Improved Prediction Accuracy for Late-Onset Preeclampsia Using cfRNA Profiles: A Comparative Study of Marker Selection Strategies
Abstract
1. Introduction
- Early-Onset Preeclampsia (EO-PE): This form is predominantly driven by placental abnormalities and immune dysregulation that begin early in gestation; distinct differential expression of cfRNA has been reported. For example, Moufarrej et al. demonstrated a high-accuracy model (AUC ≈ 0.9) using cfRNA derived from maternal plasma, suggesting an impairment of immune response and angiogenic pathways [9].
- Late-Onset Preeclampsia (LO-PE): Maternal comorbidities such as obesity or chronic hypertension play a substantial role, often diminishing the utility of purely placental biomarkers for high-sensitivity prediction. Indeed, many studies investigating cfRNA- or metabolite-based tests focus on overall PE risk and do not provide separate metrics (e.g., AUC) for LO-PE alone. For example, while Maric et al. [13] report robust performance in predicting PE, their models do not isolate late-onset cases. As a result, the true accuracy for LO-PE remains unclear, and some data even suggest that maternal factors may overshadow direct placental signals, leading to potentially lower AUCs for late-onset compared to early-onset PE. Moving forward, it will be crucial to refine LO-PE-specific molecular signatures—possibly through multi-omics approaches integrated with maternal clinical data—and