conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Ojeda, B.; Dueñas, M.; Salazar, A.; Mico, J.A.; Torres, L.M.; Failde, I. Factors Influencing Cognitive Impairment in Neuropathic and Musculoskeletal Pain and Fibromyalgia. Pain Med. 2018, 19, 499–510. [Google Scholar] [CrossRef]
- Begasse de Dhaem, O.; Robbins, M.S. Cognitive Impairment in Primary and Secondary Headache Disorders. Curr. Pain Headache Rep. 2022, 26, 391–404. [Google Scholar] [CrossRef]
- Boscher, C.; Joly, F.; Clarisse, B.; Humbert, X.; Grellard, J.-M.; Binarelli, G.; Tron, L.; Licaj, I.; Lange, M. Perceived Cognitive Impairment in Breast Cancer Survivors and Its Relationships with Psychological Factors. Cancers 2020, 12, 3000. [Google Scholar] [CrossRef]
- Von Ah, D.; Storey, S.; Crouch, A. Relationship between Self-Reported Cognitive Function and Work-Related Outcomes in Breast Cancer Survivors. J. Cancer Surviv. 2018, 12, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wan, H.; Pan, H.; Xu, Y. Postoperative Cognitive Dysfunction—Current Research Progress. Front. Behav. Neurosci. 2024, 18, 1328790. [Google Scholar] [CrossRef]
- Steinmetz, J.; Christensen, K.B.; Lund, T.; Lohse, N.; Rasmussen, L.S.; ISPOCD Group. Long-Term Consequences of Postoperative Cognitive Dysfunction. Anesthesiology 2009, 110, 548–555. [Google Scholar] [CrossRef]
- Piskin, D.; Benjaminse, A.; Dimitrakis, P.; Gokeler, A. Neurocognitive and Neurophysiological Functions Related to ACL Injury: A Framework for Neurocognitive Approaches in Rehabilitation and Return-to-Sports Tests. Sports Health 2022, 14, 549–555. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Cuthbertson, F.C.; Welch, S.J.V.; Mehta, Z.; Rothwell, P.M. Underestimation of Cognitive Impairment by Mini-Mental State Examination Versus the Montreal Cognitive Assessment in Patients With Transient Ischemic Attack and Stroke. Stroke 2010, 41, 1290–1293. [Google Scholar] [CrossRef]
- Ferraro, M.C.; O’Connell, N.E.; Sommer, C.; Goebel, A.; Bultitude, J.H.; Cashin, A.G.; Moseley, G.L.; McAuley, J.H. Complex Regional Pain Syndrome: Advances in Epidemiology, Pathophysiology, Diagnosis, and Treatment. Lancet Neurol. 2024, 23, 522–533. [Google Scholar] [CrossRef]
- Sofuoglu, M.; Elise, E.D.; Andrew, J.W.; Carroll, K.M. Cognitive Function as a Transdiagnostic Treatment Target in Stimulant Use Disorders. J. Dual Diagn. 2016, 12, 90–106. [Google Scholar] [CrossRef]
- Baker, K.S.; Gibson, S.; Georgiou-Karistianis, N.; Roth, R.M.; Giummarra, M.J. Everyday Executive Functioning in Chronic Pain: Specific Deficits in Working Memory and Emotion Control, Predicted by Mood, Medications, and Pain Interference. Clin. J. Pain 2016, 32, 673–680. [Google Scholar] [CrossRef]
- Brennecke, A.B.; Barreto, E.S.R.; Lins-Kusterer, L.; de Araujo Azi, L.M.T.; Kraychete, D. Impact of Different Treatments for Chronic Pain on Cognitive Function: A Systematic Review. Br. J. Pain, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Flor, H.; Noguchi, K.; Treede, R.-D.; Turk, D.C. The Role of Evolving Concepts and New Technologies and Approaches in Advancing Pain Research, Management, and Education since the Establishment of the International Association for the Study of Pain. Pain 2023, 164, S16–S21. [Google Scholar] [CrossRef]
- Spiegel, B.M.R.; Rizzo, A.; Persky, S.; Liran, O.; Wiederhold, B.; Woods, S.; Donovan, K.; Sarkar, K.; Xiang, H.; Ahn, S.J. (Grace); et al. What Is Medical Extended Reality? A Taxonomy Defining the Current Breadth and Depth of an Evolving Field. J. Med. Ext. Real. 2024, 1, 4–12. [Google Scholar] [CrossRef]
