Efficacy and Safety of Combined Treatment with Traditional Herbal Medicine and Western Medicine for Children with Pertussis-like Syndrome: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.2.1. Types of Studies
2.2.2. Types of Participants
2.2.3. Types of Interventions
2.2.4. Types of Comparisons
2.2.5. Types of Outcome Measurements
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Extraction
2.4.1. Study Selection
2.4.2. Data Extraction
2.5. Statistical Analysis
2.5.1. Assessment of Heterogeneity
2.5.2. Assessment of Reporting Bias
2.5.3. Subgroup and Sensitivity Analysis
2.6. Quality Assessment
3. Results
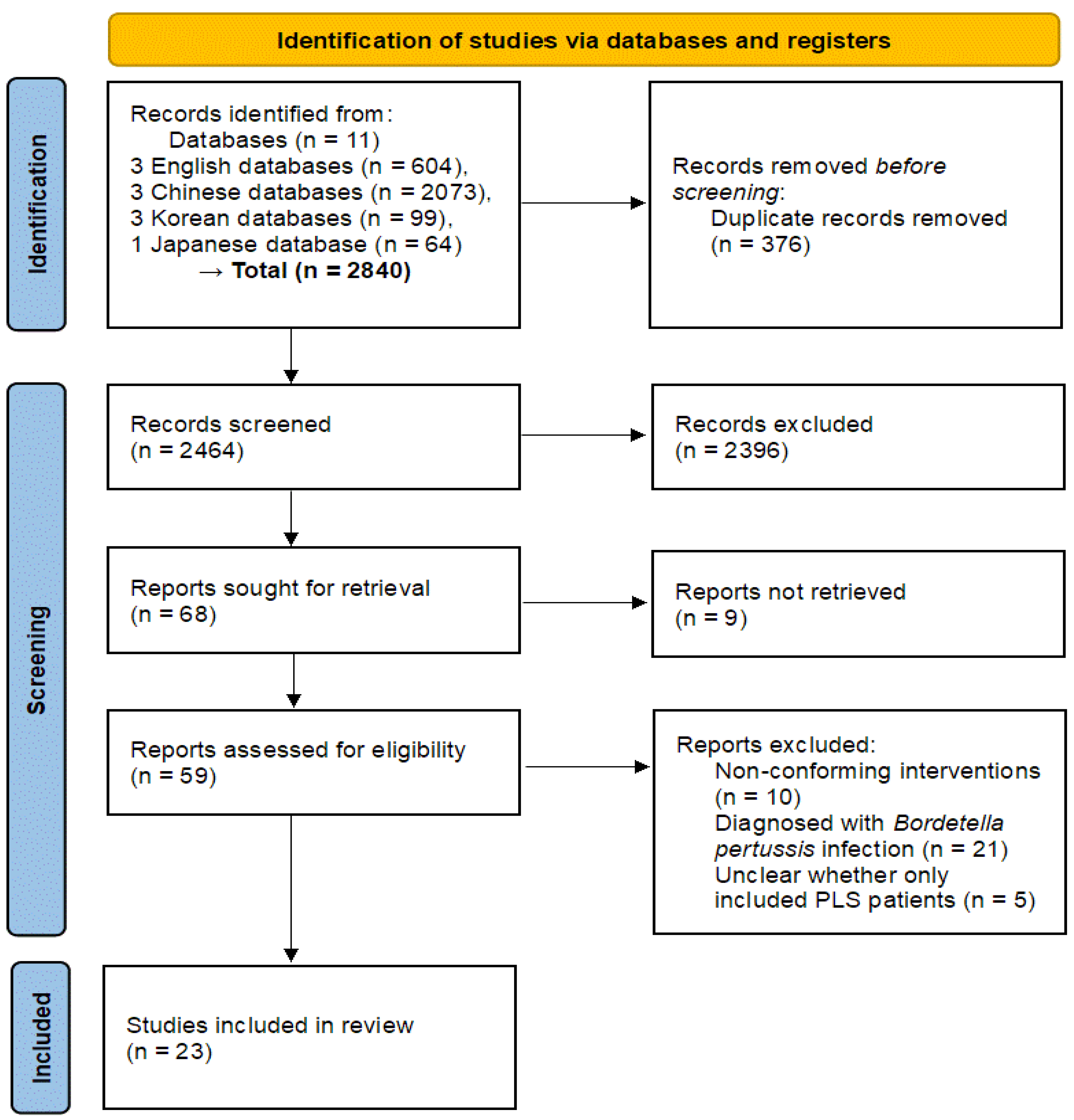
3.1. Study Selection
3.2. The Characteristics of the Study
3.3. Interventions
3.4. Outcome Measures
3.5. Meta-Analysis
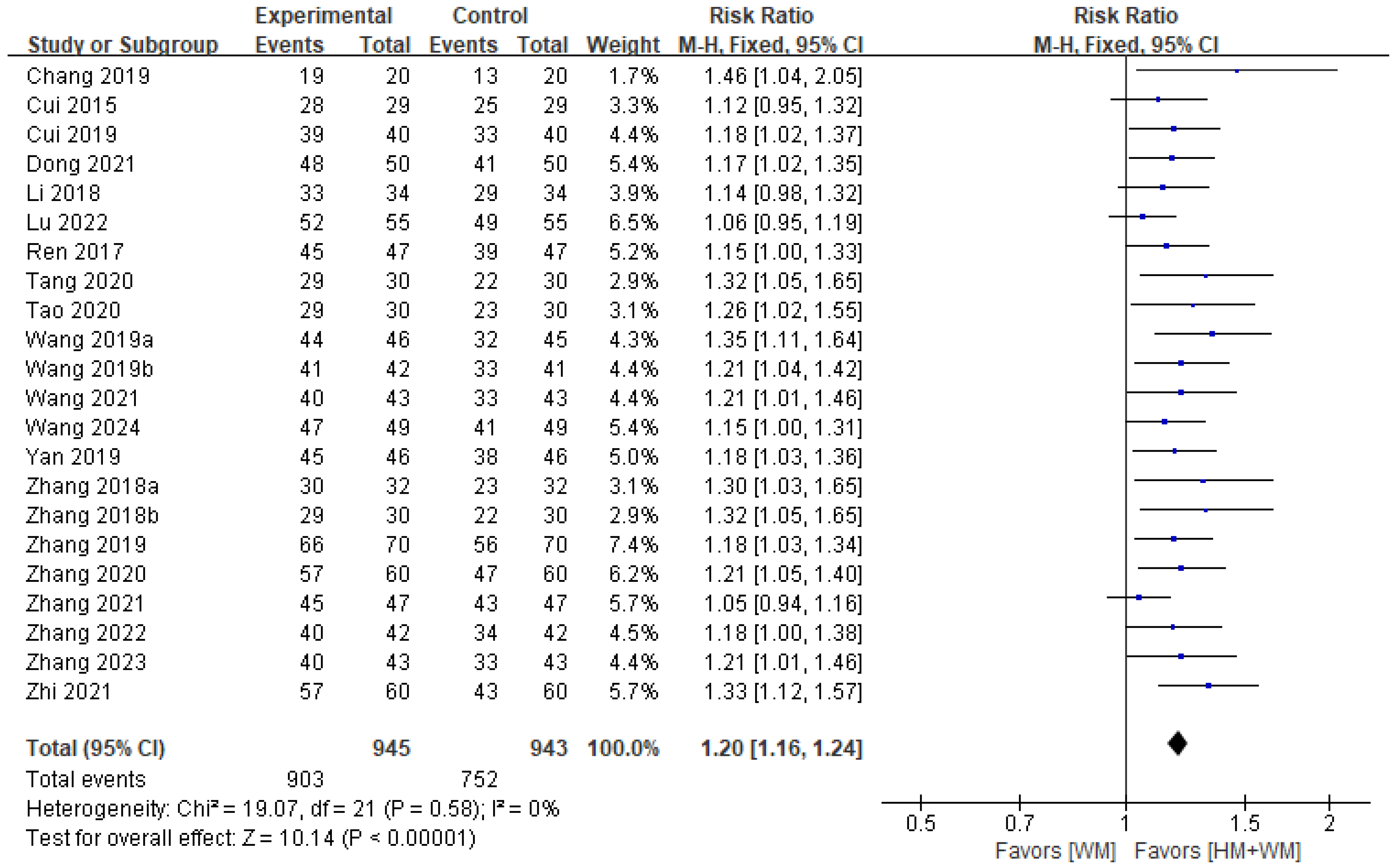
3.5.1. TER
