1. Introduction
There is a lack of a standardized operative definition of drug shortage globally, as stated by the experts in the sector worldwide, who either define it from the supply side or from the user side. The World Health Organization (WHO) in 2016 announced two definitions: shortage occurs “when the supply of medicines, health products, and vaccines identified as essential by the health system is considered to be insufficient to meet public health and patient needs” [
1]. While on the demand side, a shortage occurs “when demand exceeds supply at any point in the supply chain and may ultimately create a stock-out at the point of appropriate service delivery to the patient if the cause of the shortage cannot be resolved promptly relative to the clinical needs of the patient” [
2]. This problem is affecting many countries worldwide, and is experienced in relation to all types of drugs, with sterile injectable formulations, essential medicines, and emergency medicines being more susceptible [
2]. In particular, it has been outlined that the medicines presenting the highest risk of shortages are medicinal products (MPs) characterized by a low price and manufacturing complexity [
3]. Drug shortages can occur due to many factors, including supply issues, demand issues, and regulatory issues. Supply issues consist of manufacturing and quality problems, unavailability of raw materials, logistic problems, and business decisions. In contrast, demand issues include just-in-time inventory, unexpected higher demand for a product, and demand fluctuations due, for instance, to seasonal necessities. Finally, regulatory issues may lead to delays in drug approval [
4,
5]. Examples of commercial issues causing a lack of medicines include medicine withdrawals due to the presence of noxious materials (i.e., valsartan [
6]) or drug misuse (i.e., semaglutide employed for obesity treatment [
7]). The supply problems of medicines and starting materials have become more acute with the relocation of chemical and pharmaceutical production to countries with less impactful labour costs and environmental, socio-economic, and pharmaceutical-specific regulations. Moreover, as evidenced in the 2023 US Pharmacopeia (USP) Annual Drug Shortages Report [
3], the geographic concentration of pharmaceutical production, particularly in China and India, increases the vulnerability of the drug supply chain [
8]. More than 50% of global active pharmaceutical ingredient (API) production is concentrated in five producer countries, and this strong concentration makes the European supply chains extremely vulnerable and affected by security-relevant weaknesses.
Another problem of medicine shortage is linked to the mechanism for fixing the price of generic medicines, in which insufficient revenues can be determined, leading to the potential discontinuation of the marketing of the product by the interested company. In the European Union (EU), parallel trade has been identified as an additional risk factor for medicine shortages in low-price Member States (like Poland, Slovakia, Greece, and Spain), even if the number of studies regarding the correlation between parallel trade and medicine shortage is currently insufficient [
9,
10,
11].
Moreover, new economic plans in pharmaceutical companies may also be responsible for limitations in drug supplies (i.e., low investments in low-profit drugs such as generics).
Patients are the stakeholders mainly affected by the consequences of shortfalls in medicine supply: besides suboptimal treatments, they may experience delayed care, extended hospitalization, surgery cancellations, etc. [
2,
12].
Moreover, drug shortages have a conspicuous economic impact. For example, the cost of the annual management of drug shortages in the United States might be approx. USD 416 million, to which a further USD 215 million is to be added for the purchase of alternative medications [
13].
The management of drug shortages may include the following: restrictions of the use of current stocks, accelerated drug approval, use of medicines with minor defects that cannot normally be employed, and the extension of expiry dates. Several States have developed medical platforms, providing information to physicians, pharmacists, and final users about forthcoming shortages and their management, and guidelines to be applied at national and possibly at international levels, as frequently the responses from single countries, and even at the level of health facilities within the same country, are uncoordinated [
2,
13].
The main regulatory agencies, like the Food and Drug Administration (FDA), which has been researching this issue since 1999, and, more recently, the European Medicines Agency (EMA), have been studying the phenomenon to identify drug shortages and potential remedies, both adopting several measures to mitigate them [
1,
2].
EU regulations require the marketing authorization (MA) holder to notify the national agency of any temporary or permanent discontinuation of an MP’s marketing within the national territory no less than two months before the interruption, except in the case of unforeseeable and exceptional circumstances [
14,
15].
Countermeasures such as an appropriate management of communication on drug shortages and the promotion of the use of equivalent, imported, and compounded medicines are essential to convey accurate information, counter hoarding practices, and prevent supply tensions for medicines [
16]. Besides these initiatives, a rational tool aiding healthcare professionals in the management of drug shortages might be highly beneficial.
