Metabolic, Hematological, and Functional Health in Adults with Down Syndrome and Significance of Parental Health Literacy: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Variables and Measurement
2.3. Statistical Analysis
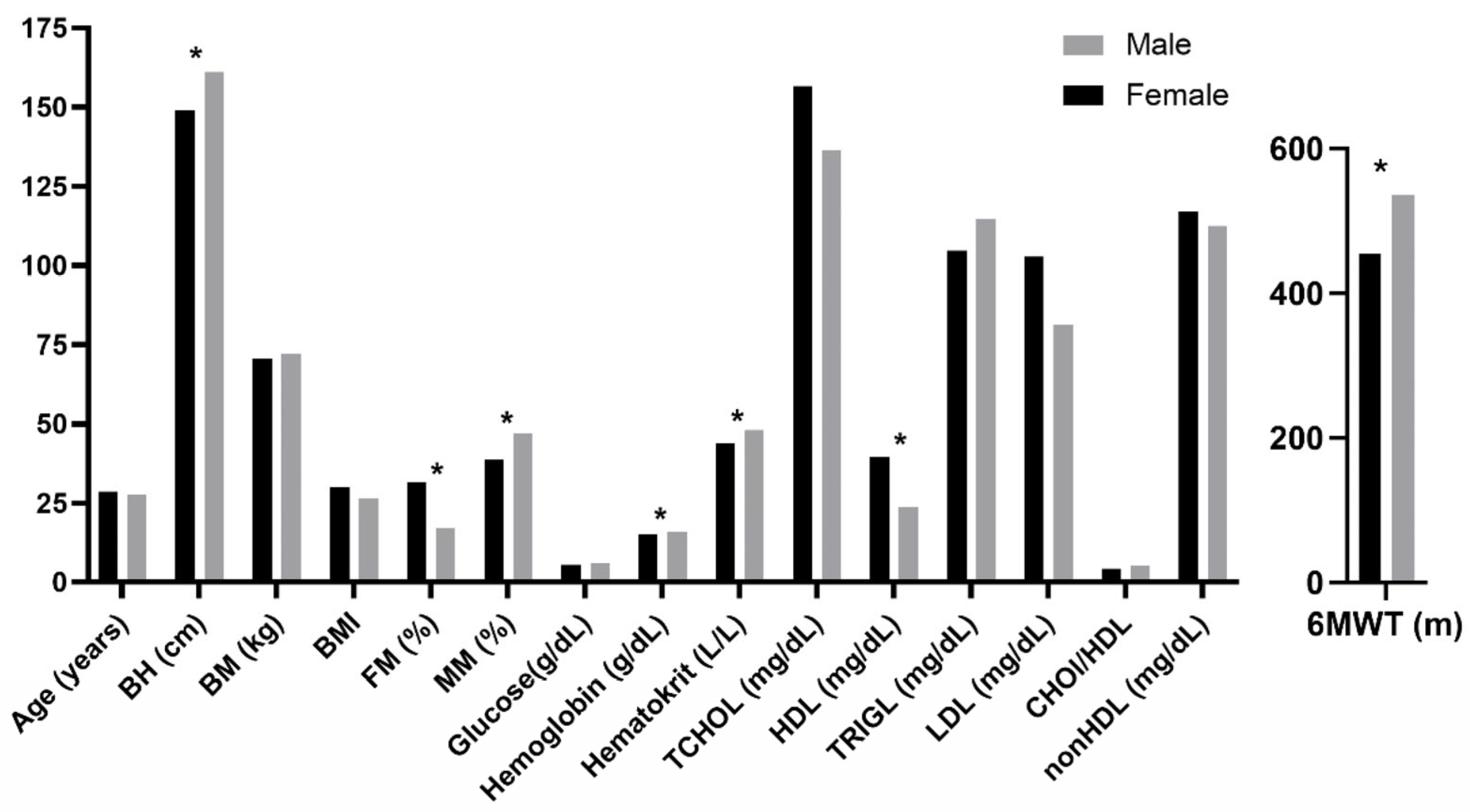
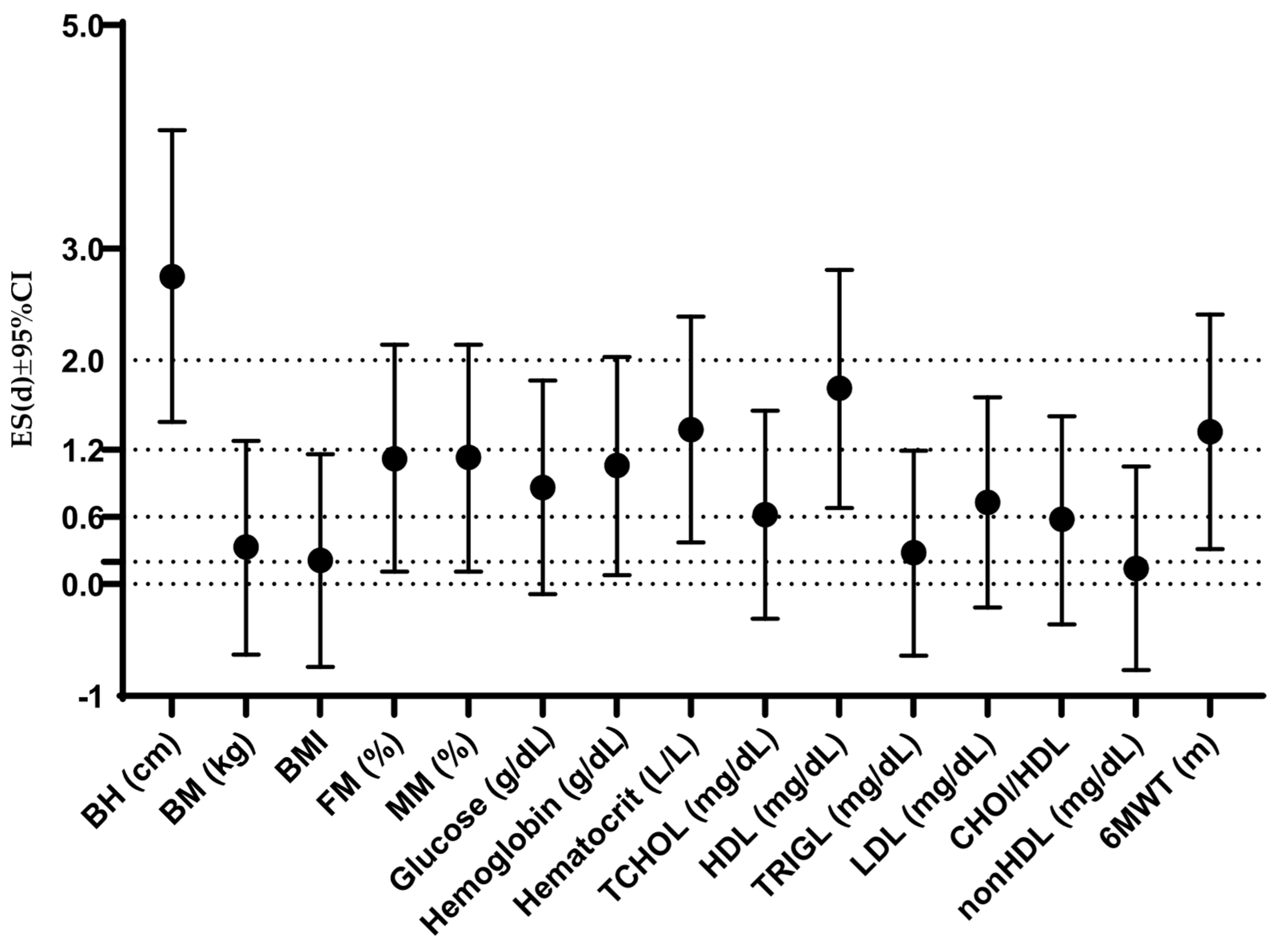
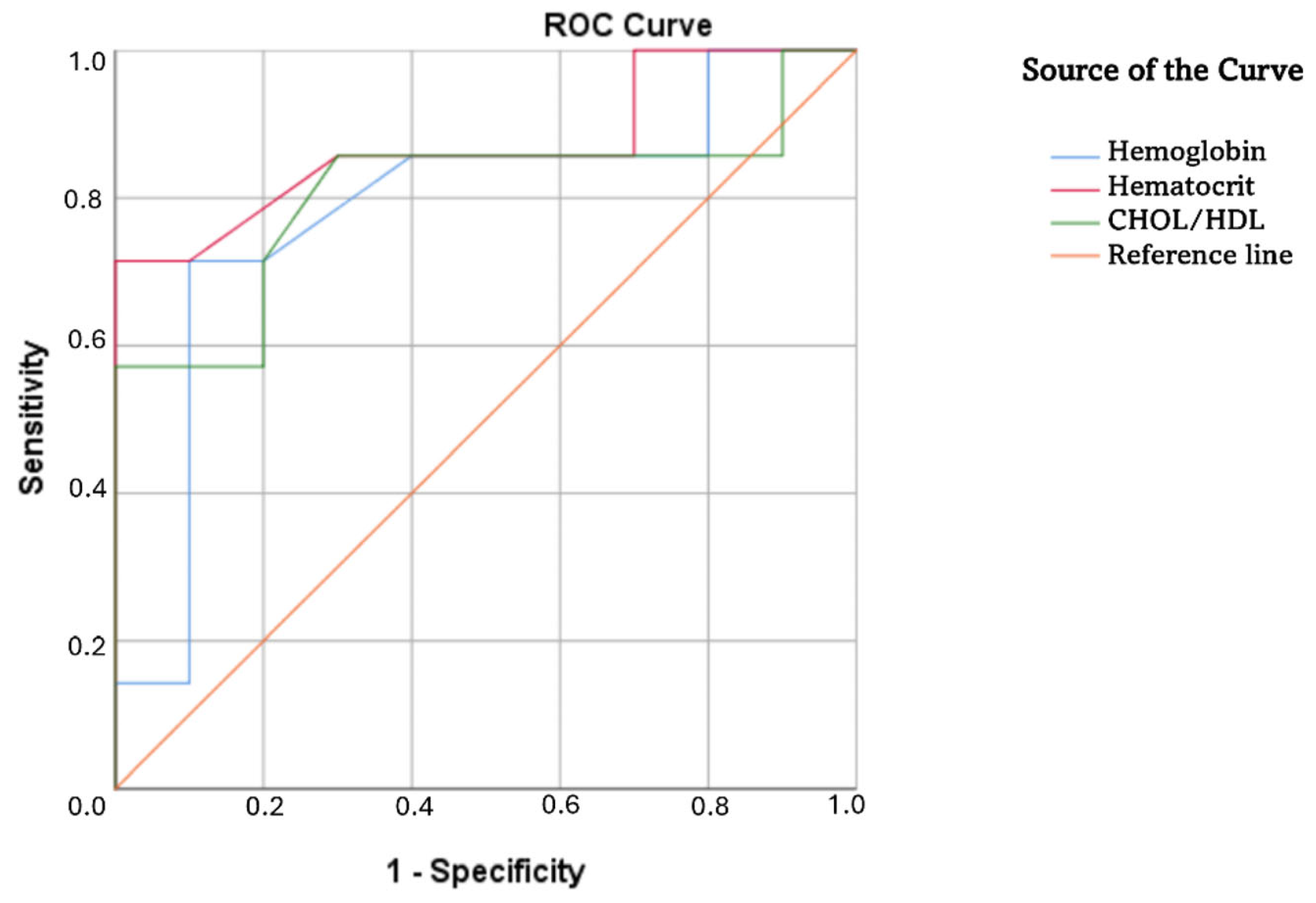
3. Results
4. Discussion
4.1. Comprehensive Profile of Participants Across Anthropometric, Metabolic, and Functional Variables: Comparisons with Previous Studies and Gender-Based Differences
4.2. Health Literacy of Parents/Legal Guardians of Adults with Down Syndrome: Status, Comparisons, and Factors Influencing Differences
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| DS | Down Syndrome |
| FM% | Fat mass percentage |
| HL | Health literacy |
| TG | Triglyceride |
| TCHOL | Total cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| 6MWT | Six-minute walk test |
References
- Koul, A.M.; Ahmad, F.; Bhat, A.; Aein, Q.-u.; Ahmad, A.; Reshi, A.A.; Kaul, R.-u.-R. Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis. Biomedicines 2023, 11, 3284. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the number of people with Down syndrome in Europe. Eur. J. Hum. Genet. 2021, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Agarwal Gupta, N.; Kabra, M. Diagnosis and management of Down syndrome. Indian J. Pediatr. 2014, 81, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Baumer, N.T.; Hojlo, M.A.; Pawlowski, K.G.; Milliken, A.L.; Lombardo, A.M.; Sargado, S.; Soccorso, C.; Davidson, E.J.; Barbaresi, W.J. Co-occurring conditions in Down syndrome: Findings from a clinical database. Am. J. Med. Genet. C Semin. Med. Genet. 2023, 193, e32072. [Google Scholar] [CrossRef] [PubMed]
- Esbensen, A.J. Health conditions associated with aging and end of life of adults with Down syndrome. Int. Rev. Res. Ment. Retard. 2010, 39, 107–126. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Lefter, N.; Abdulan, I.M.; Maștaleru, A.; Leon, M.M.; Rusu, C. Demographic Profile and Clinical Characteristics of Adults with Down Syndrome in North-Eastern Romania. Clin. Pract. 2024, 14, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Manfredo, J.; Capone, G.; Yanek, L.; McCarter, R.; Zemel, B.; Kelly, A.; Magge, S.N. Cardiometabolic risk in young adults with Down syndrome. Am. J. Med. Genet. A 2023, 191, 1758–1768. [Google Scholar] [CrossRef]