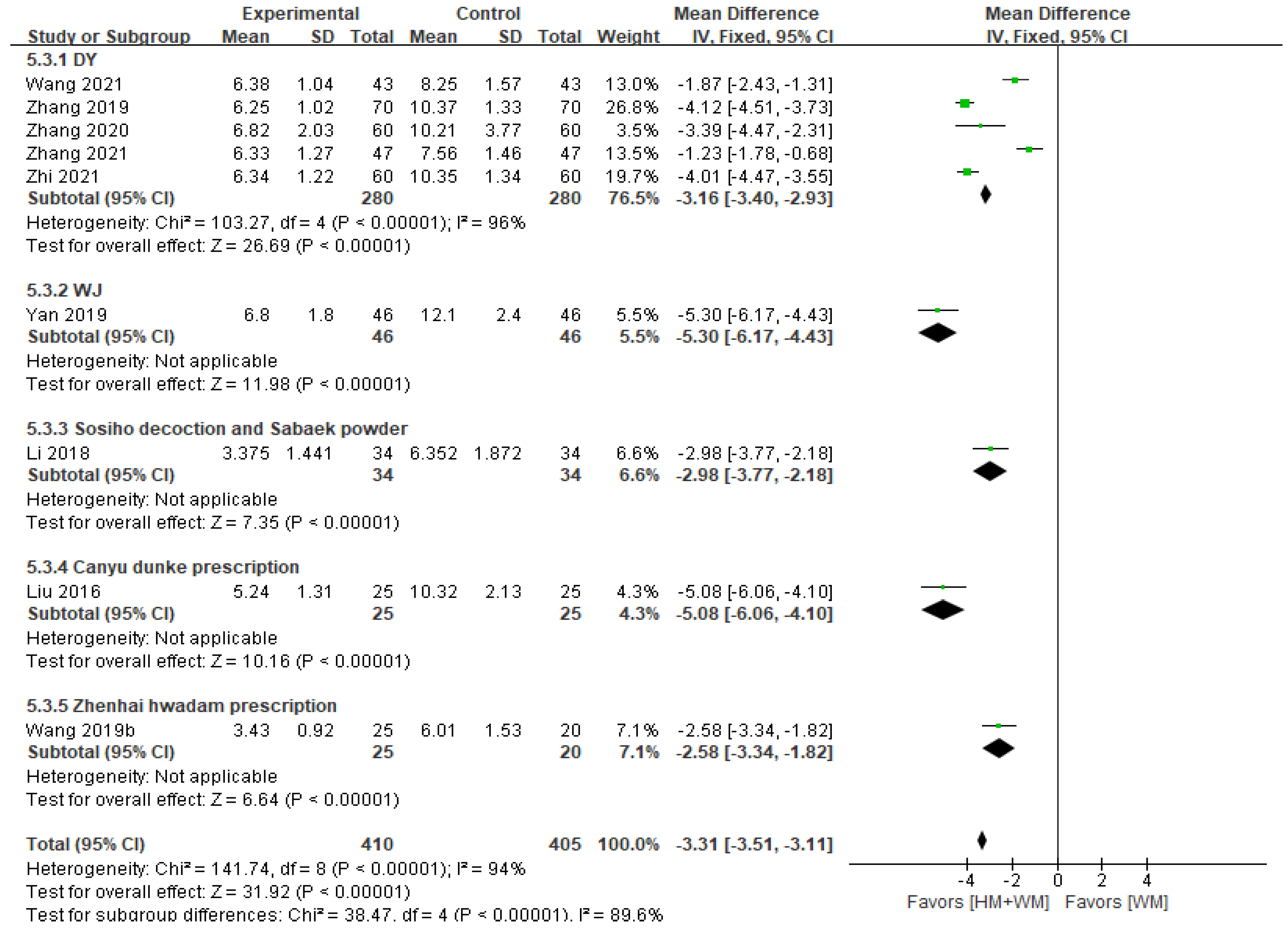
3.5.2. Disappearance Time of Main Symptoms (Spastic Cough)
3.5.3. Recovery Time of Blood Routine Parameters to Normal Range
3.5.4. Hospitalization Time
3.6. Adverse Events
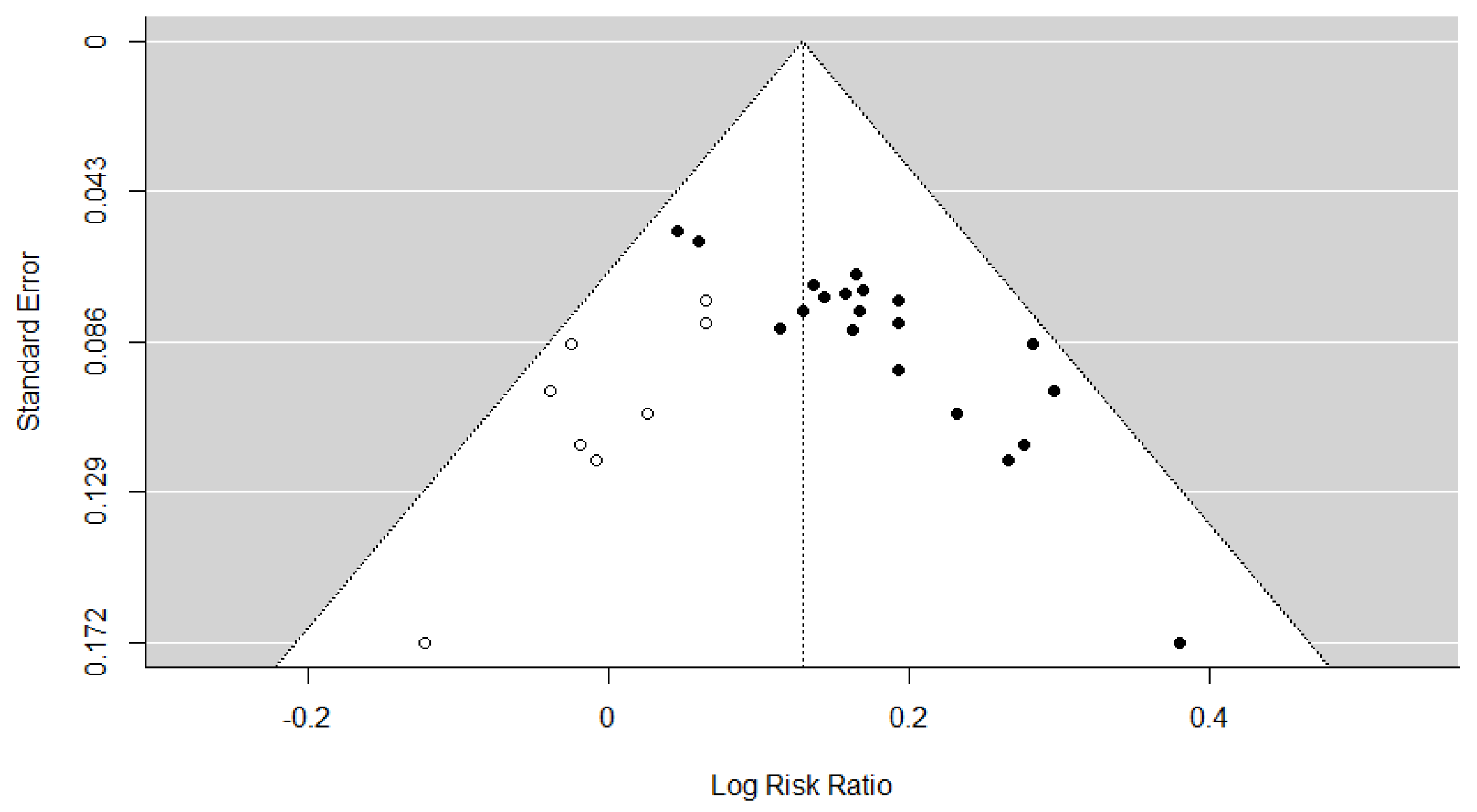
3.7. Assessment of Reporting Bias
3.8. Subgroup and Sensitivity Analyses
3.9. Quality Assessment
3.10. GRADE Certainty of Evidence
4. Discussion
4.1. Summary of Findings
4.2. Clinical Implications
4.3. Limitations and Suggestions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| DY | Dengtai ye granule |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| HM | herbal medicine |
| MD | mean difference |
| PLS | pertussis-like syndrome |
| RCTs | randomized clinical trials |
| ROB | risk of bias |
| RR | risk ratio |
| SB | Sangbaipi decoction |
| SMD | standardized mean difference |
