The Influence of the Menstrual Cycle on Electrical Thresholds for Sensory and Pain Perception: Implications for Exercise and Rehabilitation in Women With and Without Primary Dysmenorrhea—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
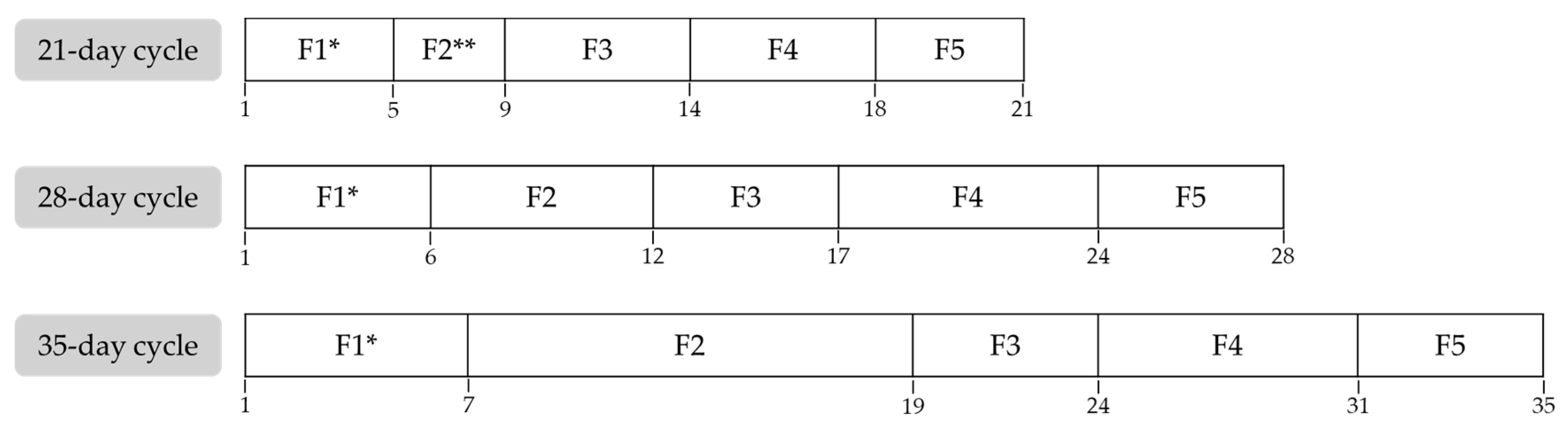
2.1. Study Design
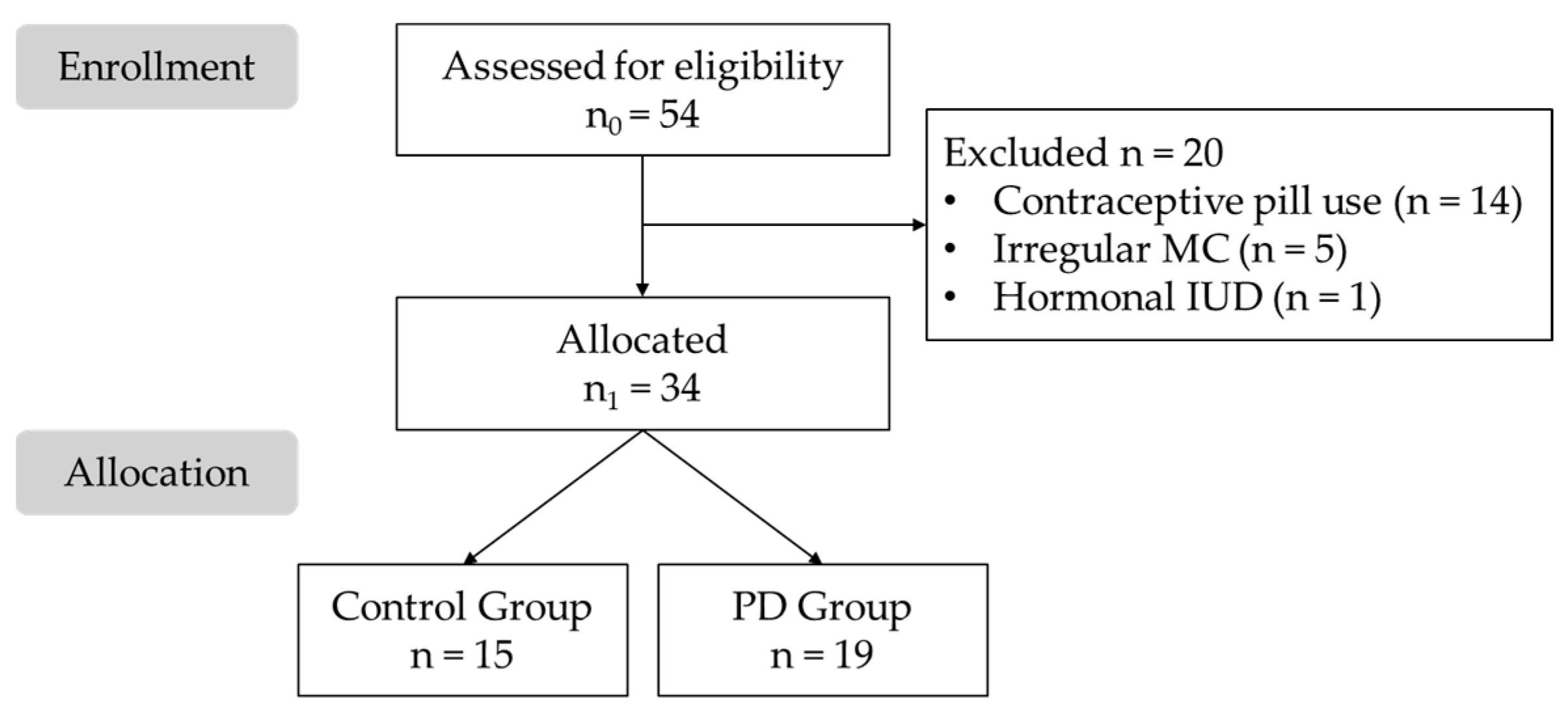
2.2. Participants
2.3. Procedure
2.3.1. Participant Assessment and Group Allocation
2.3.2. Data Collection
2.3.3. Study Variables
2.4. Statistical Analysis
3. Results
3.1. Sample Description
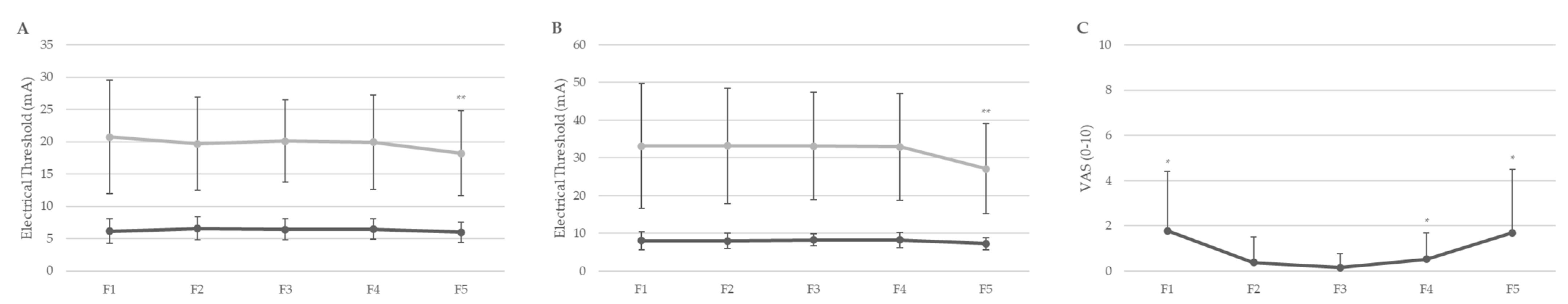
3.2. Descriptive and Comparative Analysis Across Menstrual Cycle Phases
3.3. Comparative Analysis Between the Primary Dysmenorrhea and Control Groups
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiyagarajan, D.K.; Basit, H.; Jeanmonod, R. Physiology, Menstrual Cycle; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Patricio, B.P.; Brantes, S. Normal Menstrual Cycle; Intech Open: Vienna, Austria, 2018. [Google Scholar]
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual Cycle Rhythmicity: Metabolic Patterns in Healthy Women. Sci. Rep. 2018, 8, 14568. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, R.C.; Bruinvels, G.; Pedlar, C.R. Variations in Strength-Related Measures during the Menstrual Cycle in Eumenorrheic Women: A Systematic Review and Meta-Analysis. J. Sci. Med. Sport 2020, 23, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Y.; Liu, X.; Chen, J.; Sun, M.; Zhang, J.; Zhang, W. Changes in Sex Hormones and Their Interactions Are Related to Pain Perception between Different Menstrual Subphases. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R280–R289. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.A.; Afify, E.A. Sex Differences in Pain and Opioid Mediated Antinociception: Modulatory Role of Gonadal Hormones. Life Sci. 2019, 237, 116926. [Google Scholar] [CrossRef]
- Miller, K.B.; Moir, M.E.; Fico, B.G. Vascular Health and Exercise in Females throughout the Lifespan: Exploring Puberty, Pregnancy and Menopause. Exp. Physiol. 2025, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.; Triplett, N.T. (Eds.) Essentials of Strength Training and Conditioning; Human Kinetics: Champaign, IL, USA, 2016. [Google Scholar]
- Morenas-Aguilar, M.D.; Ruiz-Alias, S.A.; Blanco, A.M.; Lago-Fuentes, C.; García-Pinillos, F.; Pérez-Castilla, A. Does the Menstrual Cycle Impact the Maximal Neuromuscular Capacities of Women? An Analysis Before and After a Graded Treadmill Test to Exhaustion. J. Strength Cond. Res. 2023, 37, 2185–2191. [Google Scholar] [CrossRef]