validate such signatures in large external cohorts. This line of research is expected to clarify whether dedicated LO-PE models can outperform current one-size-fits-all approaches and ultimately improve risk stratification in this patient population.
- To characterize cfRNA profiles in LO-PE and compare them with known markers predominantly associated with EO-PE.
- To apply two feature selection strategies—(A) an approach based on differential expression analysis and (B) an approach leveraging prediction errors (via the elastic-net solution path)—and then assess LO-PE prediction performance in terms of AUC, sensitivity, and specificity.
- To examine the performance trade-offs involved in simultaneously predicting both EO- and LO-PE, and to investigate how immune tolerance and metabolic pathways might be affected.
2. Materials and Methods
2.1. Dataset
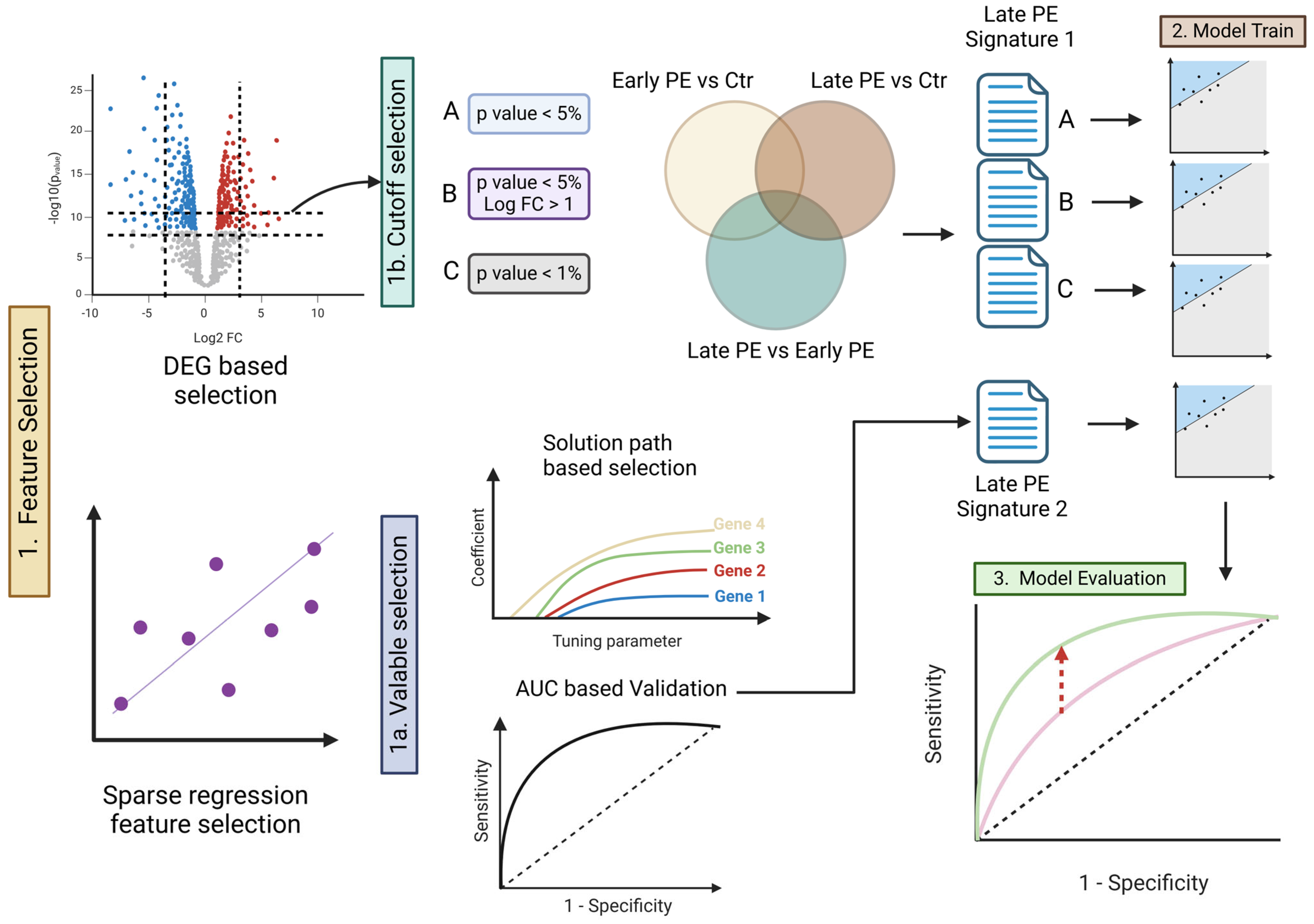
2.2. Strategy for Selecting Signature Genes
2.3. Building and Evaluating the Predictive Model
2.4. Searching for Biomarker Candidates
2.5. Performance Metrics
2.6. Model Evaluation
3. Results
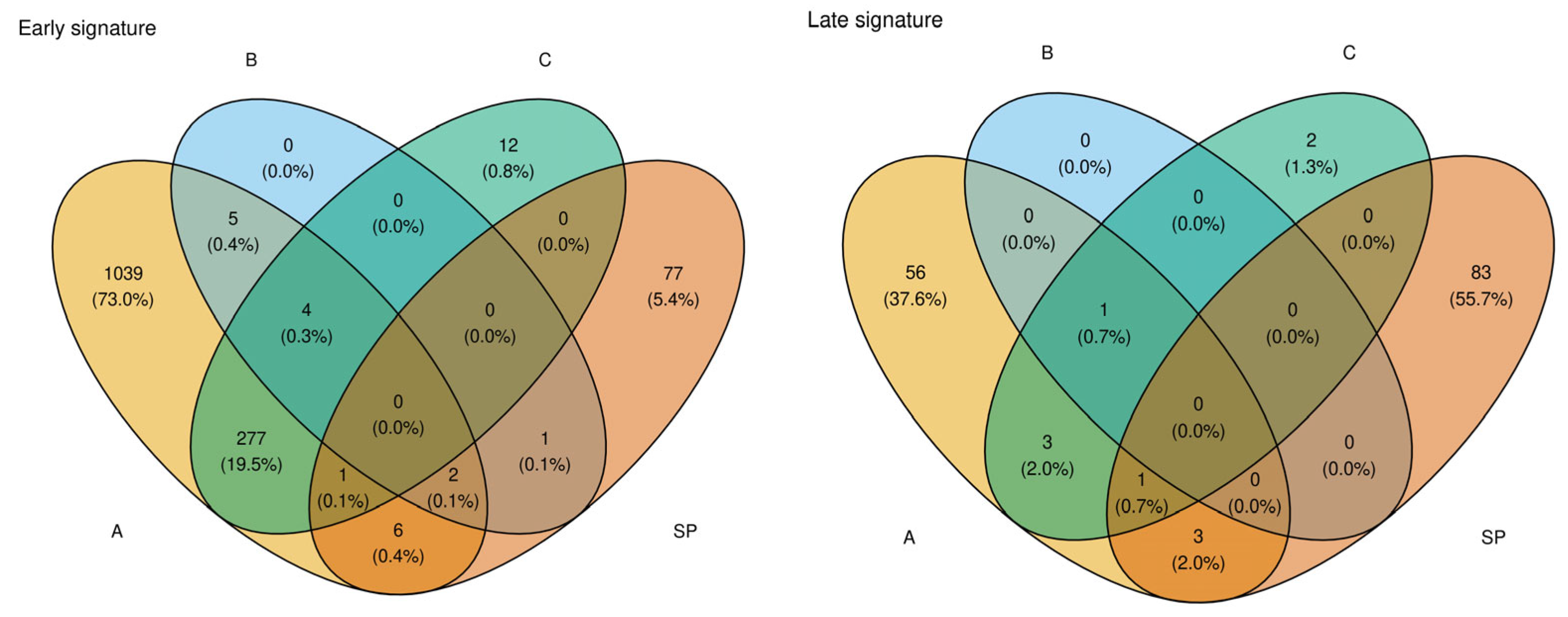
3.1. Identification of Signature Genes and Feature Selection
3.2. Candidate Selection via Prediction Error (Elastic-Net Solution Path)
3.3. Comparative Performance of Prediction Models
- Training on early-onset samples yielded an AUC of 0.9375 for predicting early-onset PE but only 0.6875 for predicting late-onset PE.