- Fleming, V.; Piro-Gambetti, B.; Handen, B.; Christian, B.T.; Cohen, A.; Tudorascu, D.; Plante, D.T.; Okonkwo, O.; Hartley, S.L. Physical Activity and Physical and Mental Health in Middle-Aged Adults with Down Syndrome. J. Policy Pract. Intellect. Disabil. 2022, 19, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Oreskovic, N.M.; Cottrell, C.; Torres, A.; Patsiogiannis, V.; Santoro, S.; Nichols, D.; Moore, C.; Skotko, B.G. Physical activity patterns in adults with Down Syndrome. J. Appl. Res. Intellect. Disabil. 2020, 33, 1457–1464. [Google Scholar] [CrossRef]
- Franklin, B.A.; Eijsvogels, T.M.H.; Pandey, A.; Quindry, J.; Toth, P.P. Physical activity, cardiorespiratory fitness, and cardiovascular health: A clinical practice statement of the American Society for Preventive Cardiology Part II: Physical activity, cardiorespiratory fitness, minimum and goal intensities for exercise training, prescriptive methods, and special patient populations. Am. J. Prev. Cardiol. 2022, 12, 100425. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Constantine, A.; Clift, P.; Condliffe, R.; Moledina, S.; Jansen, K.; Inuzuka, R.; Veldtman, G.R.; Cua, C.L.; Tay, E.L.W.; et al. Cardiovascular Complications of Down Syndrome: Scoping Review and Expert Consensus. Circulation 2023, 147, 425–441. [Google Scholar] [CrossRef] [PubMed]
- van den Driessen Mareeuw, F.A.; Coppus, A.M.W.; Delnoij, D.M.J.; de Vries, E. Quality of health care according to people with Down syndrome, their parents and support staff-A qualitative exploration. J. Appl. Res. Intellect. Disabil. 2020, 33, 496–514. [Google Scholar] [CrossRef]
- Akça, G.; Sanri, A.; Akca, U. Health literacy in parents of children with Down syndrome. Adv. Ment. Health Intellect. Disabil. 2024, 18, 88–97. [Google Scholar] [CrossRef]
- Sørensen, K.; Van den Broucke, S.; Fullam, J.; Doyle, G.; Pelikan, J.; Slonska, Z.; Brand, H. Health literacy and public health: A systematic review and integration of definitions and models. BMC Public Health 2012, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Geets-Kesić, M.; Maras, N.; Gilić, B. Analysis of the Association Between Health Literacy, Physical Literacy, and Scholastic Achievement; A Preliminary Cross-Sectional Study Among High-School Students From Southern Croatia. Montenegrin J. Sports Sci. Med. 2023, 12, 3–9. [Google Scholar] [CrossRef]
- Alahmadi, Y.M. Evaluation of Health Literacy and Associated Factors Among Adults Living in Saudi Arabia: A Cross-Sectional Study. INQ. J. Health Care Organ. Provis. Financ. 2023, 60, 00469580231161428. [Google Scholar] [CrossRef]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E.; Halpern, D.J.; Crotty, K. Low health literacy and health outcomes: An updated systematic review. Ann. Intern. Med. 2011, 155, 97–107. [Google Scholar] [CrossRef]