- Jaleel, G.; Shaphe, M.A.; Khan, A.R.; Malhotra, D.; Khan, H.; Parveen, S.; Qasheesh, M.; Beg, R.A.; Chahal, A.; Ahmad, F.; et al. Effect of Exercises on Central and Endocrine System for Pain Modulation in Primary Dysmenorrhea. J. Lifestyle Med. 2022, 12, 15–25. [Google Scholar] [CrossRef]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M.J. Primary Dysmenorrhea: Pathophysiology, Diagnosis, and Treatment Updates. Korean J. Fam. Med. 2022, 43, 101–108. [Google Scholar] [CrossRef]
- Carroquino-Garcia, P.; Jiménez-Rejano, J.J.; Medrano-Sanchez, E.; de la Casa-Almeida, M.; Diaz-Mohedo, E.; Suarez-Serrano, C. Therapeutic Exercise in the Treatment of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1371–1380. [Google Scholar] [CrossRef]
- López-Liria, R.; Torres-Álamo, L.; Vega-Ramírez, F.A.; García-Luengo, A.V.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Rocamora-Pérez, P. Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 7832. [Google Scholar] [CrossRef]
- Randell, R.K.; Clifford, T.; Drust, B.; Moss, S.L.; Unnithan, V.B.; De Ste Croix, M.B.A.; Datson, N.; Martin, D.; Mayho, H.; Carter, J.M.; et al. Physiological Characteristics of Female Soccer Players and Health and Performance Considerations: A Narrative Review. Sports Med. 2021, 51, 1377–1399. [Google Scholar] [CrossRef]
- Herreira-Ferreira, M.; Costa, Y.; Cunha, C.; Conti, A.; Conti, P.; Bonjardim, L. Experimental Pain Thresholds and Psychosocial Features across Menstrual Cycle in Myofascial Orofacial Pain Compared to Healthy Individuals: Cross-Sectional Study. Braz. J. Pain 2023, 6, 107–112. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Drescher, C.; Englbrecht, J.S.; Klein, T.; Magerl, W.; Zahn, P.K. Progesterone Relates to Enhanced Incisional Acute Pain and Pinprick Hyperalgesia in the Luteal Phase of Female Volunteers. Pain 2019, 160, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, W.J.; Sullivan, M.A.; Evans, S.M.; Bisaga, A.M.; Vosburg, S.K.; Comer, S.D. Sex Differences and Hormonal Influences on Response to Mechanical Pressure Pain in Humans. J. Pain 2010, 11, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.-H.; Koo, S.-J.; Choi, J.-K. Pain Threshold & Taste Threshold Variations across the Menstrual Cycle. J. Oral Med. 2001, 26, 253–260. [Google Scholar]