- Moreau, S.; Thérond, A.; Cerda, I.H.; Studer, K.; Pan, A.; Tharpe, J.; Crowther, J.E.; Abd-Elsayed, A.; Gilligan, C.; Tolba, R.; et al. Virtual Reality in Acute and Chronic Pain Medicine: An Updated Review. Curr. Pain Headache Rep. 2024, 28, 893–928. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Kobayashi, T.; Hirata, H.; Otani, K.; Sugimoto, M.; Tsukamoto, M.; Yoshihara, T.; Ueno, M.; Mawatari, M. XR (Extended Reality: Virtual Reality, Augmented Reality, Mixed Reality) Technology in Spine Medicine: Status Quo and Quo Vadis. J. Clin. Med. 2022, 11, 470. [Google Scholar] [CrossRef]
- Matthie, N.S.; Giordano, N.A.; Jenerette, C.M.; Magwood, G.S.; Leslie, S.L.; Northey, E.E.; Webster, C.I.; Sil, S. Use and Efficacy of Virtual, Augmented, or Mixed Reality Technology for Chronic Pain: A Systematic Review. Pain Manag. 2022, 12, 859–878. [Google Scholar] [CrossRef]
- Opara Zupančič, M.; Šarabon, N. The Current State of Virtual Reality in the Management of Musculoskeletal Conditions and Associated Chronic Pain: Terminology, Technology, and Associations. Appl. Sci. 2025, 15, 2564. [Google Scholar] [CrossRef]
- Turnbull, D.; Chugh, R.; Luck, J. Systematic-Narrative Hybrid Literature Review: A Strategy for Integrating a Concise Methodology into a Manuscript. Social. Sci. Humanit. Open 2023, 7, 100381. [Google Scholar] [CrossRef]
- Alashram, A.R.; Annino, G.; Padua, E.; Romagnoli, C.; Mercuri, N.B. Cognitive Rehabilitation Post Traumatic Brain Injury: A Systematic Review for Emerging Use of Virtual Reality Technology. J. Clin. Neurosci. 2019, 66, 209–219. [Google Scholar] [CrossRef]
- Austin, P.D. The Analgesic Effects of Virtual Reality for People with Chronic Pain: A Scoping Review. Pain Med. 2022, 23, 105–121. [Google Scholar] [CrossRef]
- Saquib, J.; AlMohaimeed, H.A.; AlOlayan, S.A.; AlRebdi, N.A.; AlBulaihi, J.I.; AlMugbel, R.A.; AlDughaishm, Y.M.; AlBahli, H.K.; Saquib, N. Effect of Interactive vs. Passive Virtual Reality on Pain Threshold and Tolerance. Scand. J. Pain 2022, 22, 167–172. [Google Scholar] [CrossRef]
- Lier, E.J.; Oosterman, J.M.; Assmann, R.; de Vries, M.; Van Goor, H. The Effect of Virtual Reality on Evoked Potentials Following Painful Electrical Stimuli and Subjective Pain. Sci. Rep. 2020, 10, 9067. [Google Scholar] [CrossRef]
- Wender, R.; Hoffman, H.G.; Hunner, H.H.; Seibel, E.J.; Patterson, D.R.; Sharar, S.R. Interactivity Influences the Magnitude of Virtual Reality Analgesia. J. Cyber Ther. Rehabil. 2009, 2, 27. [Google Scholar]
- Matamala-Gomez, M.; Donegan, T.; Bottiroli, S.; Sandrini, G.; Sanchez-Vives, M.V.; Tassorelli, C. Immersive Virtual Reality and Virtual Embodiment for Pain Relief. Front. Hum. Neurosci. 2019, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Brepohl, P.C.A.; Leite, H. Virtual Reality Applied to Physiotherapy: A Review of Current Knowledge. Virtual Real. 2023, 27, 71–95. [Google Scholar] [CrossRef]
- Levin, M.F. What Is the Potential of Virtual Reality for Post-Stroke Sensorimotor Rehabilitation? Expert. Rev. Neurother. 2020, 20, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Viderman, D.; Tapinova, K.; Dossov, M.; Seitenov, S.; Abdildin, Y.G. Virtual Reality for Pain Management: An Umbrella Review. Front. Med. 2023, 10, 1203670. [Google Scholar] [CrossRef]
- Trost, Z.; France, C.; Anam, M.; Shum, C. Virtual Reality Approaches to Pain: Toward a State of the Science. Pain 2021, 162, 325–331. [Google Scholar] [CrossRef]
- Giacomelli, L.; Martin Sölch, C.; Ledermann, K. The Effect of Virtual Reality Interventions on Reducing Pain Intensity in Chronic Pain Patients: A Systematic Review. Virtual Real. 2024, 28, 126. [Google Scholar] [CrossRef]
- Kenney, M.P.; Milling, L.S. The Effectiveness of Virtual Reality Distraction for Reducing Pain: A Meta-Analysis. Psychol. Conscious. Theory Res. Pract. 2016, 3, 199. [Google Scholar] [CrossRef]