- Zaidman, E.A.; Scott, K.M.; Hahn, D.; Bennett, P.; Caldwell, P.H. Impact of parental health literacy on the health outcomes of children with chronic disease globally: A systematic review. J. Paediatr. Child Health 2023, 59, 12–31. [Google Scholar] [CrossRef]
- Lander, J.; Dierks, M.-L.; Hawkins, M. Health Literacy Development among People with Chronic Diseases: Advancing the State of the Art and Learning from International Practices. Int. J. Environ. Res. Public Health 2022, 19, 7315. [Google Scholar] [CrossRef]
- Assembly, U.G. Convention on the Rights of Persons with Disabilities. Ga. Res. 2006, 61, 106. [Google Scholar]
- Gordon, S.; Gardiner, T.; Gledhill, K.; Tamatea, A.; Newton-Howes, G. From Substitute to Supported Decision Making: Practitioner, Community and Service-User Perspectives on Privileging Will and Preferences in Mental Health Care. Int. J. Environ. Res. Public Health 2022, 19, 6002. [Google Scholar] [CrossRef] [PubMed]
- Kohn, N.A.; Blumenthal, J.A.; Campbell, A.T. Supported decision-making: A viable alternative to guardianship. Penn St. L. Rev. 2012, 117, 1111. [Google Scholar] [CrossRef]
- Sørensen, K.; Van den Broucke, S.; Pelikan, J.M.; Fullam, J.; Doyle, G.; Slonska, Z.; Kondilis, B.; Stoffels, V.; Osborne, R.H.; Brand, H. Measuring health literacy in populations: Illuminating the design and development process of the European Health Literacy Survey Questionnaire (HLS-EU-Q). BMC Public Health 2013, 13, 948. [Google Scholar] [CrossRef]
- Geets-Kesić, M. Health Literacy, Physical Activity and Health Status of High School Adolescents; University of Split, Faculty of Kinesiology: Split, Croatia, 2024. [Google Scholar]
- González-Correa, C.; Caicedo-Eraso, J.C. Bioelectrical impedance analysis (BIA): A proposal for standardization of the classical method in adults. Proc. J. Phys. Conf. Ser. 2012, 407, 012018. [Google Scholar] [CrossRef]
- Larsson, A.; Greig-Pylypczuk, R.; Huisman, A. The state of point-of-care testing: A European perspective. Upsala J. Med. Sci. 2015, 120, 1–10. [Google Scholar] [CrossRef]
