Effects of Exercise on Balance Function in People with Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Methodological Quality Assessment
2.5. Statistical Analysis
3. Results
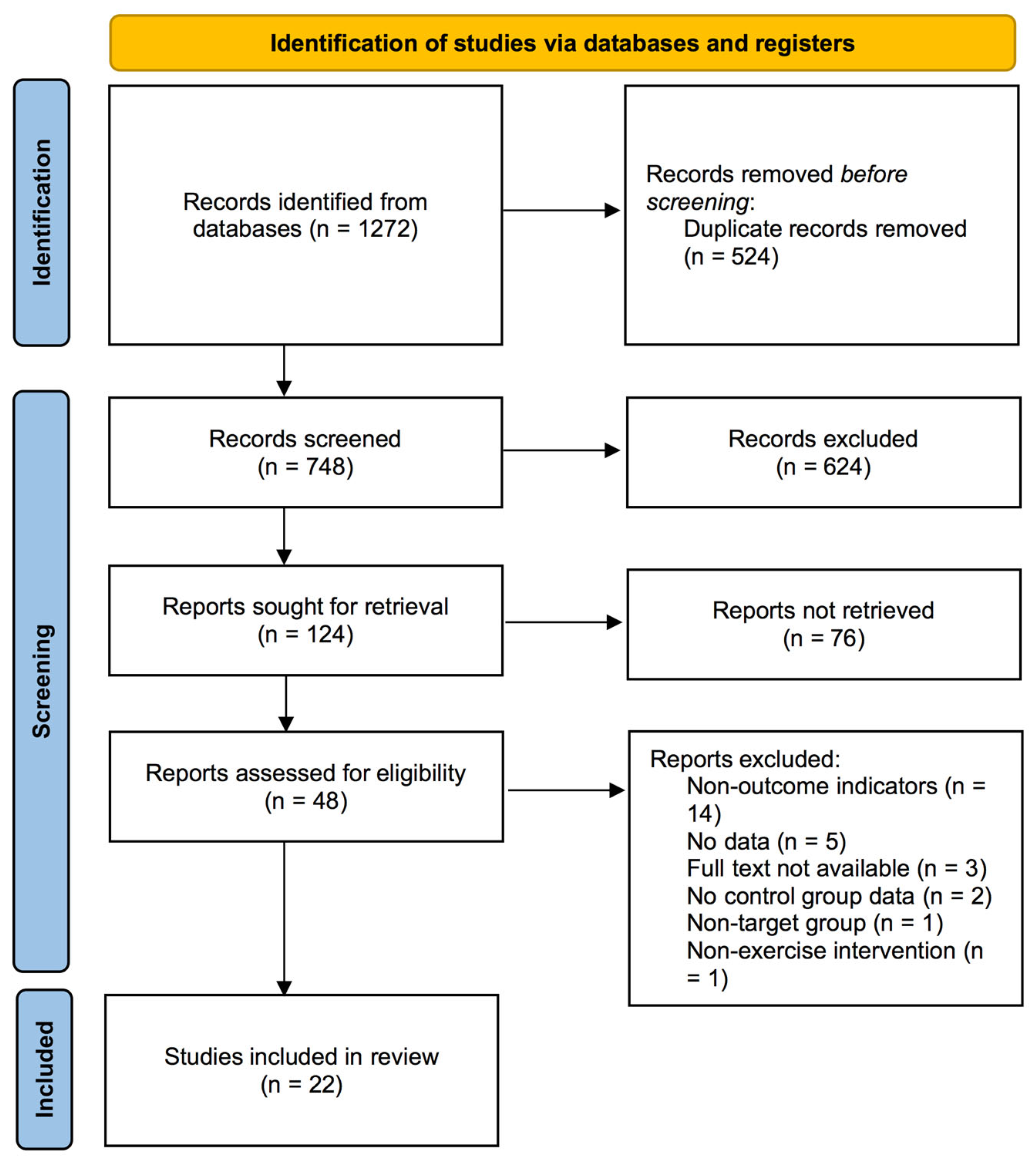
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Risk of Bias
3.4. Meta-Analysis Results
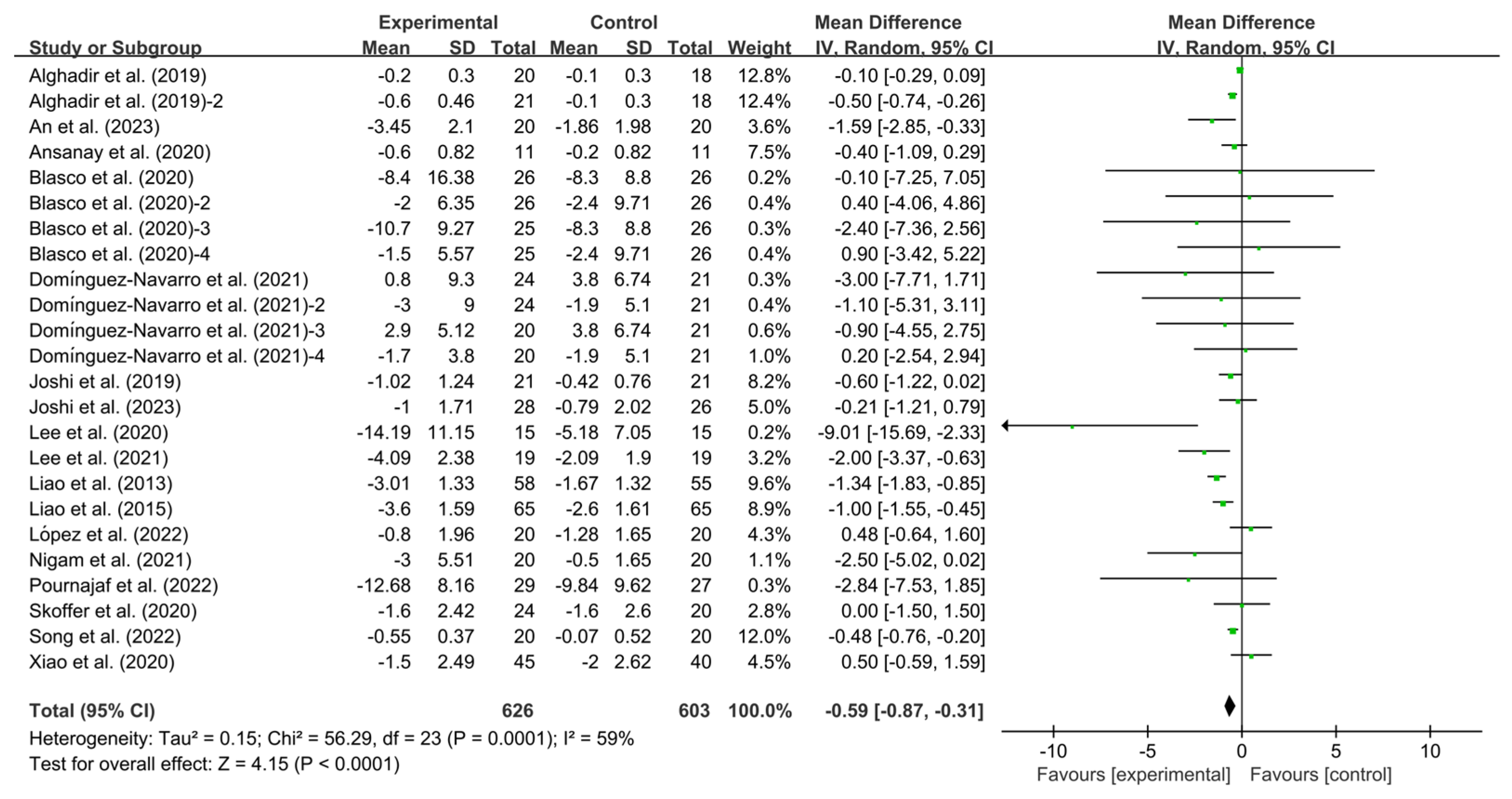
3.4.1. Effects of Exercise on TUG in KOA Patients
3.4.2. Effects of Exercise on BBS in KOA Patients
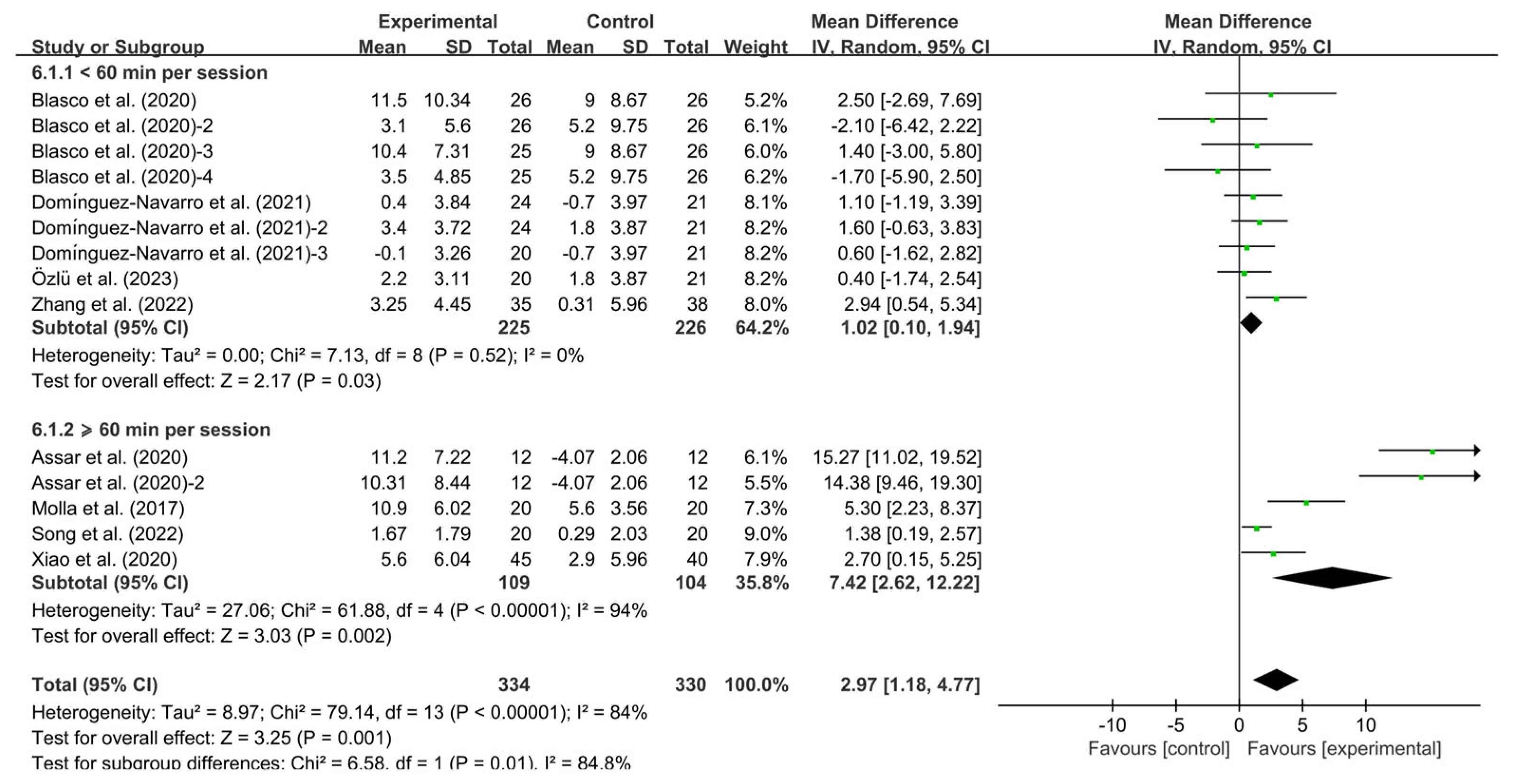
3.5. Subgroup Analysis
3.6. Meta-Regression
3.7. Publication Bias
3.8. Sensitivity Analysis
4. Discussion
4.1. Main Findings
4.2. Effects of Exercise on Balance Function in KOA Patients
4.3. Effects of Different Exercise Modalities on Balance Function in KOA Patients
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KOA | Knee osteoarthritis |
| BBS | Berg balance scale |
| TUG | Timed up and go test |
| RCT | Randomized controlled trial |
| PRISMA | Preferred Reporting Items for Systematic Evaluation and Meta-Analyses |
| MESH | Medical Subject Headings |
| SD | Standard deviation |
| SE | Standard error |
| CI | Confidence interval |
| WMD | Weighted mean difference |
| EULAR | European Alliance of Associations for Rheumatology |
| LLOA | Hong Kong League Against Osteoarthritis |
| ACSM | American College of Sports Medicine |
References
- Yohannes, A.M.; Caton, S. Management of depression in older people with osteoarthritis: A systematic review. Aging Ment. Health 2010, 14, 637–651. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Huang, H.; Liu, C.; Wan, Q.; Shang, S. The effects of a home-based exercise intervention on elderly patients with knee osteoarthritis: A quasi-experimental study. BMC Musculoskelet. Disord. 2019, 20, 160. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.M.; Pendergast, D.R. Reduced muscle function in patients with osteoarthritis. Scand. J. Rehabil. Med. 1997, 29, 213–221. [Google Scholar] [CrossRef]
- Williams, S.B.; Brand, C.A.; Hill, K.D.; Hunt, S.B.; Moran, H. Feasibility and outcomes of a home-based exercise program on improving balance and gait stability in women with lower-limb osteoarthritis or rheumatoid arthritis: A pilot study. Arch. Phys. Med. Rehabil. 2010, 91, 106–114. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Liu, H.; Qu, J.; Wang, Y.; Chen, P.; Zhang, T.; Luo, J. The impact of Otago exercise programme on the prevention of falls in older adult: A systematic review. Front. Public. Health 2022, 10, 953593. [Google Scholar] [CrossRef]
- Mort, J.S.; Billington, C.J. Articular cartilage and changes in arthritis: Matrix degradation. Arthritis Res. 2001, 3, 337–341. [Google Scholar] [CrossRef]