- Goudman, L.; Jansen, J.; Billot, M.; Vets, N.; De Smedt, A.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-Analysis. JMIR Serious Games 2022, 10, e34402. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Zhou, X.; Zhan, L.; Wu, F.; Huang, Z.; Sun, Y.; Feng, Y.; Du, Q. Virtual Reality Therapy for the Management of Chronic Spinal Pain: Systematic Review and Meta-Analysis. JMIR Serious Games 2024, 12, e50089. [Google Scholar] [CrossRef]
- Baker, N.A.; Polhemus, A.H.; Ospina, E.H.; Feller, H.; Zenni, M.; Deacon, M.; DeGrado, G.; Basnet, S.; Driscoll, M. The State of Science in the Use of Virtual Reality in the Treatment of Acute and Chronic Pain: A Systematic Scoping Review. Clin. J. Pain 2022, 38, 424–441. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Yin, X.; Li, H. Effect of Virtual Reality Technology as Intervention for People with Kinesiophobia: A Meta-analysis of Randomised Controlled Trials. J. Clin. Nurs. 2023, 32, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Warty, R.R.; Sursas, J.A.; Payne, O.; Nair, A.; Krishnan, S.; da Silva Costa, F.; Wallace, E.M.; Vollenhoven, B. The Effectiveness of Virtual Reality in Managing Acute Pain and Anxiety for Medical Inpatients: Systematic Review. J. Med. Internet Res. 2020, 22, e17980. [Google Scholar] [CrossRef]
- Ioannou, A.; Papastavrou, E.; Avraamides, M.N.; Charalambous, A. Virtual Reality and Symptoms Management of Anxiety, Depression, Fatigue, and Pain: A Systematic Review. SAGE Open Nurs. 2020, 6, 2377960820936163. [Google Scholar] [CrossRef]
- Gautama, M.S.N.; Huang, T.-W.; Haryani, H. A Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effectiveness of Immersive Virtual Reality in Cancer Patients Receiving Chemotherapy. Eur. J. Oncol. Nurs. 2023, 67, 102424. [Google Scholar] [CrossRef]
- Porta, X.; Nieto, R.; Serrat, M.; Bourdin-Kreitz, P. Exploring User Perspectives on Virtual Reality as a Therapeutic Tool for Chronic Pain Management. Musculoskelet. Care 2025, 23, e70101. [Google Scholar] [CrossRef]
- Jahn, F.S.; Skovbye, M.; Obenhausen, K.; Jespersen, A.E.; Miskowiak, K.W. Cognitive Training with Fully Immersive Virtual Reality in Patients with Neurological and Psychiatric Disorders: A Systematic Review of Randomized Controlled Trials. Psychiatry Res. 2021, 300, 113928. [Google Scholar] [CrossRef]
- Moreno, A.; Wall, K.J.; Thangavelu, K.; Craven, L.; Ward, E.; Dissanayaka, N.N. A Systematic Review of the Use of Virtual Reality and Its Effects on Cognition in Individuals with Neurocognitive Disorders. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 834–850. [Google Scholar] [CrossRef]
- Manivannan, S.; Al-Amri, M.; Postans, M.; Westacott, L.J.; Gray, W.; Zaben, M. The Effectiveness of Virtual Reality Interventions for Improvement of Neurocognitive Performance After Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2019, 34, E52–E65. [Google Scholar] [CrossRef]
- Moulaei, K.; Sharifi, H.; Bahaadinbeigy, K.; Dinari, F. Efficacy of Virtual Reality-Based Training Programs and Games on the Improvement of Cognitive Disorders in Patients: A Systematic Review and Meta-Analysis. BMC Psychiatry 2024, 24, 116. [Google Scholar] [CrossRef]
- Riva, G.; Valentina, M.; Silvia, C.; Stramba-Badiale, C. Virtual Reality in Neurorehabilitation: A Review of Its Effects on Multiple Cognitive Domains. Expert. Rev. Med. Devices 2020, 17, 1035–1061. [Google Scholar] [CrossRef]