- Yu-Fei, W.; Wei-Ping, J.; Ming-Hsun, W.; Miao, O.C.; Ming-Chang, H.; Chi-Pin, W.; Ming-Shih, L. Accuracy Evaluation of 19 Blood Glucose Monitoring Systems Manufactured in the Asia-Pacific Region: A Multicenter Study. J. Diabetes Sci. Technol. 2017, 11, 953–965. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Calling, S.; Johansson, S.E.; Wolff, M.; Sundquist, J.; Sundquist, K. Total cholesterol/HDL-C ratio versus non-HDL-C as predictors for ischemic heart disease: A 17-year follow-up study of women in southern Sweden. BMC Cardiovasc. Disord. 2021, 21, 163. [Google Scholar] [CrossRef]
- Sigdel, M.; Yadav, B.K.; Gyawali, P.; Regmi, P.; Baral, S.; Regmi, S.R.; Jha, B. Non-high density lipoprotein cholesterol versus low density lipoprotein cholesterol as a discriminating factor for myocardial infarction. BMC Res. Notes 2012, 5, 640. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Procedures and Devices for the Collection of Diagnostic Capillary Blood Specimens; Approved Standard—Sixth Edition; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- de Gonzalo-Calvo, D.; Barroeta, I.; Nan, M.N.; Rives, J.; Garzón, D.; Carmona-Iragui, M.; Benejam, B.; Videla, L.; Fernández, S.; Altuna, M.; et al. Evaluation of biochemical and hematological parameters in adults with Down syndrome. Sci. Rep. 2020, 10, 13755. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Ruiz, R.; Alcántara-Cordero, F.J.; Ruiz-Gavilán, I.; Sánchez-López, A.M. Feasibility and Reliability of a Physical Fitness Test Battery in Individuals with Down Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 2685. [Google Scholar] [CrossRef] [PubMed]
- Gastelum Guerrero, C.; Cháidez Fernández, Y.L.; Magaña Ordorica, D.; Berger, H.; Vazquez Landrove, M.; Guadrón Llanos, A.; Angulo Rojo, C.; Magaña Gómez, J. A systematic review and meta-analysis of serum lipid concentrations in people with Down syndrome. J. Intellect. Disabil. Res. 2024, 68, 553–563. [Google Scholar] [CrossRef]
- Real de Asua, D.; Parra, P.; Costa, R.; Moldenhauer, F.; Suarez, C. Evaluation of the impact of abdominal obesity on glucose and lipid metabolism disorders in adults with Down syndrome. Res. Dev. Disabil. 2014, 35, 2942–2949. [Google Scholar] [CrossRef]
- Brantmüller, É.; Gyuró, M.; Karácsony, I. Development of walking and self-sufficiency ability related to nutrition among people with Down syndrome. Pract. Theory Syst. Educ. 2015, 10, 165–176. [Google Scholar] [CrossRef]
- Real de Asua, D.; Parra, P.; Costa, R.; Moldenhauer, F.; Suarez, C. A cross-sectional study of the phenotypes of obesity and insulin resistance in adults with down syndrome. Diabetes Metab. J. 2014, 38, 464–471. [Google Scholar] [CrossRef]