- Roos, E.M.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 92–101. [Google Scholar] [CrossRef]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports Med. 2015, 49, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Tirasci, E.; Sarpel, T.; Coskun Benlidayi, I.; Deniz, V. The effect of balance exercises on central sensitization in patients with knee osteoarthritis. Rheumatol. Int. 2024, 44, 795–804. [Google Scholar] [CrossRef]
- You, Y.; Liu, J.; Tang, M.; Wang, D.; Ma, X. Effects of Tai Chi exercise on improving walking function and posture control in elderly patients with knee osteoarthritis: A systematic review and meta-analysis. Medicine 2021, 100, e25655. [Google Scholar] [CrossRef]
- Kramarow, E.; Chen, L.H.; Hedegaard, H.; Warner, M. Deaths from Unintentional Injury Among Adults Aged 65 and Over: United States, 2000–2013; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2015; p. 199.
- Fortinsky, R.H.; Baker, D.; Gottschalk, M.; King, M.; Trella, P.; Tinetti, M.E. Extent of implementation of evidence-based fall prevention practices for older patients in home health care. J. Am. Geriatr. Soc. 2008, 56, 737–743. [Google Scholar] [CrossRef]
- Berg, K.O.; Maki, B.E.; Williams, J.I.; Holliday, P.J.; Wood-Dauphinee, S.L. Clinical and laboratory measures of postural balance in an elderly population. Arch. Phys. Med. Rehabil. 1992, 73, 1073–1080. [Google Scholar]
- Keskin, D.; Borman, P.; Ersöz, M.; Kurtaran, A.; Bodur, H.; Akyüz, M. The risk factors related to falling in elderly females. Geriatr. Nurs. 2008, 29, 58–63. [Google Scholar] [CrossRef]
- Arnold, C.M.; Gyurcsik, N.C. Risk factors for falls in older adults with lower extremity arthritis: A conceptual framework of current knowledge and future directions. Physiother. Can. 2012, 64, 302–314. [Google Scholar] [CrossRef]
- Nnodim, J.O.; Yung, R.L. Balance and its Clinical Assessment in Older Adults—A Review. J. Geriatr. Med. Gerontol. 2015, 1, 3. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S. Land-based exercise for osteoarthritis of the knee: A metaanalysis of randomized controlled trials. J. Rheumatol. 2009, 36, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.; Close, J.C.; Lord, S.R. Exercise to prevent falls in older adults: An updated meta-analysis and best practice recommendations. NSW Public. Health Bull. 2011, 22, 78–83. [Google Scholar] [CrossRef]
- Pirayeh, N.; Kazemi, K.; Rahimi, F.; Mostafaee, N.; Shaterzadeh-Yazdi, M.J. The Effect of Balance Training on Functional Outcomes in Patients with Knee Osteoarthritis: A Systematic Review. Med. J. Islam. Repub. Iran. 2022, 36, 107. [Google Scholar] [CrossRef]