A “Pilot Project on Drug Shortages in Regione Liguria”, involving various actors, including the Italian Medicines Agency (AIFA), the regional offices for drug policies of Regione Liguria (A.Li.Sa.), experts and trainees of the Specialization School in Hospital Pharmacy from the University of Genoa, and main professional stakeholders, was established with various objectives, including to provide informatic support to healthcare professionals in selecting the most suitable alternative when an MP is unavailable on the market. The development of an algorithm which allows for a comparison of the different MPs by using a novel code able to describe them might be helpful in pointing out available pharmaceutical alternatives to physicians.
The aim of this paper is to present the algorithm developed for this goal, which can provide a ranking of possible substitutes for a drug in shortage according to the availability of equivalent MPs, or alternative MPs with different dosages, dose unit numbers, pharmaceutical forms, or administration routes. This algorithm can fill a gap in day-to-day drug substitution decisions, being easily adapted to any market areas or healthcare systems. In the literature, a number of publications have dealt with drug shortages by analyzing causes, trends, and impacts in different national systems [
17,
18,
19,
20,
21,
22,
23], but, to the best of our knowledge, no algorithms similar to ours have ever been disclosed, nor have similar rational tools been developed and implemented, and this underlines the novelty of this approach.
3. Results and Discussion
The algorithm proposed here is designed based on the descriptive strings of the MPs, assembling internationally standardized codes for the identification of the API and the description of the characteristic pharmaceutical properties of the MPs, which, to the best of our knowledge, have never been used before in this context as is conceived here. The developed algorithm is applied and validated using MPs authorized for the Italian market and, in particular, provided by the Italian Health Service (Servizio Sanitario Nazionale, SSN). It is noteworthy that we use a localized Italian dataset as the only accessible source of MPs on the market available to us. The datasets of other countries may only change in terms of the quantitative composition (number of MPs), but in worldwide national administrative datasets the registration number of each marketed product is associated with the information useful for the application of the universally recognized codes ATC/DDD and ST. The combined code that we propose allows for the use of this or other future algorithms, enabling the interoperability among the different MP databases of different national health systems.
The algorithm provides a flexible tool to help operators identify potential substitutions in drug shortages, though additional region-specific adaptations or validations might be required, and can be managed by a computerized system. To consider the real value of the dataset used, it has to be considered that as of December 31, 2022, the population in Italy was 58,997,201 residents, of which more than 60% had received at least one prescription for drugs in the previous 12 months [
28]. In the same year, public pharmaceutical expenditure represented 68.9% of total pharmaceutical expenditure with a value of EUR 23.5 billion.
The concept of the therapeutic equivalence of a drug has consolidated over the years at a global level, certainly thanks to the work carried out by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), WHO, FDA, and EMA. The approach to the problem by the FDA was pioneering, with the “
Approved Drug Products With Therapeutic Equivalence Evaluations”, now commonly known as the
Orange Book, being published since October 1980 and currently on its 45th edition [
29].
The equivalence-related terms and definitions used in this paper are those reported in the introduction of the Orange Book. Pharmaceutical equivalents are drug products in identical dosage forms and route(s) of administration that contain the same amount of the same API; pharmaceutical alternatives are drug products that contain the identical therapeutic moiety, or its precursor, but not necessarily in the same quantity or dosage form, or the same derivative; approved MPs are considered to be therapeutic equivalents if they are pharmaceutical equivalents for which bioequivalence has been demonstrated, and they can be expected to have the same clinical effect and safety profile when administered to patients under the conditions specified in the label. The concept of therapeutic equivalence applies only to MPs containing the same API(s) and does not encompass a comparison of different therapeutic agents used for the same condition: in this paper, this last case is indicated as the therapeutic alternative MP.
The developed algorithm, given an unavailable MP, allows for the pharmaceutical equivalents present on the market to be found and listed by attributing to them a degree of substitutability (DS) of 100%, together with the found pharmaceutical alternatives, sorted in decreasing order of DS.