- Bredella, M.A. Sex Differences in Body Composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Crisafulli, A. Gender Differences in Hemodynamic Regulation and Cardiovascular Adaptations to Dynamic Exercise. Curr. Cardiol. Rev. 2020, 16, 65–72. [Google Scholar] [CrossRef]
- Ballenger, B.K.; Schultz, E.E.; Dale, M.; Fernhall, B.; Motl, R.W.; Agiovlasitis, S. Health Outcomes of Physical Activity Interventions in Adults With Down Syndrome: A Systematic Review. Adapt. Phys. Act. Q. 2023, 40, 378–402. [Google Scholar] [CrossRef]
- Baynard, T.; Pitetti, K.H.; Guerra, M.; Unnithan, V.B.; Fernhall, B. Age-related changes in aerobic capacity in individuals with mental retardation: A 20-yr review. Med. Sci. Sports Exerc. 2008, 40, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Lochbaum, M.; Sherburn, M.; Sisneros, C.; Cooper, S.; Lane, A.M.; Terry, P.C. Revisiting the Self-Confidence and Sport Performance Relationship: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6381. [Google Scholar] [CrossRef] [PubMed]
- Vazirian, F.; Darroudi, S.; Rahimi, H.R.; Latifi, M.; Shakeri, B.; Abolbashari, S.; Mohammadpour, A.H.; Esmaily, H.; Mouhebati, M.; Samadi, S.; et al. Non-HDL cholesterol and long-term follow-up outcomes in patients with metabolic syndrome. Lipids Health Dis. 2023, 22, 165. [Google Scholar] [CrossRef]
- Soler Marín, A.; Xandri Graupera, J.M. Nutritional status of intellectual disabled persons with Down syndrome. Nutr. Hosp. 2011, 26, 1059–1066. [Google Scholar] [CrossRef]
- Eberhard, Y.; Eterradossi, J.; Debû, B. Biological Changes Induced by Physical Activity in Individuals with Down’s Syndrome. Adapt. Phys. Act. Q. 1997, 14, 166–175. [Google Scholar] [CrossRef]
- Pecoraro, L.; Zadra, M.; Cavallin, F.; Lauriola, S.; Piacentini, G.; Pietrobelli, A. Lipid Profile, Eating Habit, and Physical Activity in Children with Down Syndrome: A Prospective Study. Diseases 2024, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.W.d.; Greguol, M. Lipid profile in people with Down syndrome: A literature review. J. Human Growth Dev. 2020, 30, 197–208. [Google Scholar] [CrossRef]
- Adelekan, T.; Magge, S.; Shults, J.; Stallings, V.; Stettler, N. Lipid profiles of children with Down syndrome compared with their siblings. Pediatrics 2012, 129, e1382–e1387. [Google Scholar] [CrossRef]
- Ordóñez-Munoz, F.J.; Rosety-Rodríguez, M.; Rosety-Rodríguez, J.M.; Rosety-Plaza, M. Medidas antropométricas como predictores del comportamiento lipídico sérico en adolescentes con síndrome de Down. Rev. Investig. Clin. 2005, 57, 691–694. [Google Scholar]