- Nascimento, S.S.; Oliveira, L.R.; DeSantana, J.M. Correlations between Brain Changes and Pain Management after Cognitive and Meditative Therapies: A Systematic Review of Neuroimaging Studies. Complement. Ther. Med. 2018, 39, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Čeko, M.; Baeuerle, T.; Webster, L.; Wager, T.D.; Lumley, M.A. The Effects of Virtual Reality Neuroscience-Based Therapy on Clinical and Neuroimaging Outcomes in Patients with Chronic Back Pain: A Randomized Clinical Trial. Pain 2024, 165, 1860–1874. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.G.; Seibel, C.C.; Coron, L.; Simons, L.E.; Drever, S.; Le May, S.; Mason, K.P.; Flor, H. Increasing Presence via a More Immersive VR System Increases Virtual Reality Analgesia and Draws More Attention into Virtual Reality in a Randomized Crossover Study. Front. Virtual Real. 2024, 5, 1452486. [Google Scholar] [CrossRef]
- Holzer, K.J.; Todorovic, M.S.; Wilson, E.A.; Steinberg, A.; Avidan, M.S.; Haroutounian, S. Cognitive Flexibility Training for Chronic Pain: A Randomized Clinical Study. Pain Rep. 2024, 9, e1120. [Google Scholar] [CrossRef]
- Gamito, P.; Oliveira, J.; Alves, C.; Santos, N.; Coelho, C.; Brito, R. Virtual Reality-Based Cognitive Stimulation to Improve Cognitive Functioning in Community Elderly: A Controlled Study. Cyberpsychol. Behav. Soc. Netw. 2020, 23, 150–156. [Google Scholar] [CrossRef]
- Hoffman, H.G. Interacting with Virtual Objects via Embodied Avatar Hands Reduces Pain Intensity and Diverts Attention. Sci. Rep. 2021, 11, 10672. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Ren, Z. Rehabilitative Effects of Virtual Reality Technology for Mild Cognitive Impairment: A Systematic Review With Meta-Analysis. Front. Psychol. 2020, 11, 1811. [Google Scholar] [CrossRef]
- Porras-Garcia, B.; Rojas-Rincón, J.; Adams, A.; Garolera, M.; Chang, R. Immersive Virtual Reality Cognitive Training for Improving Cognition and Depressive Symptoms Among Older Adults. Current Evidence and Future Recommendations. A Systematic Review. Cyberpsychol. Behav. Soc. Netw. 2024, 27, 692–703. [Google Scholar] [CrossRef]
- Yen, H.-Y.; Chiu, H.-L. Virtual Reality Exergames for Improving Older Adults’ Cognition and Depression: A Systematic Review and Meta-Analysis of Randomized Control Trials. J. Am. Med. Dir. Assoc. 2021, 22, 995–1002. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Tseng, H.-Y.; Lin, Y.-J.; Wang, C.-J.; Hsu, W.-C. Using Virtual Reality-Based Training to Improve Cognitive Function, Instrumental Activities of Daily Living and Neural Efficiency in Older Adults with Mild Cognitive Impairment. Eur. J. Phys. Rehabil. Med. 2020, 56, 47–57. [Google Scholar] [CrossRef]
- Maggio, M.G.; Latella, D.; Maresca, G.; Sciarrone, F.; Manuli, A.; Naro, A.; De Luca, R.; Calabrò, R.S. Virtual Reality and Cognitive Rehabilitation in People With Stroke: An Overview. J. Neurosci. Nurs. 2019, 51, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Montana, J.I.; Tuena, C.; Serino, S.; Cipresso, P.; Riva, G. Neurorehabilitation of Spatial Memory Using Virtual Environments: A Systematic Review. J. Clin. Med. 2019, 8, 1516. [Google Scholar] [CrossRef] [PubMed]
- Gür, O.; Başar, S. The Effect of Virtual Reality on Pain, Kinesiophobia and Function in Total Knee Arthroplasty Patients: A Randomized Controlled Trial. Knee 2023, 45, 187–197. [Google Scholar] [CrossRef]
- Harvie, D.S.; Kelly, J.; Kluver, J.; Deen, M.; Spitzer, E.; Coppieters, M.W. A Randomized Controlled Pilot Study Examining Immediate Effects of Embodying a Virtual Reality Superhero in People with Chronic Low Back Pain. Disabil. Rehabil. Assist. Technol. 2024, 19, 851–858. [Google Scholar] [CrossRef]
- Morales Tejera, D.; Beltran-Alacreu, H.; Cano-de-la-Cuerda, R.; Leon Hernandez, J.V.; Martín-Pintado-Zugasti, A.; Calvo-Lobo, C.; Gil-Martínez, A.; Fernández-Carnero, J. Effects of Virtual Reality versus Exercise on Pain, Functional, Somatosensory and Psychosocial Outcomes in Patients with Non-Specific Chronic Neck Pain: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 5950. [Google Scholar]