- Training on late-onset samples resulted in an AUC of 0.6875 for predicting late-onset PE.
3.4. Candidate Biomarkers and Functional Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUC | Area Under the Receiver Operating Characteristic Curve |
| BMI | Body Mass Index |
| cfRNA | Cell-Free RNA |
| DEG | Differentially Expressed Gene |
| EHR | Electronic Health Record |
| EVT | Extravillous Trophoblast |
| HLA-G | Human Leukocyte Antigen-G |
| IL17RB | Interleukin-17 Receptor B |
| EO-PE | Early-Onset Preeclampsia |
| LO-PE | Late-Onset Preeclampsia |
| ML | Machine Learning |
| NGS | Next-Generation Sequencing |
| PE | Preeclampsia |
| PlGF | Placental Growth Factor |
| ROC | Receiver Operating Characteristic |
| sFlt-1 | Soluble fms-like Tyrosine Kinase-1 |
References
- Liu, M.; Yang, X.; Chen, G.; Ding, Y.; Shi, M.; Sun, L.; Huang, Z.; Liu, J.; Liu, T.; Yan, R.; et al. Development of a Prediction Model on Preeclampsia Using Machine Learning: A Retrospective Cohort Study in China. Front. Physiol. 2022, 13, 896969. [Google Scholar] [CrossRef] [PubMed]
- Erez, O.; Romero, R.; Maymon, E.; Chaemsaithong, P.; Done, B.; Pacora, P.; Panaitescu, B.; Chaiworapongsa, T.; Hassan, S.S.; Tarca, A.L. The Prediction of Late-Onset Preeclampsia: Results from a Longitudinal Proteomics Study. PLoS ONE 2017, 12, e0181468. [Google Scholar] [CrossRef]
- Baylis, A.; Zhang, W.; Menkhorst, E.; Dimitriadis, E. Prediction and Prevention of Late-Onset Pre-Eclampsia: A Systematic Review. Front. Med. 2024, 10, 1459289. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the Prevention of Preterm and Term Preeclampsia: Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e1. [Google Scholar] [CrossRef]
- Tan, M.Y.; Syngelaki, A.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; de Alvarado, M.; Carbone, I.F.; Dutemeyer, V.; Fiolna, M.; Frick, A.; et al. Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 11–13 Weeks’ Gestation. Ultrasound Obstet. Gynecol. 2018, 52, 186–195. [Google Scholar] [CrossRef]
- Jhee, J.H.; Lee, S.; Park, Y.; Lee, S.E.; Kim, Y.A.; Kang, S.-W.; Kwon, J.-Y.; Park, J.T. Prediction Model Development of Late-Onset Preeclampsia Using Machine Learning-Based Methods. PLoS ONE 2019, 14, e0221202. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Wedenoja, S.; Yoshihara, M.; Teder, H.; Sariola, H.; Gissler, M.; Katayama, S.; Wedenoja, J.; Häkkinen, I.M.; Ezer, S.; Linder, N.; et al. Fetal HLA-G Mediated Immune Tolerance and Interferon Response in Preeclampsia. EBioMedicine 2020, 59, 102872. [Google Scholar] [CrossRef] [PubMed]
- Moufarrej, M.N.; Vorperian, S.K.; Wong, R.J.; Campos, A.A.; Quaintance, C.C.; Sit, R.V.; Tan, M.; Detweiler, A.M.; Mekonen, H.; Neff, N.F.; et al. Early Prediction of Preeclampsia in Pregnancy with Cell-Free RNA. Nature 2022, 602, 689–694. [Google Scholar] [CrossRef]
- Robillard, P.-Y.; Dekker, G.; Scioscia, M.; Saito, S. Progress in the Understanding of the Pathophysiology of Immunologic Maladaptation Related to Early-Onset Preeclampsia and Metabolic Syndrome Related to Late-Onset Preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S867–S875. [Google Scholar] [CrossRef]
- Layton, A.T. Artificial Intelligence and Machine Learning in Preeclampsia. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, V.P.; Eberhard, B.W.; Cohen, R.Y.; Maher, M.; Saxena, R.; Gray, K.J. Preeclampsia Prediction Using Machine Learning and Polygenic Risk Scores from Clinical and Genetic Risk Factors in Early and Late Pregnancies. Hypertension 2024, 81, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Marić, I.; Contrepois, K.; Moufarrej, M.N.; Stelzer, I.A.; Feyaerts, D.; Han, X.; Tang, A.; Stanley, N.; Wong, R.J.; Traber, G.M.; et al. Early Prediction and Longitudinal Modeling of Preeclampsia from Multiomics. Patterns 2022, 3, 100655. [Google Scholar] [CrossRef]