- de B. Barbosa, M.; de O. Guirro, E.C.; Nunes, F.R. Evaluation of Sensitivity, Motor and Pain Thresholds across the Menstrual Cycle through Medium-Frequency Transcutaneous Electrical Nerve Stimulation. Clinics 2013, 68, 901–908. [Google Scholar] [CrossRef]
- Toprak Celenay, S.; Ozcelikel, G.; Bayrakli, A. Efficacy of Progressive Muscle Relaxation Technique in Primary Dysmenorrhea: A Randomized Controlled Trial. Taiwan. J. Obstet. Gynecol. 2024, 63, 329–335. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Chesney, M.A.; Tasto, D.L. The Development of the Menstrual Symptom Questionnaire. Behav. Res. Ther. 1975, 13, 237–244. [Google Scholar] [CrossRef]
- Melzack, R. The Short-Form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Boztaş Elverişli, G.; Armağan, N.; Atilgan, E. Comparison of the Efficacy of Pharmacological and Nonpharmacological Treatments in Women with Primary Dysmenorrhea: Randomized Controlled Parallel-Group Study. Ginekol. Pol. 2023, 94, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. Women with Dysmenorrhoea Are Hypersensitive to Experimentally Induced Forearm Ischaemia during Painful Menstruation and during the Pain-Free Follicular Phase. Eur. J. Pain 2015, 19, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Madsen, H.; Arendt-Nielsen, L. A Comparison of Modality-Specific Somatosensory Changes during Menstruation in Dysmenorrheic and Nondysmenorrheic Women. Clin. J. Pain 2002, 18, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Soshi, S.; Kubota, M.; Marumo, K. Efficacy of Laminoplasty in Improving Sensory Disturbances in Patients with Cervical Spondylotic Myelopathy: A Prospective Study. World Neurosurg. 2020, 134, e581–e588. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.B.; Lee, J.W.; Woo, A.M.; Kim, C.J.; Chung, M.Y.; Moon, H.S. A Quantitative Measure of Pain with Current Perception Threshold, Pain Equivalent Current, and Quantified Pain Degree: A Retrospective Study. J. Clin. Med. 2023, 12, 5476. [Google Scholar] [CrossRef]
- Shimoda, O.; Ikuta, Y. The current perception thresholds vary between horizontal and 70 degrees tilt-up positions. Anesth. Analg. 2000, 91, 398–402. [Google Scholar] [CrossRef]
- Lázaro, C.; Caseras, X.; Whizar-Lugo, V.M.; Wenk, R.; Baldioceda, F.; Bernal, R.; Ovalle, A.; Torrubia, R.; Baños, J.E. Psychometric Properties of a Spanish Version of the McGill Pain Questionnaire in Several Spanish-Speaking Countries. Clin. J. Pain 2001, 17, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Rhudy, J.L. Comparing pain sensitivity and the nociceptive flexion reflex threshold across the mid-follicular and late-luteal menstrual phases in healthy women. Clin. J. Pain 2013, 29, 154–161. [Google Scholar] [CrossRef]