- Matamala-Gomez, M.; Gonzalez, A.M.D.; Slater, M.; Sanchez-Vives, M. V Decreasing Pain Ratings in Chronic Arm Pain through Changing a Virtual Body: Different Strategies for Different Pain Types. J. Pain 2019, 20, 685–697. [Google Scholar] [CrossRef]
- Garcia, L.; Birckhead, B.; Krishnamurthy, P.; Mackey, I.; Sackman, J.; Salmasi, V.; Louis, R.; Castro, C.; Maddox, R.; Maddox, T.; et al. Durability of the Treatment Effects of an 8-Week Self-Administered Home-Based Virtual Reality Program for Chronic Low Back Pain: 6-Month Follow-up Study of a Randomized Clinical Trial. J. Med. Internet Res. 2022, 24, e37480. [Google Scholar] [CrossRef]
- Sharifpour, S.; Manshaee, G.R.; Sajjadian, I. Effects of Virtual Reality Therapy on Perceived Pain Intensity, Anxiety, Catastrophising and Self-Efficacy among Adolescents with Cancer. Couns. Psychother. Res. 2021, 21, 218–226. [Google Scholar] [CrossRef]
- Darnall, B.D.; Krishnamurthy, P.; Tsuei, J.; Minor, J.D. Self-Administered Skills-Based Virtual Reality Intervention for Chronic Pain: Randomized Controlled Pilot Study. JMIR Form. Res. 2020, 4, e17293. [Google Scholar] [CrossRef]
- Gava, V.; Fialho, H.R.F.; Calixtre, L.B.; Barbosa, G.M.; Kamonseki, D.H. Effects of Gaming on Pain-Related Fear, Pain Catastrophizing, Anxiety, and Depression in Patients with Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Games Health J. 2022, 11, 369–384. [Google Scholar] [CrossRef]
- Cai, H.; Guichen, L.; Shanshan, H.; Yufei, L.; Chen, L. Effect of Exercise on Cognitive Function in Chronic Disease Patients: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Clin. Interv. Aging 2017, 12, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Hao, S. Exercise-Induced Neuroplasticity: A New Perspective on Rehabilitation for Chronic Low Back Pain. Front. Mol. Neurosci. 2024, 17, 1407445. [Google Scholar] [CrossRef]
- Jolles, D.; Crone, E.A. Training the Developing Brain: A Neurocognitive Perspective. Front. Hum. Neurosci. 2012, 6, 76. [Google Scholar] [CrossRef]
- Sala, G.; Gobet, F. Cognitive Training Does Not Enhance General Cognition. Trends Cogn. Sci. 2019, 23, 9–20. [Google Scholar] [CrossRef]
- Brugada-Ramentol, V.; Bozorgzadeh, A.; Jalali, H. Enhance VR: A Multisensory Approach to Cognitive Training and Monitoring. Front. Digit. Health 2022, 4, 916052. [Google Scholar] [CrossRef]
- Hong, X.L.; Cheng, L.J.; Feng, R.C.; Goh, J.; Gyanwali, B.; Itoh, S.; TAM, W.S.W.; Wu, X.V. Effect of Physio-Cognitive Dual-Task Training on Cognition in Pre-Ageing and Older Adults with Neurocognitive Disorders: A Meta-Analysis and Meta-Regression of Randomized Controlled Trial. Arch. Gerontol. Geriatr. 2024, 116, 105161. [Google Scholar] [CrossRef]
- Chowdhary, N.; Barbui, C.; Anstey, K.J.; Kivipelto, M.; Barbera, M.; Peters, R.; Zheng, L.; Kulmala, J.; Stephen, R.; Ferri, C.P.; et al. Reducing the Risk of Cognitive Decline and Dementia: WHO Recommendations. Front. Neurol. 2022, 12, 765584. [Google Scholar] [CrossRef]
- Sáez-Gutiérrez, S.; Fernandez-Rodriguez, E.J.; Sanchez-Gomez, C.; Garcia-Martin, A.; Polo-Ferrero, L.; Barbero-Iglesias, F.J. Effectiveness of Different Neurocognitive Intervention Approaches on Functionality in Healthy Older Adults: A Systematic Review. Behav. Sci. 2024, 14, 87. [Google Scholar] [CrossRef]
- Mitsea, E.; Drigas, A.; Skianis, C. Virtual Reality Mindfulness for Meta-Competence Training among People with Different Mental Disorders: A Systematic Review. Psychiatry Int. 2023, 4, 324–353. [Google Scholar] [CrossRef]