- Gomiero, A.B.; Kayo, A.; Abraão, M.; Peccin, M.S.; Grande, A.J.; Trevisani, V.F. Sensory-motor training versus resistance training among patients with knee osteoarthritis: Randomized single-blind controlled trial. Sao Paulo Med. J. 2018, 136, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, H.; Hou, X.; Dong, X.; Zhang, S.; Lv, Y.; Li, C.; Yu, L. The effect of exercise on balance function in stroke patients: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2024, 271, 4751–4768. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Li, G.; Tao, X.; Lei, B.; Hou, X.; Yang, X.; Wang, L.; Zhang, S.; Lv, Y.; Wang, T.; Yu, L. Effects of exercise on post-stroke cognitive function: A systematic review and meta-analysis of randomized controlled trials. Top. Stroke Rehabil. 2024, 31, 645–666. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z.; Zhou, H.; Wang, L.; Li, X.; Lv, Y.; Sun, T.; Yu, L. Effects of Acute Ingestion of Caffeine Capsules on Muscle Strength and Muscle Endurance: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1146. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, Y.; Tao, X.; Hou, X.; Du, L.; Lv, Y.; Yu, L. The effect of exercise on flow-mediated dilation in people with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 2024, 15, 1347399. [Google Scholar] [CrossRef]
- Özlü, A.; Ünver, G.; Tuna, H.U.; Menekşeoǧlu, A.K. The Effect of a Virtual Reality-Mediated Gamified Rehabilitation Program on Pain, Disability, Function, and Balance in Knee Osteoarthritis: A Prospective Randomized Controlled Study. Games Health J. 2023, 12, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ansanay, R.L.; Tinduh, D.; Kusharyaningsih, R.H. The effect of dexterity and perturbation exercise on knee osteoarthritis through functional balance and power improvement of quadriceps and hamstring. Indian J. Forensic Med. Toxicol. 2020, 14, 2307–2312. [Google Scholar]
- Yousefian Molla, R.; Sadeghi, H.; Kahlaee, A.H. The Effect of Early Progressive Resistive Exercise Therapy on Balance Control of Patients with Total Knee Arthroplasty. Top. Geriatr. Rehabil. 2017, 33, 286–294. [Google Scholar] [CrossRef]
- Sayers, S.P.; Gibson, K.; Cook, C.R. Effect of high-speed power training on muscle performance, function, and pain in older adults with knee osteoarthritis: A pilot investigation. Arthritis Care Res. 2012, 64, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Blasco, J.M.; Acosta-Ballester, Y.; Martínez-Garrido, I.; García-Molina, P.; Igual-Camacho, C.; Roig-Casasús, S. The effects of preoperative balance training on balance and functional outcome after total knee replacement: A randomized controlled trial. Clin. Rehabil. 2020, 34, 182–193. [Google Scholar] [CrossRef]