- Edwards, H.E.; Burnham, W.M.; Mendonca, A.; Bowlby, D.A.; MacLusky, N.J. Steroid Hormones Affect Limbic Afterdischarge Thresholds and Kindling Rates in Adult Female Rats. Brain Res. 1999, 838, 136–150. [Google Scholar] [CrossRef]
- Recacha-Ponce, P.; Collado-Boira, E.; Suarez-Alcazar, P.; Montesinos-Ruiz, M.; Hernando-Domingo, C. Is It Necessary to Adapt Training According to the Menstrual Cycle? Influence of Contraception and Physical Fitness Variables. Life 2023, 13, 1764. [Google Scholar] [CrossRef]
- Witkoś, J.; Hartman-Petrycka, M.; Błażejewski, G. The Effects of Sex, Women’s Body Composition and Monthly Cycle Phases on the Sensory Threshold of Upper Limb to Transcutaneous Electrical Nerve Stimulation in Healthy Subjects. Appl. Sci. 2023, 13, 8365. [Google Scholar] [CrossRef]
- Krunic, J.; Mladenovic, I.; Radovic, I.; Stojanovic, N. Changes in pulp sensitivity across the menstrual cycle in healthy women and women with temporomandibular disorders. J. Oral Rehabil. 2021, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Bastiaanssen, T.F.S.; Peterson, V.; Sajjad, J.; Murphy, A.; Stanton, C.; McNamara, B.; Shorten, G.D.; Cryan, J.F.; O’Mahony, S.M. Sex, pain, and the microbiome: The relationship between baseline gut microbiota composition, gender and somatic pain in healthy individuals. Brain Behav. Immun. 2022, 104, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Kuba, T.; Quinones-Jenab, V. The Role of Female Gonadal Hormones in Behavioral Sex Differences in Persistent and Chronic Pain: Clinical versus Preclinical Studies. Brain Res. Bull. 2005, 66, 179–188. [Google Scholar] [CrossRef]
- Fortún-Rabadán, R.; Boudreau, S.A.; Bellosta-López, P.; Herrero, P.; Graven-Nielsen, T.; Doménech-García, V. Facilitated Central Pain Mechanisms Across the Menstrual Cycle in Dysmenorrhea and Enlarged Pain Distribution in Women With Longer Pain History. J. Pain 2023, 24, 1541–1554. [Google Scholar] [CrossRef]
- Wei, S.Y.; Chen, L.F.; Lin, M.W.; Li, W.C.; Low, I.; Yang, C.J.; Chao, H.T.; Hsieh, J.C. The OPRM1 A118G polymorphism modulates the descending pain modulatory system for individual pain experience in young women with primary dysmenorrhea. Sci. Rep. 2017, 7, 39906. [Google Scholar] [CrossRef]