The algorithm works by identifying the MP through the code conceived by the Authors as a union of the ATC code [
30] with five of the STs proposed and managed by EDQM ver. 1.2.0-28 January 2019 [
26], as listed in
Table 1. The identification code is completed with the number of DDDs for the presentation unit (NDXUP), calculated as the number of DDDs referring to the single unit pharmaceutical dosage form (e.g., tablet, capsule) or referring to volume (mL) or weight (g) for liquids and solids in single and multiple dose forms (e.g., syrup, solution).
The EDQM ST code has been recognized as the leading system in pharmaceutical product description, initially drawn from the European Pharmacopoeia Commission for use in drug labelling, summary of product characteristics, and digital communication, as a result of the implementation of ISO 11239:2012 and ISO/TS 20440:2016 [
31]. Since 2017, the scope of the ST database has widened to allow for the inclusion of different aspects, like adverse event reporting and clinical trials. It can be used for many other purposes in digital communication or pharmaceutical data analysis, or when an accurate description of an MP pharmaceutical characteristics is necessary. The algorithm presented here uses the five main or traditional STs: basic or generalized dosage form or group of related pharmaceutical dosage forms (BDF); Administration Method (AME); Intended Site or the site at which a pharmaceutical product is intended to be administered (ISI); Release Characteristic (RCA); and Transformation or procedure that must be carried out to convert a manufactured dosage form to its administrable dosage form (TRN). Each ST is associated with a four-digit numeric code.
Table 2 shows an example of ST codification for some MPs containing risperidone, an atypical antipsychotic mainly used in schizophrenia and bipolar disorder.
Each code, like those reported in
Table 2, is preceded by the five levels of the ATC code (for risperidone, ATC = N05AX08). For example, for the first item in
Table 2, the code is reported in
Table 3.
When the algorithm is queried with the MA number of a lacking or unavailable MP, as a first step, it converts the characteristics of the pharmaceutical product in the above-described code, and uses it to search in the database for the pharmaceutical equivalents (with the same digital string) and the alternatives (same ATC, but with some differences in the ST or NDXUP part of the digital string), returning a list of MPs in descending DS order.
The DS score for pharmaceutical equivalence is set at 100, a value from which penalties are deducted in the case of alternatives with differences from the factor classes of the compared MPs, as calculated in Equation (1):
For the class NDXUP, the maximum deduction is set at 10 points. For the ST classes, the maximum deduction is set at 80 points.
In
Table 4, the criteria to attribute the penalty scores for any difference in the number of DDDs for the presentation unit (NDXUP) between the unavailable or lacking MP (lak) and its potential substitute MP (sub) are reported.
In identifying a pharmaceutical alternative, beyond some choices of score attribution that can be considered reasonable, even if arbitrary, it is preferred to give less weight to the difference in the dose contained in the pharmaceutical form compared to the other characteristics of the pharmaceutical form described by the STs. In fact, the maximum penalty of 10 is attributed to dosages of API which are very different from one of the lacking MPs (i.e., to dosages more than twice higher or less than twice lower).
For the ST class score, the lists in each ST are grouped together depending on the similarity of the characteristics or properties, attributing them a numerical value for each relative position (RP), as shown in
Table 1. The distance between the RPs varies from zero, in the case of exact correspondence of the MPlak and MPsub STs, to a maximum value that is a function of the ST scale, which is the whole range of the RPs for that specific ST. Every relative difference in absolute value is normalized to 100. For example, for BDF, the normalized relative distance (NRD) between tablet (lacking) and syrup (alternative) was calculated as in Equation (2):
Each contribution for ST is weighed with a different weight, as shown in
Table 5.