- Xiao, C.; Zhuang, Y.; Kang, Y. Effects of Wu Qin xi Qigong exercise on physical functioning in elderly people with knee osteoarthritis: A randomized controlled trial. Geriatr. Gerontol. Int. 2020, 20, 899–903. [Google Scholar] [CrossRef]
- Domínguez-Navarro, F.; Silvestre-Muñoz, A.; Igual-Camacho, C.; Díaz-Díaz, B.; Torrella, J.V.; Rodrigo, J.; Payá-Rubio, A.; Roig-Casasús, S.; Blasco, J.M. A randomized controlled trial assessing the effects of preoperative strengthening plus balance training on balance and functional outcome up to 1 year following total knee replacement. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 838–848. [Google Scholar] [CrossRef]
- Song, J.; Wei, L.; Cheng, K.; Lin, Q.; Xia, P.; Wang, X.; Wang, X.; Yang, T.; Chen, B.; Ding, A.; et al. The Effect of Modified Tai Chi Exercises on the Physical Function and Quality of Life in Elderly Women with Knee Osteoarthritis. Front. Aging Neurosci. 2022, 14, 860762. [Google Scholar] [CrossRef]
- Assar, S.; Gandomi, F.; Mozafari, M.; Sohaili, F. The effect of Total resistance exercise vs. aquatic training on self-reported knee instability, pain, and stiffness in women with knee osteoarthritis: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2020, 12, 27. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, G.; Li, X.; Yao, F.; Wu, Z.; Zhu, Q.; Fang, M. The Effectiveness of Traditional Chinese Yijinjing Qigong Exercise for the Patients with Knee Osteoarthritis on the Pain, Dysfunction, and Mood Disorder: A Pilot Randomized Controlled Trial. Front. Med. 2022, 8, 792436. [Google Scholar] [CrossRef]
- Joshi, S.; Kolke, S. Effects of progressive neuromuscular training on pain, function, and balance in patients with knee osteoarthritis: A randomised controlled trial. Eur. J. Physiother. 2023, 25, 179–186. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, S.K.; Vij, J.S. Effect of retrowalking, a non-pharmacological treatment on pain, disability, balance and gait in knee osteoarthritis: A randomized controlled trial. Indian J. Public Health Res. Dev. 2019, 10, 214–219. [Google Scholar] [CrossRef]
- López, L.L.; Benítez, P.O.; López, J.C.; Martos, I.C.; Torres, J.R.; Santiago, M.G.; Valenza, M.C. Effectiveness of an individualized comprehensive rehabilitation program in women with chronic knee osteoarthritis: A randomized controlled trial. Menopause 2022, 29, 687–692. [Google Scholar] [CrossRef]