- Lima, V.; Arruda, G.; Strelow, C.; Froelich, M.; Saccol, M.; Braz, M. Comparison of the Pain Pressure Threshold on the Pelvic Floor in Women with and without Primary Dysmenorrhea. Braz. J. Pain 2019, 2, 101–104. [Google Scholar] [CrossRef]
- Payne, L.A.; Rapkin, A.J.; Seidman, L.C.; Zeltzer, L.K.; Tsao, J.C. Experimental and Procedural Pain Responses in Primary Dysmenorrhea: A Systematic Review. J. Pain Res. 2017, 10, 2233–2246. [Google Scholar] [CrossRef]
- Bartley, E.J.; Palit, S.; Kuhn, B.L.; Kerr, K.L.; Terry, E.L.; DelVentura, J.L.; Rhudy, J.L. Nociceptive processing in women with premenstrual dysphoric disorder (PMDD): The role of menstrual phase and sex hormones. Clin. J. Pain 2015, 31, 304–314. [Google Scholar] [CrossRef]
- Teepker, M.; Kunz, M.; Peters, M.; Kundermann, B.; Schepelmann, K.; Lautenbacher, S. Endogenous pain inhibition during menstrual cycle in migraine. Eur. J. Pain 2014, 18, 989–998. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Berkley, K.J.; Iezzi, S.; de Bigontina, P.; Vecchiet, L. Pain Threshold Variations in Somatic Wall Tissues as a Function of Menstrual Cycle, Segmental Site and Tissue Depth in Non-Dysmenorrheic Women, Dysmenorrheic Women and Men. Pain 1997, 71, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Hellman, K.M.; Roth, G.E.; Dillane, K.E.; Garrison, E.F.; Oladosu, F.A.; Clauw, D.J.; Tu, F.F. Dysmenorrhea Subtypes Exhibit Differential Quantitative Sensory Assessment Profiles. Pain 2020, 161, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.B. Central Sensitivity Syndromes: A New Paradigm and Group Nosology for Fibromyalgia and Overlapping Conditions, and the Related Issue of Disease versus Illness. Semin. Arthritis Rheum. 2008, 37, 339–352. [Google Scholar] [CrossRef]
- As-Sanie, S.; Harris, R.E.; Napadow, V.; Kim, J.; Neshewat, G.; Kairys, A.; Williams, D.; Clauw, D.J.; Schmidt-Wilcke, T. Changes in Regional Gray Matter Volume in Women with Chronic Pelvic Pain: A Voxel-Based Morphometry Study. Pain 2012, 153, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Dawood, M.Y.; Fuchs, F. Prostaglandins in Primary Dysmenorrhea. Comparison of Prophylactic and Nonprophylactic Treatment with Ibuprofen and Use of Oral Contraceptives. Am. J. Med. 1981, 70, 535–541. [Google Scholar] [CrossRef]
- Söderman, L.; Edlund, M.; Böttiger, Y.; Marions, L. Adjuvant Use of Melatonin for Pain Management in Dysmenorrhea—A Randomized Double-Blinded, Placebo-Controlled Trial. Eur. J. Clin. Pharmacol. 2022, 78, 191–196. [Google Scholar] [CrossRef]