- García-Betances, R.I.; Cabrera-Umpiérrez, M.F.; Arredondo, M.T. Computerized Neurocognitive Interventions in the Context of the Brain Training Controversy. Prog. Neurobiol. 2018, 29, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; Maresca, G.; De Luca, R.; Stagnitti, M.C.; Porcari, B.; Ferrera, M.C.; Galletti, F.; Casella, C.; Manuli, A.; Calabrò, R.S. The Growing Use of Virtual Reality in Cognitive Rehabilitation: Fact, Fake or Vision? A Scoping Review. J. Natl. Med. Assoc. 2019, 111, 457–463. [Google Scholar] [CrossRef]
- Glueck, A.C.; Han, D.Y. Improvement Potentials in Balance and Visuo-Motor Reaction Time after Mixed Reality Action Game Play: A Pilot Study. Virtual Real. 2020, 24, 223–229. [Google Scholar] [CrossRef]
- Béraud-Peigné, N.; Maillot, P.; Perrot, A. The Effects of a New Immersive Multidomain Training on Cognitive, Dual-Task and Physical Functions in Older Adults. Geroscience 2024, 46, 1825–1841. [Google Scholar] [CrossRef]
- Bulle-Smid, L.; Keuning, W.; Van Den Heuvel, R.; Hakvoort, G.; Verhoeven, F.; Daniels, R.; Hettinga, M. The Use of Extended Reality in Rehabilitation for Patients with Acquired Brain Injury: A Scoping Review. Technol. Disabil. 2024, 37, 3–44. [Google Scholar] [CrossRef]
- Sokołowska, B. Impact of Virtual Reality Cognitive and Motor Exercises on Brain Health. Int. J. Environ. Res. Public Health 2023, 20, 4150. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, K.A.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined Physical and Cognitive Training for Older Adults with and without Cognitive Impairment: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef]
- Herold, F.; Hamacher, D.; Schega, L.; Müller, N.G. Thinking While Moving or Moving While Thinking—Concepts of Motor-Cognitive Training for Cognitive Performance Enhancement. Front. Aging Neurosci. 2018, 10, 228. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Chen, I.-H.; Lin, Y.-J.; Chen, Y.; Hsu, W.-C. Effects of Virtual Reality-Based Physical and Cognitive Training on Executive Function and Dual-Task Gait Performance in Older Adults With Mild Cognitive Impairment: A Randomized Control Trial. Front. Aging Neurosci. 2019, 11, 162. [Google Scholar] [CrossRef]
- Alfieri, F.M.; da Silva Dias, C.; de Oliveira, N.C.; Battistella, L.R. Gamification in Musculoskeletal Rehabilitation. Curr. Rev. Musculoskelet. Med. 2022, 15, 629–636. [Google Scholar] [CrossRef]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to Treatment Adherence in Physiotherapy Outpatient Clinics: A Systematic Review. Man. Ther. 2010, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.H.; Pot-Kolder, R.; Rizzo, A.; Rus-Calafell, M.; Cardi, V.; Cella, M.; Ward, T.; Riches, S.; Reinoso, M.; Thompson, A.; et al. Advances in the Use of Virtual Reality to Treat Mental Health Conditions. Nat. Rev. Psychol. 2024, 3, 552–567. [Google Scholar] [CrossRef]
- Pallavicini, F.; Ferrari, A.; Mantovani, F. Video Games for Well-Being: A Systematic Review on the Application of Computer Games for Cognitive and Emotional Training in the Adult Population. Front. Psychol. 2018, 9, 2127. [Google Scholar] [CrossRef] [PubMed]
- Vermeir, J.F.; White, M.J.; Johnson, D.; Crombez, G.; Van Ryckeghem, D.M.L. The Effects of Gamification on Computerized Cognitive Training: Systematic Review and Meta-Analysis. JMIR Serious Games 2020, 8, e18644. [Google Scholar] [CrossRef]
- Guzmán, D.E.; Rengifo, C.F.; Guzmán, J.D.; Garcia Cena, C.E. Virtual Reality Games for Cognitive Rehabilitation of Older Adults: A Review of Adaptive Games, Domains and Techniques. Virtual Real. 2024, 28, 92. [Google Scholar] [CrossRef]
- Swinnen, N.; Mathieu, V.; Vancampfort, D. Exergames in People with Major Neurocognitive Disorder: A Systematic Review. Disabil. Rehabil. Assist. Technol. 2022, 17, 376–389. [Google Scholar] [CrossRef]