- Liao, C.D.; Liou, T.H.; Huang, Y.Y.; Huang, Y.C. Effects of balance training on functional outcome after total knee replacement in patients with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2013, 27, 697–709. [Google Scholar] [CrossRef]
- Skoffer, B.; Maribo, T.; Mechlenburg, I.; Korsgaard, C.G.; Søballe, K.; Dalgas, U. Efficacy of preoperative progressive resistance training in patients undergoing total knee arthroplasty: 12-month follow-up data from a randomized controlled trial. Clin. Rehabil. 2020, 34, 82–90. [Google Scholar] [CrossRef]
- Liao, C.D.; Lin, L.F.; Huang, Y.C.; Huang, S.W.; Chou, L.C.; Liou, T.H. Functional outcomes of outpatient balance training following total knee replacement in patients with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2015, 29, 855–867. [Google Scholar] [CrossRef]
- Nigam, A.; Satpute, K.H.; Hall, T.M. Long term efficacy of mobilisation with movement on pain and functional status in patients with knee osteoarthritis: A randomised clinical trial. Clin. Rehabil. 2021, 35, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Pournajaf, S.; Goffredo, M.; Pellicciari, L.; Piscitelli, D.; Criscuolo, S.; Le Pera, D.; Damiani, C.; Franceschini, M.; Franceschini, M. Effect of balance training using virtual reality-based serious games in individuals with total knee replacement: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2022, 65, 101609. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Anwer, S.; Sarkar, B.; Paul, A.K.; Anwar, D. Effect of 6-week retro or forward walking program on pain, functional disability, quadriceps muscle strength, and performance in individuals with knee osteoarthritis: A randomized controlled trial (retro-walking trial). BMC Musculoskelet. Disord. 2019, 20, 159. [Google Scholar] [CrossRef]
- An, J.; Son, Y.W.; Lee, B.H. Effect of Combined Kinematic Chain Exercise on Physical Function, Balance Ability, and Gait in Patients with Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 3524. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H.; Lee, B.H. Effect of dynamic balance exercises based on visual feedback on physical function, balance ability, and depression in women after bilateral total knee arthroplasty: A randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 3203. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; An, J.; Lee, B.H. The effect of progressive dynamic balance training on physical function, the ability to balance and quality of life among elderly women who underwent a total knee arthroplasty: A double-blind randomized control trial. Int. J. Environ. Res. Public Health 2021, 18, 2513. [Google Scholar] [CrossRef]