- Romero-Parra, N.; Alfaro-Magallanes, V.M.; Rael, B.; Cupeiro, R.; Rojo-Tirado, M.A.; Benito, P.J.; Peinado, A.B. Indirect Markers of Muscle Damage Throughout the Menstrual Cycle. Int. J. Sports Physiol. Perform. 2021, 16, 190–198. [Google Scholar] [CrossRef]
- Santos, C.; Kryger, K.O.; Wilke, C.; Travassos, B. Impact of the Menstrual Cycle and Barriers to Football and Futsal Performance in Portuguese Players: A Survey-Based Cross-Sectional Study. Front. Psychol. 2025, 16, 1576752. [Google Scholar] [CrossRef]
| Sample (n = 34) | CG (n = 15) | PD (n = 19) | p Value | |
|---|---|---|---|---|
| Age (years) | 22.12 ± 4.39 | 22.27 ± 4.35 | 22.00 ± 4.53 | 0.793 |
| Height (cm) | 164.71 ± 6.21 | 163.80 ± 6.77 | 165.42 ± 5.81 | 0.570 |
| Weight (Kg) | 61.07 ± 10.35 | 59.40 ± 8.36 | 62.39 ± 11.74 | 0.102 |
| Painful cycles | 6.88 ± 3.64 | 3.73 ± 2.87 | 9.37 ± 1.77 | 0.327 |
| MSQ | 66.15 ± 16.39 | 52.13 ± 12.57 | 77.21 ± 8.75 | 0.147 |
| SF-MPQ | 21.71 ± 12.76 | 11.60 ± 9.10 | 29.68 ± 9.06 | 0.855 |
| F1 | F2 | F3 | F4 | F5 | p Value | |
|---|---|---|---|---|---|---|
| SETf | 6.14 ± 1.88 | 6.59 ± 1.81 | 6.42 ± 1.61 | 6.45 ± 1.57 | 5.89 ± 1.61 | <0.001 ** |
| PETf | 20.72 ± 8.77 | 19.67 ± 7.21 | 20.10 ± 6.38 | 19.91 ± 7.35 | 18.20 ± 6.56 | <0.001 ** |
| SETa | 8.04 ± 2.46 | 8.03 ± 2.12 | 8.26 ± 1.60 | 8.22 ± 2.01 | 7.26 ± 1.59 | <0.001 ** |
| PETa | 33.10 ± 16.56 | 33.21 ± 15.31 | 33.10 ± 14.26 | 32.95 ± 14.15 | 27.15 ± 11.94 | <0.001 ** |
| VAS | 1.79 ± 2.63 * | 0.38 ± 1.12 | 0.16 ± 0.62 | 0.53 ± 1.17 * | 1.70 ± 2.80 * | p = 0.002–0.212 |
| MP | CG (n = 15) | PD (n = 19) | p Value | CI 95% | |
|---|---|---|---|---|---|
| F1 | SETf | 6.32 ± 1.79 | 6.00 ± 2.01 | 0.950 | (−1.28, 1.92) |
| PETf | 21.23 ± 11.80 | 20.32 ± 5.88 | 0.240 | (−6.55, 8.37) | |
| SETa | 7.82 ± 2.03 | 8.21 ± 2.81 | 0.121 | (−2.48, 1.69) | |
| PETa | 33.77 ± 21.34 | 32.57 ± 12.47 | 0.218 † | (−12.89, 15.30) | |
| VAS | 0.58 ± 1.73 | 2.82 ± 2.88 | 0.005 * † | (−4.14, −0.33) | |
| F2 | SETf | 6.50 ± 1.46 | 6.68 ± 2.17 | 0.526 | (−1.58, 1.22) |
| PETf | 19.83 ± 7.92 | 19.50 ± 6.65 | 0.808 † | (−5.26, 5.93) | |
| SETa | 7.90 ± 1.73 | 8.18 ± 2.53 | 0.173 | (−1.92, 1.36) | |
| PETa | 31.43 ± 17.02 | 35.11 ± 13.62 | 0.987 | (−15.47, 8.12) | |
| VAS | 0.73 ± 1.49 | 0.00 ± 0.00 | <0.001 ** † | (−0.09, 1.56) | |
| F3 | SETf | 5.57 ± 1.55 | 6.87 ± 1.53 | 0.743 | (−2.41, 0.18) |
| PETf | 18.20 ± 5.13 | 21.37 ± 6.97 | 0.440 | (−8.50, 2.17) | |
| SETa | 7.40 ± 1.22 | 8.83 ± 1.59 | 0.668 | (−2.66, −0.20) | |