- Munchel, S.; Rohrback, S.; Randise-Hinchliff, C.; Kinnings, S.; Deshmukh, S.; Alla, N.; Tan, C.; Kia, A.; Greene, G.; Leety, L.; et al. Circulating Transcripts in Maternal Blood Reflect a Molecular Signature of Early-Onset Preeclampsia. Sci. Transl. Med. 2020, 12, eaaz0131. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A Scaling Normalization Method for Differential Expression Analysis of RNA-seq Data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Smyth, G.K. Limma: Linear Models for Microarray Data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R., Carey, V., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005; pp. 397–420. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Andrietti, S.; Silva, M.; Wright, A.; Wright, D.; Nicolaides, K.H. Competing-Risks Model in Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 35–37 Weeks’ Gestation. Ultrasound Obstet. Gynecol. 2016, 48, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Tang, Y.; Hu, R.; Zhou, Q.; Li, X. IL-25 Promotes Trophoblast Proliferation and Invasion via Binding with IL-17RB and Associated with PE. Hypertens. Pregnancy 2021, 40, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qian, Y.; Jiang, H.; Meng, H.; Wang, Y.; Yang, Y. Combined Maternal KIR2DL4 and Fetal HLA-G Polymorphisms Were Associated with Preeclampsia in a Han Chinese Population. Front. Genet. 2024, 15, 1442938. [Google Scholar] [CrossRef]
- Aisagbonhi, O.; Morris, G.P. Human Leukocyte Antigens in Pregnancy and Preeclampsia. Front. Genet. 2022, 13, 884275. [Google Scholar] [CrossRef] [PubMed]



| Prediction Without Signature | Training Data | Early | Late | Early | Late | |
|---|---|---|---|---|---|---|
| Early prediction AUC | 0.9375 | 0.6875 | ||||
| Late prediction AUC | 0.6875 | 0.6875 | ||||
| Signature | Early | Late | Early and Late | |||
| DEG Signature | ||||||
| (a) p value < 0.05 | ||||||
| Number of Early Signature | 1334 | Early prediction AUC | 0.9944 | 0.6826 | 0.9938 | 0.6493 |
| Number of Late Signature | 64 | Late prediction AUC | 0.6625 | 0.9563 | 0.65 | 0.6979 |
| (b) p value < 0.05 and logFC > 1 | ||||||
| Number of Early Signature | 11 | Early prediction AUC | 0.915 | 0.653 | 0.905 | 0.738 |
| Number of Late Signature | 1 | Late prediction AUC | 0.687 | 0.736 | 0.68 | 0.786 |
| (c) p value < 0.01 | ||||||
| Number of Early Signature | 295 | Early prediction AUC | 0.997 | 0.66 | 0.995 | 0.606 |
| Number of Late Signature | 7 | Late prediction AUC | 0.67 | 0.755 | 0.656 | 0.651 |
| Elastic Net–Based Signatures | ||||||
| Number of Early Signature | 87 | Early prediction AUC | 0.988 | 0.828 | 0.869 | 0.856 |
| Number of Late Signature | 87 | Late prediction AUC | 0.71 | 0.988 | 0.687 | 0.869 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, A.; Uno, K.; Matsui, Y. Improved Prediction Accuracy for Late-Onset Preeclampsia Using cfRNA Profiles: A Comparative Study of Marker Selection Strategies. Healthcare 2025, 13, 1162. https://doi.org/10.3390/healthcare13101162
Nakano A, Uno K, Matsui Y. Improved Prediction Accuracy for Late-Onset Preeclampsia Using cfRNA Profiles: A Comparative Study of Marker Selection Strategies. Healthcare. 2025; 13(10):1162. https://doi.org/10.3390/healthcare13101162
Chicago/Turabian StyleNakano, Akiha, Kohei Uno, and Yusuke Matsui. 2025. "Improved Prediction Accuracy for Late-Onset Preeclampsia Using cfRNA Profiles: A Comparative Study of Marker Selection Strategies" Healthcare 13, no. 10: 1162. https://doi.org/10.3390/healthcare13101162
APA StyleNakano, A., Uno, K., & Matsui, Y. (2025). Improved Prediction Accuracy for Late-Onset Preeclampsia Using cfRNA Profiles: A Comparative Study of Marker Selection Strategies. Healthcare, 13(10), 1162. https://doi.org/10.3390/healthcare13101162