The difference in weight factors among the STs is necessary to offset the strong leverage effect of STs that contain fewer terms, such as RCA and TRN, and at the same time to attribute to BDF, AME and ISI a minimum advantage in the selection criteria for the choice of pharmaceutical alternative. The calculated weighed contribution for the previous example is 41.30 × 0.46 = 18.998. To calculate the score of the ST class, the weighed NRD contribution of each of the five STs is normalized to 80, the maximum score reserved for this class, as in Equation (3):
During the development and evaluation of the algorithm, the problem of the ambiguous codification of the ATC of combination products, or otherwise defined fixed combinations (FCs), was afforded. In the Guidelines for ATC classification and DDD assignment 2024 [
30], the FCs containing two or more APIs belonging to the same fourth level are normally classified using the fifth level codes 20 or 30; the FCs containing two or more APIs not belonging to the same fourth level are normally classified using the 50-series as the fifth level; and FC products containing psycholeptic drugs not classified as N05 or N06 are classified at separate fifth levels using the 70-series. It may be difficult to establish a rule for all FCs and it is not easy to decide how an FC should be classified. For example, an MP containing an analgesic and a tranquillizer used primarily to ease pain should be classified for its main therapeutic indication, i.e., as an analgesic; likewise, an FC of an analgesic and an antispasmodic drug will be classified in A03 (drug for functional gastrointestinal disorders). This algorithm, in order to run correctly, needs an unambiguous recognition of the APIs in FCs. Therefore, only as a proof of concept of the operation of the algorithm, for FCs with ambiguous codes, fictitious ATC codes are used, reporting as fourth level the one of the API with the main therapeutical effect and using for the fifth level a number in the range from 99 to 80 that has never been used before. Another criterion for the fourth level could also be to refer to the component present in a larger quantity, but, in this case, there is the possibility of losing therapeutic information. Some examples of this new attribution are reported in
Table 6, along with the original ATCs, to identify the FCs unambiguously. For example, in the case of a combination birth control pill (desogestrel and ethinylestradiol), the code G03AA09 is unambiguous, while for A03DB04, the ATC/WHO classification describes butylscopolamine and analgesics without indicating the analgesic drug, so for the FC of butylscopolamine and paracetamol the fictitious code A03DB95 is chosen.
In the case where some of these codes have already been used to describe an API molecule, for the fifth level, a letter of the English alphabet (26 characters) associated with a number chosen in the range from 0 to 9 could be used, thus providing 260 unique codes for FCs with the same ATC; by exchanging the position of the letter with the number, the possibility of univocal identification could double to 520. This notation at the fifth ATC level would also allow for the recognition of fixed combinations, because in the ATC code the fifth level is represented only by numbers. Anyway, this aspect should be addressed at the international level by the WHO Collaborating Centre for Drug Statistics Methodology [
32], with the considerable advantage of having an unambiguous code for each type of MP.
Another issue that arises is due to there being no definition of DDDs for FCs. To overcome this issue, the DDDs for FCs are calculated as the sum of the DDDs of the APIs (referring to the solid dosage unit, or volume or weight) in the FC. This allows for a specific DDD to be assigned to the FC, which enables the assignment of different scores, using Equation (4):
The probability to attribute the same NDXUP to two APIs in an FC is remote, because two FC MPs marketed with inverted dosage would need to exist, which seems to be unrealistic.
In
Table 7, the NDXUP sum of the DDDs of the two APIs in the FCs is reported.
Also, for electrolytic solutions, the same issue has arisen, so the use of a univocal fictitious ATC code that unambiguously identifies the unique qualitative–quantitative composition of the MP and attributes 1 as a formal value to the sum of NDXUP is conceived (
Table S2).
In
Table 8, as an example, the output of the Excel macro implementing the algorithm searching for a film tablet containing 2 mg of risperidone (Italian MA number 037599230), being lacking/unavailable on the Italian market is reported.
The Excel macro result returns 32 items as being potential MP substitutes. The first four are equivalent pharmaceutical products, having a DS score of 100. From item 5 to item 19 in ranking order, the macro finds alternative MPs differing only for dosage, but with identical STs: in this case, the score attributed by the algorithm favours (98%) MPs with half the required dosage (double intake), with respect to those with twice the content (96%; symmetrical division of the tablet) and those containing 3 mg (92%; only two-thirds of the entire tablet must be taken). In any case, the algorithm considers an MP with a DS score > 90% as a potential candidate for substitution. Scrolling the list of the outputs, it can be observed that the DS score decreases from oral tablets to oral solutions (still > 80%), and decreases even more to parenteral solutions (<60%).
The validation phase includes two different stages: an “internal validation”, conducted using the algorithm internal database, and an “external validation”, involving the recruitment of a “panel group” to evaluate the algorithm responses from a clinical perspective.
Based on the analysis of the outcomes of the internal validation phase, the algorithm provides reliable responses for all types of MPs. Only 13 of the 598 items used as the validation test set had no alternative, corresponding to 2%.