- Moseng, T.; Vlieland, T.P.M.V.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Kan, H.; Chan, P.; Chiu, K.; Yan, C.; Yeung, S.; Ng, Y.; Shiu, K.; Ho, T. Non-surgical treatment of knee osteoarthritis. Hong Kong Med. J. 2019, 25, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Perlman, A.I.; Sabina, A.; Williams, A.L.; Njike, V.Y.; Katz, D.L. Massage therapy for osteoarthritis of the knee: A randomized controlled trial. Arch. Intern. Med. 2006, 166, 2533–2538. [Google Scholar] [CrossRef]
- Gunn, A.H.; Schwartz, T.A.; Arbeeva, L.S.; Callahan, L.F.; Golightly, Y.; Goode, A.; Hill, C.H.; Huffman, K.; Iversen, M.D.; Pathak, A.; et al. Fear of Movement and Associated Factors Among Adults with Symptomatic Knee Osteoarthritis. Arthritis Care Res. 2017, 69, 1826–1833. [Google Scholar] [CrossRef]
- Slemenda, C.; Heilman, D.K.; Brandt, K.D.; Katz, B.P.; Mazzuca, S.A.; Braunstein, E.M.; Byrd, D. Reduced quadriceps strength relative to body weight: A risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998, 41, 1951–1959. [Google Scholar]
- Øiestad, B.E.; Juhl, C.B.; Eitzen, I.; Thorlund, J.B. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, Y.; Liu, H.; Bao, T.; Wang, C.; Wang, Z.; Huang, J.; Jiang, Y.; He, C.; Zhu, S. Effect of the telemedicine-supported multicomponent exercise therapy in patients with knee osteoarthritis: Study protocol for a randomized controlled trial. Trials 2023, 24, 729. [Google Scholar] [CrossRef]
- An, J.; Cheon, S.J.; Lee, B.H. The Effect of Combined Balance Exercise on Knee Range of Motion, Balance, Gait, and Functional Outcomes in Acute Phase Following Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial. Medicina 2024, 60, 1389. [Google Scholar] [CrossRef]
- Juhl, C.; Christensen, R.; Roos, E.M.; Zhang, W.; Lund, H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Gür, H.; Cakin, N.; Akova, B.; Okay, E.; Küçükoğlu, S. Concentric versus combined concentric-eccentric isokinetic training: Effects on functional capacity and symptoms in patients with osteoarthrosis of the knee. Arch. Phys. Med. Rehabil. 2002, 83, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Jehu, D.A.; Daneshjoo, A.; Shakoor, E.; Razeghi, M.; Amani, A.; Hakim, M.N.; Yusof, A. Effects of 8 Weeks of Balance Training, Virtual Reality Training, and Combined Exercise on Lower Limb Muscle Strength, Balance, and Functional Mobility Among Older Men: A Randomized Controlled Trial. Sports Health 2021, 13, 606–612. [Google Scholar] [CrossRef]