| PETa | 26.00 ± 11.83 | 37.83 ± 14.09 | 0.511 | (−23.03, −0.64) | |
| VAS | 0.00 ± 0.00 | 0.27 ± 0.80 | 0.033 * † | (−0.71, 0.18) | |
| F4 | SETf | 6.45 ± 1.62 | 6.45 ± 1.58 | 0.879 | (−1.23, 1.24) |
| PETf | 20.00 ± 7.34 | 19.87 ± 7.56 | 0.945† | (−5.67, 5.94) | |
| SETa | 8.27 ± 2.09 | 8.18 ± 2.02 | 0.323 | (−1.50, 1.67) | |
| PETa | 32.82 ± 15.60 | 33.03 ± 13.69 | 0.702 | (−11.38, 10.97) | |
| VAS | 0.00 ± 0.00 | 0.84 ± 1.38 | <0.001 ** † | (−1.50, −0.17) | |
| F5 | SETf | 5.59 ± 0.97 | 6.17 ± 2.04 | 0.005 * | (−1.97, 0.82) |
| PETf | 19.45 ± 8.54 | 17.04 ± 4.10 | 0.326 | (−3.32, 8.14) | |
| SETa | 7.64 ± 1.47 | 6.92 ± 1.68 | 0.532 | (−0.65, 2.09) | |
| PETa | 30.05 ± 13.67 | 24.50 ± 9.96 | 0.913 | (−4.76, 15.85) | |
| VAS | 0.09 ± 0.30 | 3.17 ± 3.26 | <0.001 ** † | (−5.15, −1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Lalaguna, A.C.; Ríos-Asín, I.; Pardos-Aguilella, P.; Pérez-Rey, J.; Estébanez-de-Miguel, E.; Malo-Urriés, M. The Influence of the Menstrual Cycle on Electrical Thresholds for Sensory and Pain Perception: Implications for Exercise and Rehabilitation in Women With and Without Primary Dysmenorrhea—A Pilot Study. Healthcare 2025, 13, 1240. https://doi.org/10.3390/healthcare13111240
Morales-Lalaguna AC, Ríos-Asín I, Pardos-Aguilella P, Pérez-Rey J, Estébanez-de-Miguel E, Malo-Urriés M. The Influence of the Menstrual Cycle on Electrical Thresholds for Sensory and Pain Perception: Implications for Exercise and Rehabilitation in Women With and Without Primary Dysmenorrhea—A Pilot Study. Healthcare. 2025; 13(11):1240. https://doi.org/10.3390/healthcare13111240
Chicago/Turabian StyleMorales-Lalaguna, Ana Cristina, Izarbe Ríos-Asín, Pilar Pardos-Aguilella, Jorge Pérez-Rey, Elena Estébanez-de-Miguel, and Miguel Malo-Urriés. 2025. "The Influence of the Menstrual Cycle on Electrical Thresholds for Sensory and Pain Perception: Implications for Exercise and Rehabilitation in Women With and Without Primary Dysmenorrhea—A Pilot Study" Healthcare 13, no. 11: 1240. https://doi.org/10.3390/healthcare13111240
APA StyleMorales-Lalaguna, A. C., Ríos-Asín, I., Pardos-Aguilella, P., Pérez-Rey, J., Estébanez-de-Miguel, E., & Malo-Urriés, M. (2025). The Influence of the Menstrual Cycle on Electrical Thresholds for Sensory and Pain Perception: Implications for Exercise and Rehabilitation in Women With and Without Primary Dysmenorrhea—A Pilot Study. Healthcare, 13(11), 1240. https://doi.org/10.3390/healthcare13111240