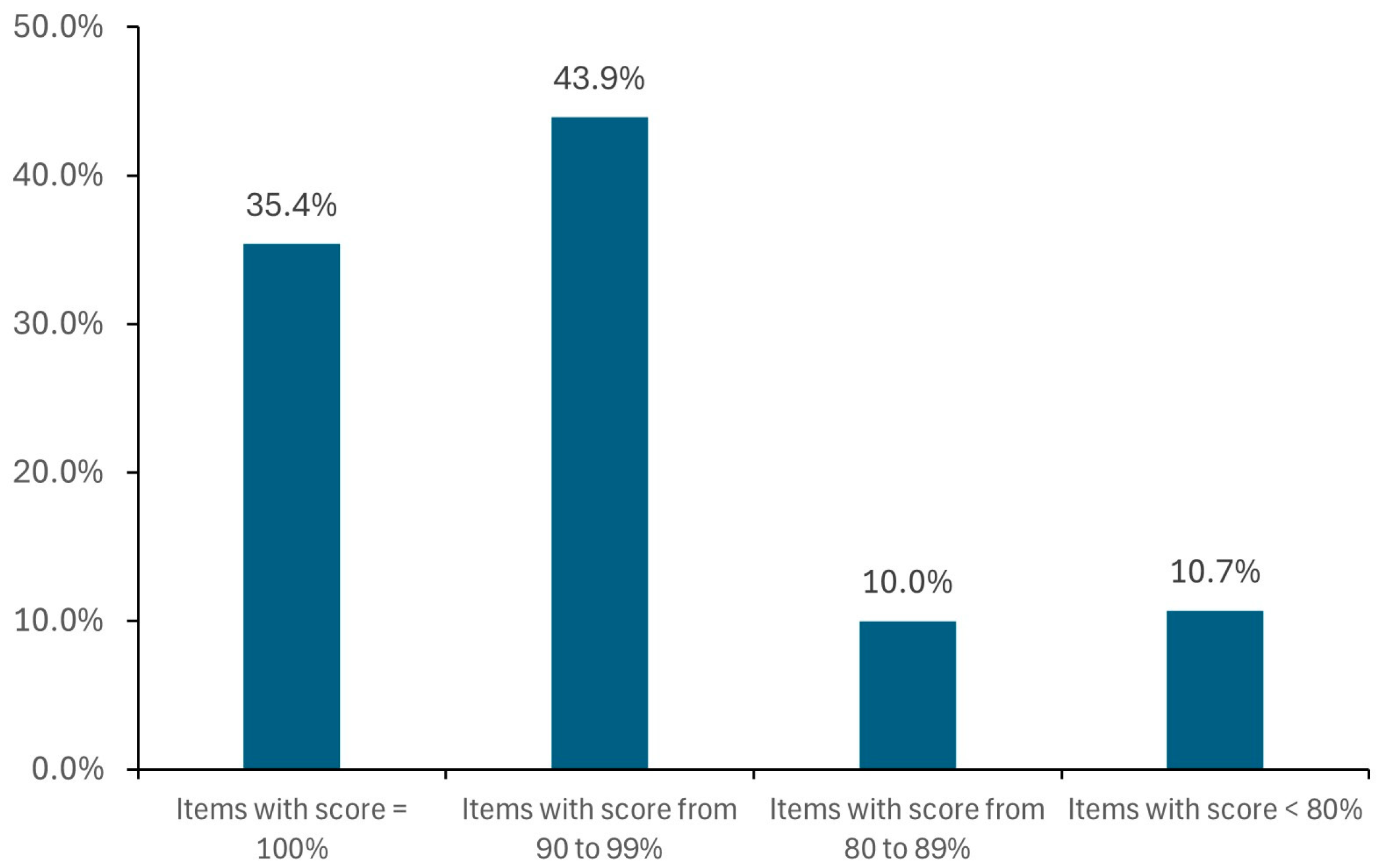
For each item, a different number of alternatives are found (from 1 to more than 100, e.g., for ibuprofen, pantoprazole, and paracetamol), in most cases with DS score ≥ 90% (
Figure 1).
The number of items examined during the test involving the randomized sample of 598 MPs authorized in Italy is noteworthy. Considering that the macro suggests a variable number of alternative items for each searched MP (from a few items to several tens of items), there is a different multiplication factor for each of the 598 searched MPs; thus, the evaluation is performed on a number of alternative items largely higher than 598.