- Samarkandy, M.M.; Mohamed, B.A.; Al-Hamdan, A.A. Nutritional assessment and obesity in Down syndrome children and their siblings in Saudi Arabia. Saudi Med. J. 2012, 33, 1216–1221. [Google Scholar]
- Dörner, K.; Gaethke, A.S.; Tolksdorf, M.; Schumann, K.P.; Gustmann, H. Cholesterol fractions and triglycerides in children and adults with Down’s syndrome. Clin. Chim. Acta 1984, 142, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Marrugat, J.; Elosua, R.; Covas, M.I. Relationship between body mass index, serum cholesterol, leisure-time physical activity, and diet in a Mediterranean Southern-Europe population. Br. J. Nutr. 2003, 90, 431–439. [Google Scholar] [CrossRef]
- Mierlo, A.I.V.; Queje, I.C.; Simionatto, M.; Brito, P.S.; Santos, F.A.D.; Vellosa, J.C.R.; Borato, D.C.K. Biochemical and hematological evaluation in subjects with intellectual disability associated or not to Down syndrome. J. Bras. Patol. Med. Lab. 2018, 54, 9–13. [Google Scholar] [CrossRef]
- Fonseca, C.T.; Amaral, D.M.; Ribeiro, M.G.; Beserra, I.C.R.; Guimarães, M.M. Insulin resistance in adolescents with Down syndrome: A cross-sectional study. BMC Endocr. Disord. 2005, 5, 6. [Google Scholar] [CrossRef]
- Aslam, A.A.; Baksh, R.A.; Pape, S.E.; Strydom, A.; Gulliford, M.C.; Chan, L.F. Diabetes and Obesity in Down Syndrome Across the Lifespan: A Retrospective Cohort Study Using U.K. Electronic Health Records. Diabetes Care 2022, 45, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Culp-Hill, R.; Zheng, C.; Reisz, J.A.; Smith, K.; Rachubinski, A.; Nemkov, T.; Butcher, E.; Granrath, R.; Hansen, K.C.; Espinosa, J.M.; et al. Red blood cell metabolism in Down syndrome: Hints on metabolic derangements in aging. Blood Adv. 2017, 1, 2776–2780. [Google Scholar] [CrossRef]
- Maclean, G.A.; Menne, T.F.; Guo, G.; Sanchez, D.J.; Park, I.H.; Daley, G.Q.; Orkin, S.H. Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc. Natl. Acad. Sci. USA 2012, 109, 17567–17572. [Google Scholar] [CrossRef]
- Bobinac, A.; Dukić Samaržija, N.; Ribarić, E. Zdravstvena pismenost u Republici Hrvatskoj. Rev. Soc. Politiku 2022, 29, 427–443. [Google Scholar] [CrossRef]
- Baccolini, V.; Rosso, A.; Di Paolo, C.; Isonne, C.; Salerno, C.; Migliara, G.; Prencipe, G.P.; Massimi, A.; Marzuillo, C.; De Vito, C.; et al. What is the Prevalence of Low Health Literacy in European Union Member States? A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2021, 36, 753–761. [Google Scholar] [CrossRef]
- World Health Organization. Anemia. Available online: https://www.who.int/news-room/fact-sheets/detail/anaemia (accessed on 2 January 2025).