- Husby, V.S.; Foss, O.A.; Husby, O.S.; Winther, S.B. Randomized controlled trial of maximal strength training vs. standard rehabilitation following total knee arthroplasty. Eur. J. Phys. Rehabil. Med. 2018, 54, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Schmid, C.H.; Iversen, M.D.; Harvey, W.F.; Fielding, R.A.; Driban, J.B.; Price, L.L.; Wong, J.B.; Reid, K.F.; Rones, R. Comparative Effectiveness of Tai Chi Versus Physical Therapy for Knee Osteoarthritis: A Randomized Trial. Ann. Intern. Med. 2016, 165, 77–86. [Google Scholar] [CrossRef]
- Chaipinyo, K.; Karoonsupcharoen, O. No difference between home-based strength training and home-based balance training on pain in patients with knee osteoarthritis: A randomised trial. Aust. J. Physiother. 2009, 55, 25–30. [Google Scholar] [CrossRef]
- American College of Sports Medicine Position Stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Kehlet, H.; Søballe, K. Fast-track hip and knee replacement--what are the issues? Acta Orthop. 2010, 81, 271–272. [Google Scholar] [CrossRef]
- Forte, P.; Encarnação, S.G.; Branquinho, L.; Barbosa, T.M.; Monteiro, A.M.; Pecos-Martín, D. The Effects of an 8-Month Multicomponent Training Program in Body Composition, Functional Fitness, and Sleep Quality in Aged People: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 6603. [Google Scholar] [CrossRef]
- Kalebota, N.; Žerjavić, N.L.; Durmiš, K.K.; Milošević, M.; Andreić, A.; Končar, B.; Vedriš, M.; Turković, P.; Žura, N.; Žagar, I.; et al. Effects of Tai Chi exercise on pain, functional status, and quality of life in patients with osteoarthritis or inflammatory arthritis. Turk. J. Phys. Med. Rehabil. 2024, 70, 300–308. [Google Scholar] [CrossRef]
- Mihalko, S.L.; Cox, P.; Beavers, D.P.; Miller, G.D.; Nicklas, B.J.; Lyles, M.; Hunter, D.J.; Eckstein, F.; Guermazi, A.; Loeser, R.F.; et al. Effect of intensive diet and exercise on self-efficacy in overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. Transl. Behav. Med. 2019, 9, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ha, G.C.; Yoon, J.R.; Yoo, C.G.; Kang, S.J.; Ko, K.J. Effects of 12-week aquatic exercise on cardiorespiratory fitness, knee isokinetic function, and Western Ontario and McMaster University osteoarthritis index in patients with knee osteoarthritis women. J. Exerc. Rehabil. 2018, 14, 870–876. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, D.-W.; Jeong, M.-B. Effect of a Telerehabilitation Exercise Program on the Gait, Knee function and Quality of life In Patients with Knee Osteoarthritis. J. Korean Soc. Phys. Med. 2020, 15, 143–152. [Google Scholar] [CrossRef][Green Version]
- Dong, Y.; Yan, Y.; Zhou, J.; Zhou, Q.; Wei, H. Evidence on risk factors for knee osteoarthritis in middle-older aged: A systematic review and meta analysis. J. Orthop. Surg. Res. 2023, 18, 634. [Google Scholar] [CrossRef]