Most of the alternatives (80%) have the same BDF as the reference item; only 15% of the reference items have alternatives differing for four or five STs (
Figure 2).
The analysis, performed separately on each ATC class, highlights the best performance for the ATC class of the cardiovascular system, which is the class with the highest % of drug consumption in Italy (
Figure 3).
In order to visualize, in one plot, the results of the internal validation, Principal Component Analysis (PCA) is performed, simultaneously considering the ten performance indicators and showing the ATC classes that behave similarly (
Figure 4). The analysis is limited to the seven ATC classes at the highest % of drug consumption (
Table S1) to evaluate the algorithm’s performance on an acceptable number of MPs.
The first two Principal Components explain 78.6% of the total variance in the data. The C (cardiovascular system), A (alimentary tract and metabolism), B (blood and blood-forming organs), and R (respiratory system) classes show the highest number of alternatives, with DS scores > 90%. For B and C, respectively, 99% and 98% of the alternative items have the same BDF of the reference drug; moreover, most of the reference items have alternatives that differ by a maximum of one ST (60% and 82%, respectively). The algorithm seems to underperform for class A, where 23 and 29% of the reference items have alternatives that differ by three STs and by five STs, respectively. This can be attributed to several drugs in the test set (e.g., pantoprazole, omeprazole) for which the list of alternatives includes both oral and parenteral dosage forms.
However, besides the demonstrated efficacy of this algorithm, the final substitution choice requires an in-depth clinical evaluation: it is up to the physician to decide whether a proposed alternative is clinically feasible, especially in the case where the missing and the proposed product present considerable differences in terms of dosage or formulation composition. We acknowledge that this algorithm can help with, but not replace, a healthcare operator’s professional experience. Therefore, an external validation is mandatory.
For external validation, the feedback document containing short-answer questions (YES/NO/OTHER) received from a panel of 18 professionals is shown in
Table 9, together with the evaluation results. A broader testing plan, including a higher number of healthcare practitioners belonging to a multi-regional area, is being outlined to confirm the significance of the results obtained from the preliminary external validation.
4. Conclusions
The developed algorithm tries to meet the needs of being a reliable, rational instrument to help health systems find adequate replacements for currently unavailable MPs. This tool can be considered a valuable support in the decision-making process of healthcare professionals.
The innovative aspect of this work lies in the application of the WHO ATC codes, of the DDD codes, and of the EDQM Standard Terms to codify MP identifiers usable by IT systems and to describe the APIs, the pharmaceutical characteristics of the dosage form, and the drug strength.
During validation, the algorithm proved to be able to find all the pharmaceutical equivalents of the indicated MP.
Some of the critical points found during algorithm development, such as the univocal description of FC products or electrolyte solutions and the comparison of dose strength between solid and liquid forms and mono- or multidose presentation, are tentatively addressed, though a standardization is needed at an international level.
The algorithm is structured to be flexible by being able to change either the values of arbitrary criteria or by choosing different weight factors or attributing different penalty scores. This feature allows for further adjustments to improve the algorithm performance. Studies to evaluate the robustness of the algorithm, considering sensitivity analyses or alternative scoring scenarios, are being planned.
In spite of using Italy-focused data, the use of universally recognized codes such as ATC/DDD and ST in the algorithm enables interoperability among different MP databases of different national health systems, provided that they include sufficiently detailed ATC/DDD and dosage form descriptions.
The preliminary validation, although limited in sample size, vouches for the algorithm clinical utility. An extension of the number of testing practitioners, even involving those belonging to different regulatory areas, is being planned.
The implementation of this algorithm in practical use requires overcoming some challenges, like its transfer to an informatic platform which is easily interfaceable or integrable with the most common IT health systems, its acceptance by clinicians and pharmacists, and its compliance with regulatory constraints.
This algorithm could also be used in different fields, for example, in Pharmacovigilance, Pharmacoutilization, Pharmacoepidemiology, and Pharmacoeconomics. Moreover, it can be employed in preventive risk analysis to highlight potential critical issues in an MP database of a national health system, highlighting the items that have no or few valid substitutes.
For large-scale implementation or iterative improvements, the collaboration with international standard-setting bodies, like WHO and EDQM, and the involvement of more stakeholders, will be necessary.