- Guerra-Armas, J.; Flores-Cortes, M.; Ceniza-Bordallo, G.; Matamala-Gomez, M. User Experience in Immersive Virtual Reality-Induced Hypoalgesia in Adults and Children Suffering from Pain Conditions. Multimodal Technol. Interact. 2024, 8, 66. [Google Scholar] [CrossRef]
- Parsons, T.D. Virtual Reality for Enhanced Ecological Validity and Experimental Control in the Clinical, Affective and Social Neurosciences. Front. Hum. Neurosci. 2015, 9, 660. [Google Scholar] [CrossRef]
- Huygelier, H.; Mattheus, E.; Abeele, V.V.; van Ee, R.; Gillebert, C.R. The Use of the Term Virtual Reality in Post-Stroke Rehabilitation: A Scoping Review and Commentary. Psychol. Belg. 2021, 61, 145. [Google Scholar] [CrossRef]
- Slatman, S.; Staal, J.B.; van Goor, H.; Ostelo, R.; Soer, R.; Knoop, J. Limited Use of Virtual Reality in Primary Care Physiotherapy for Patients with Chronic Pain. BMC Musculoskelet. Disord. 2024, 25, 168. [Google Scholar] [CrossRef]
- Combalia, A.; Sanchez-Vives, M.V.; Donegan, T. Immersive Virtual Reality in Orthopaedics—A Narrative Review. Int. Orthop. 2024, 48, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Glegg, S.M.N.; Levac, D.E. Barriers, Facilitators and Interventions to Support Virtual Reality Implementation in Rehabilitation: A Scoping Review. PMR 2018, 10, 1237–1251. [Google Scholar] [CrossRef]
- Garrett, B.; Taverner, T.; Gromala, D.; Tao, G.; Cordingley, E.; Sun, C. Virtual Reality Clinical Research: Promises and Challenges. JMIR Serious Games 2018, 6, e10839. [Google Scholar] [CrossRef]
- Nijs, J.; Malfliet, A.; Roose, E.; Lahousse, A.; Van Bogaert, W.; Johansson, E.; Runge, N.; Goossens, Z.; Labie, C.; Bilterys, T.; et al. Personalized Multimodal Lifestyle Intervention as the Best-Evidenced Treatment for Chronic Pain: State-of-the-Art Clinical Perspective. J. Clin. Med. 2024, 13, 644. [Google Scholar] [CrossRef]
- Mansell, W.; Harvey, A.; Watkins, E.R.; Shafran, R. Cognitive Behavioral Processes Across Psychological Disorders: A Review of the Utility and Validity of the Transdiagnostic Approach. Int. J. Cogn. Ther. 2008, 1, 181–191. [Google Scholar] [CrossRef]
- Borgne, M.L.; Boudoukha, A.H.; Petit, A.; Roquelaure, Y. Chronic Low Back Pain and the Transdiagnostic Process: How Do Cognitive and Emotional Dysregulations Contribute to the Intensity of Risk Factors and Pain? Scand. J. Pain 2017, 17, 309–315. [Google Scholar] [CrossRef]
- Mazzolenis, M.V.; Mourra, G.N.; Moreau, S.; Mazzolenis, M.E.; Cerda, I.H.; Vega, J.; Khan, J.S.; Thérond, A. The Role of Virtual Reality and Artificial Intelligence in Cognitive Pain Therapy: A Narrative Review. Curr. Pain Headache Rep. 2024, 28, 881–892. [Google Scholar] [CrossRef]
- Pons, P.; Navas-Medrano, S.; Soler-Dominguez, J.L. Extended Reality for Mental Health: Current Trends and Future Challenges. Front. Comput. Sci. 2022, 4, 1034307. [Google Scholar] [CrossRef]
- Aasdahl, L.; Granviken, F.; Meisingset, I.; Woodhouse, A.; Evensen, K.A.I.; Vasseljen, O. Recovery Trajectories in Common Musculoskeletal Complaints by Diagnosis Contra Prognostic Phenotypes. BMC Musculoskelet. Disord. 2021, 22, 455. [Google Scholar]
- Meisingset, I.; Vasseljen, O.; Vøllestad, N.K.; Robinson, H.S.; Woodhouse, A.; Engebretsen, K.B.; Glette, M.; Øverås, C.K.; Nordstoga, A.L.; Evensen, K.A.I.; et al. Novel Approach towards Musculoskeletal Phenotypes. Eur. J. Pain 2020, 24, 921–932. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Turk, D.C.; Angst, M.S.; Dionne, R.; Freeman, R.; Hansson, P.; Haroutounian, S.; Arendt-Nielsen, L.; Attal, N.; et al. Patient Phenotyping in Clinical Trials of Chronic Pain Treatments: IMMPACT Recommendations. Pain Rep. 2021, 6, e896. [Google Scholar] [CrossRef]