- Gardner, W.; Kassebaum, N. Global, Regional, and National Prevalence of Anemia and Its Causes in 204 Countries and Territories, 1990–2019. Curr. Dev. Nutr. 2020, 4, 830. [Google Scholar] [CrossRef]
- Wiafe, M.A.; Yeboah, G.B.; Gyamerah, E.; Konlaa, H.D.; Ibrahim, I.; Benewaa, A. Knowledge, prevalence and factors associated with anaemia among women of reproductive age in Tamale Metropolis, Ghana: A cross-sectional study. Womens Health 2024, 20, 17455057241263826. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.L.; Hong, A.S.; Hahm, K.; Kim, D.; Smith-Morris, C.; Zaha, V.G. Health Literacy, Individual and Community Engagement, and Cardiovascular Risks and Disparities: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Boer, P.H.; de Beer, Z. The effect of aquatic exercises on the physical and functional fitness of adults with Down syndrome: A non-randomised controlled trial. J. Intellect. Disabil. Res. 2019, 63, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Keim-Malpass, J.; Letzkus, L.C.; Kennedy, C. Parent/caregiver health literacy among children with special health care needs: A systematic review of the literature. BMC Pediatr. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Madalı-Kafes, B.; Burçin, A.Ş.; Serpil, K.; Berna, T.; Kara, H.H. Health literacy and eHealth literacy in caregivers of students with special education needs: A cross-sectional survey study. Int. J. Dev. Disabil. 2025, 1–10. [Google Scholar] [CrossRef]
- Covain, S.; Baillieul, S.; Nguyen, T.D.; Guinot, M.; Doutreleau, S.; Bricout, V.A. Gender Differences, Motor Skills and Physical Fitness Heterogeneity in Adults with Down’s Syndrome. J. Clin. Med. 2023, 12, 1367. [Google Scholar] [CrossRef]
| Analyst/Search/Index | Gender | Years | Reference Interval |
|---|---|---|---|
| Hemoglobin | male | ≥20 | 13.8–17.5 g/dL |
| female | ≥20 | 11.9–15.7 g/dL | |
| Hematocrit | male | ≥20 | 41.5–53.0 dL/dL |
| female | ≥20 | 35.6–47.0 dL/dL | |
| Glucose | male, female | 20–30 | 59.4594–93.6936 mg/dL |
| male, female | >30 | 63.063–100.900 mg/dL | |
| Cholesterol | Male, female | adult | <193.35 mg/dL |
| HDL-cholesterol | male | adult | >38.67 mg/dL |
| female | adult | >46.40 mg/dL | |
| LDL-cholesterol | male, female | adult | <116.01 mg/dL |
| Triglycerides | male, female | adult | <150.56 mg/dL |
| Non-HDL | male, female | adult | <130 mg/dL |
| Variable | HL-T | HL-1 | HL-2 | HL-3 | HL-4 | HL-5 | HL-6 | HL-7 | HL-8 | HL-9 | HL-10 | HL-11 | HL-12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | −0.03 | −0.08 | 0.02 | −0.10 | 0.25 | 0.20 | −0.11 | −0.18 | −0.30 | 0.11 | −0.21 | −0.19 | 0.10 |
| BH | 0.17 | 0.31 | 0.45 | 0.21 | 0.06 | −0.01 | 0.13 | 0.21 | 0.32 | −0.34 | 0.20 | 0.37 | −0.38 |
| BM | 0.31 | 0.43 | 0.45 | 0.08 | 0.24 | 0.33 | 0.25 | −0.07 | 0.22 | 0.15 | 0.40 | 0.29 | −0.12 |
| BMI | 0.26 | 0.30 | 0.25 | 0.03 | 0.31 | 0.45 | 0.19 | −0.21 | 0.07 | 0.28 | 0.29 | 0.11 | 0.10 |
| FM% | 0.08 | 0.01 | −0.05 | −0.01 | 0.18 | 0.28 | 0.17 | −0.35 | 0.01 | 0.21 | 0.18 | −0.16 | 0.19 |
| MM% | −0.08 | −0.01 | 0.05 | 0.01 | −0.18 | −0.28 | −0.17 | 0.35 | −0.01 | −0.21 | −0.18 | 0.16 | −0.20 |