- Koca, I.; Boyaci, A.; Tutoglu, A.; Boyaci, N.; Ozkur, A. The Relationship between Quadriceps Thickness, Radiological Staging, and Clinical Parameters in Knee Osteoarthritis. J. Phys. Ther. Sci. 2014, 26, 931–936. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, Y.C.; Tseng, Y.C.; Tsai, S.T.; Tseng, Y.H. Physical activity and successful aging among middle-aged and older adults: A systematic review and meta-analysis of cohort studies. Aging 2020, 12, 7704–7716. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef]
- Prabhakar, A.J.; Shruthi, R.; Thomas, D.T.; Nayak, P.; Joshua, A.M.; Prabhu, S.; Kamat, Y.D. Effectiveness of balance training on pain and functional outcomes in knee osteoarthritis: A systematic review and meta-analysis. F1000Research 2023, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Yan, Y.; Zhou, Q.; Ran, Q.; Chen, H.; Sun, S.; Lu, W.; Chen, W.; Wang, J. Kinesitherapy for Knee Osteoarthritis Patients Physical and Psychological Health Based on “Traditional Chinese Exercise” Management Modalities: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Orthop. Surg. 2024, 16, 3–16. [Google Scholar] [CrossRef]
- Alghadir, A.; Anwer, S.; Brismée, J.M. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1-3 knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 174. [Google Scholar] [CrossRef]
- Mostafaee, N.; Pirayeh, N.; Moosavi, S.S. Reliability, validity, responsiveness and minimal important changes of common clinical standing balance tests in individuals with knee osteoarthritis. Physiother. Theory Pract. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, Z.; Liang, Y.; Su, H.; Wang, T.; Lv, Y.; Yu, L. Effects of Exercise on Balance Function in People with Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare 2025, 13, 1312. https://doi.org/10.3390/healthcare13111312
Wang X, Chen Z, Liang Y, Su H, Wang T, Lv Y, Yu L. Effects of Exercise on Balance Function in People with Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare. 2025; 13(11):1312. https://doi.org/10.3390/healthcare13111312
Chicago/Turabian StyleWang, Xingyue, Zhuying Chen, Yin Liang, Hao Su, Tongling Wang, Yuanyuan Lv, and Laikang Yu. 2025. "Effects of Exercise on Balance Function in People with Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Healthcare 13, no. 11: 1312. https://doi.org/10.3390/healthcare13111312
APA StyleWang, X., Chen, Z., Liang, Y., Su, H., Wang, T., Lv, Y., & Yu, L. (2025). Effects of Exercise on Balance Function in People with Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare, 13(11), 1312. https://doi.org/10.3390/healthcare13111312