- Linton, S.J.; O’Sullivan, P.B.; Zetterberg, H.E.; Vlaeyen, J.W.S. The “Future” Pain Clinician: Competencies Needed to Provide Psychologically Informed Care. Scand. J. Pain 2024, 24, 20240017. [Google Scholar] [CrossRef] [PubMed]
- French, M.A.; Roemmich, R.T.; Daley, K.; Beier, M.; Penttinen, S.; Raghavan, P.; Searson, P.; Wegener, S.; Celnik, P. Precision Rehabilitation: Optimizing Function, Adding Value to Health Care. Arch. Phys. Med. Rehabil. 2022, 103, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Lier, E.J.; de Vries, M.; Steggink, E.M.; ten Broek, R.P.G.; van Goor, H. Effect Modifiers of Virtual Reality in Pain Management: A Systematic Review and Meta-Regression Analysis. Pain 2023, 164, 1658–1665. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement Items for Systematic Reviews in the Sport and Exercise Medicine, Musculoskeletal Rehabilitation and Sports Science Fields: The PERSiST (Implementing Prisma in Exercise, Rehabilitation, Sport Medicine and SporTs Science) Guidance. Br. J. Sports Med. 2022, 56, 175. [Google Scholar] [CrossRef]
- Dijkers, M.P. Overview of Reviews Using the Template for Intervention Description and Replication (TIDieR) as a Measure of Trial Intervention Reporting Quality. Arch. Phys. Med. Rehabil. 2021, 102, 1623–1632. [Google Scholar] [CrossRef]
- Butcher, N.J.; Monsour, A.; Mew, E.J.; Chan, A.-W.; Moher, D.; Mayo-Wilson, E.; Terwee, C.B.; Chee-A-Tow, A.; Baba, A.; Gavin, F. Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension. JAMA 2022, 328, 2252–2264. [Google Scholar] [CrossRef]



| Pain Targets | Authors | Key Findings in XR |
|---|---|---|
| Attention | Hoffman et al., 2024 [85] Holzer et al., 2024 [86] Gamitto et al., 2020 [87] Hoffman et al.,2021 [88] |
|
| Executive Function | Holzer et al., 2024 [86] Riva et al., 2020 [82] Wu et al., 2020 [89] Alasham et al., 2019 [58] |
|
| Memory | Porras-García et al., 2024 [90] Yen et al., 2021 [91] Riva et al., 2020 [82] Liao et al., 2019 [92] Moreno et al., 2019 [79] |
|
| Spatial Cognition | Riva et al., 2020 [82] Maggio et al., 2019 [93] Montana et al., 2019 [94] |
|
| Body Perception | Gur et al., 2024 [95] Harvie et al., 2024 [96] Morales-Tejeda et al., 2020 [97] Matamala-Gomez et al., 2019 [98] |
|
| Emotional Regulation | Goudmann et al., 2022 [70] García et al., 2022 [99] Sharifpour et al., 2021 [100] Darnall et al., 2020 [101] |
|
| Pain-Related Worries | Gava et al., 2022 [102] Ceko et al., 2022 [84] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Armas, J.; Roldán-Ruiz, A.; Flores-Cortes, M.; Harvie, D.S. Harnessing Extended Reality for Neurocognitive Training in Chronic Pain: State of the Art, Opportunities, and Future Directions. Healthcare 2025, 13, 1338. https://doi.org/10.3390/healthcare13111338
Guerra-Armas J, Roldán-Ruiz A, Flores-Cortes M, Harvie DS. Harnessing Extended Reality for Neurocognitive Training in Chronic Pain: State of the Art, Opportunities, and Future Directions. Healthcare. 2025; 13(11):1338. https://doi.org/10.3390/healthcare13111338
Chicago/Turabian StyleGuerra-Armas, Javier, Alberto Roldán-Ruiz, Mar Flores-Cortes, and Daniel S. Harvie. 2025. "Harnessing Extended Reality for Neurocognitive Training in Chronic Pain: State of the Art, Opportunities, and Future Directions" Healthcare 13, no. 11: 1338. https://doi.org/10.3390/healthcare13111338
APA StyleGuerra-Armas, J., Roldán-Ruiz, A., Flores-Cortes, M., & Harvie, D. S. (2025). Harnessing Extended Reality for Neurocognitive Training in Chronic Pain: State of the Art, Opportunities, and Future Directions. Healthcare, 13(11), 1338. https://doi.org/10.3390/healthcare13111338