| Glucose | 0.22 | 0.36 | 0.35 | 0.17 | 0.00 | 0.21 | 0.09 | 0.11 | 0.09 | 0.15 | 0.11 | 0.32 | −0.04 |
| Hemoglobin | 0.46 | 0.37 | 0.43 | 0.29 | 0.04 | 0.07 | 0.03 | 0.43 | 0.57 ** | 0.39 | 0.34 | 0.47 * | 0.21 |
| Hematocrit | 0.47 * | 0.52 * | 0.56 ** | 0.38 | 0.48 * | 0.11 | 0.16 | 0.29 | 0.54 * | 0.12 | 0.31 | 0.37 | −0.07 |
| TCHOL | −0.19 | −0.28 | −0.20 | −0.17 | −0.19 | 0.03 | −0.46 * | −0.23 | −0.26 | 0.14 | −0.36 | −0.27 | 0.39 |
| HDL | −0.32 | −0.40 | −0.52 * | −0.12 | −0.15 | −0.18 | −0.14 | −0.14 | −0.37 | −0.05 | −0.25 | −0.34 | 0.20 |
| TRIGL | 0.04 | −0.17 | −0.11 | −0.24 | −0.16 | 0.04 | −0.49 * | 0.21 | 0.08 | 0.38 | −0.09 | 0.08 | 0.46 |
| LDL | −0.16 | −0.15 | −0.17 | −0.09 | −0.06 | −0.11 | −0.34 | −0.12 | −0.34 | 0.08 | −0.29 | −0.20 | 0.20 |
| CHOl/HDL | 0.38 | 0.31 | 0.43 | 0.15 | 0.15 | 0.13 | −0.19 | 0.31 | 0.23 | 0.33 | 0.09 | 0.31 | 0.13 |
| nonHDL | −0.09 | −0.15 | −0.01 | −0.14 | −0.15 | 0.11 | −0.46 * | −0.20 | −0.16 | 0.18 | −0.32 | −0.18 | 0.36 |
| 6MWT | 0.16 | 0.06 | 0.23 | 0.26 | −0.15 | −0.27 | 0.01 | 0.50 * | 0.45 | −0.01 | 0.14 | 0.23 | −0.07 |
| Variables | AUC | SE | AS | 95% CI |
|---|---|---|---|---|
| BH | 0.70 | 0.16 | 0.19 | 0.39–1.00 |
| BM | 0.72 | 0.13 | 0.16 | 0.46–0.97 |
| BMI | 0.62 | 0.15 | 0.45 | 0.32–0.91 |
| FM% | 0.48 | 0.17 | 0.91 | 0.15–0.82 |
| Glucose | 0.61 | 0.15 | 0.46 | 0.32–0.89 |
| Hemoglobin | 0.79 | 0.12 | 0.05 | 0.54–1.00 |
| Hematocrit | 0.87 | 0.10 | 0.01 | 0.68–1.00 |
| TCHOL | 0.61 | 0.14 | 0.46 | 0.33–0.89 |
| TRIGL | 0.32 | 0.13 | 0.22 | 0.06–0.58 |
| LDL | 0.64 | 0.15 | 0.33 | 0.36–0.93 |
| nonHDL | 0.51 | 0.15 | 0.96 | 0.22–0.80 |
| HDL | 0.71 | 0.13 | 0.16 | 0.45–0.96 |
| 6MWT | 0.67 | 0.14 | 0.24 | 0.39–0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkovic Vuletic, P.; Geets-Kesic, M.; Jurcev-Savicevic, A.; Nurjanah, N.; Gilic, B. Metabolic, Hematological, and Functional Health in Adults with Down Syndrome and Significance of Parental Health Literacy: A Cross-Sectional Study. Healthcare 2025, 13, 1212. https://doi.org/10.3390/healthcare13101212
Rajkovic Vuletic P, Geets-Kesic M, Jurcev-Savicevic A, Nurjanah N, Gilic B. Metabolic, Hematological, and Functional Health in Adults with Down Syndrome and Significance of Parental Health Literacy: A Cross-Sectional Study. Healthcare. 2025; 13(10):1212. https://doi.org/10.3390/healthcare13101212
Chicago/Turabian StyleRajkovic Vuletic, Petra, Marijana Geets-Kesic, Anamarija Jurcev-Savicevic, Nurjanah Nurjanah, and Barbara Gilic. 2025. "Metabolic, Hematological, and Functional Health in Adults with Down Syndrome and Significance of Parental Health Literacy: A Cross-Sectional Study" Healthcare 13, no. 10: 1212. https://doi.org/10.3390/healthcare13101212
APA StyleRajkovic Vuletic, P., Geets-Kesic, M., Jurcev-Savicevic, A., Nurjanah, N., & Gilic, B. (2025). Metabolic, Hematological, and Functional Health in Adults with Down Syndrome and Significance of Parental Health Literacy: A Cross-Sectional Study. Healthcare, 13(10), 1212. https://doi.org/10.3390/healthcare13101212