| TER | total effective rate |
| WJ | Weijing decoction |
| WM | Western medicine |
References
- Nguyen, V.T.N.; Simon, L. Pertussis: The whooping cough. Prim. Care 2018, 45, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.M.; Smith, E.A.; Zavala, A. Pertussis: Common questions and answers. Am. Fam. Physician 2021, 104, 186–192. [Google Scholar] [PubMed]
- World Health Organization. Pertussis Reported Cases and Incidence. Available online: https://immunizationdata.who.int/global/wiise-detail-page/pertussis-reported-cases-and-incidence (accessed on 24 April 2025).
- Centers for Disease Control and Prevention (CDC). Pertussis Surveillance and Trends. Available online: https://www.cdc.gov/pertussis/php/surveillance/index.html (accessed on 24 April 2025).
- Dportal of Korea Disease Control and Prevention Agency (KDCA). Infectious Disease Reporting. Available online: https://dportal.kdca.go.kr/pot/is/summaryEDW.do (accessed on 24 April 2025).
- National Disease Control and Prevention Administration (NDCPA). Epidemic Information. Available online: https://www.ndcpa.gov.cn/jbkzzx/c100016/second/list.html (accessed on 24 April 2025).
- Gu, W.; Wang, K.; Zhang, X.; Hao, C.; Lu, Y.; Wu, M.; Chen, S.; He, Y.; Xu, J.; Shao, X.; et al. Pathogen analysis of pertussis-like syndrome in children. BMC Infect. Dis. 2020, 20, 353. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, W.; Meng, Q.; Yuan, L.; Gao, W.; Wang, L.; Yao, K. Detection of Bordetella spp. in children with pertussis-like illness from 2018 to 2024 in China. J. Infect. 2024, 89, 106222. [Google Scholar] [CrossRef]
- Saiki-Macedo, S.; Valverde-Ezeta, J.; Cornejo-Tapia, A.; Castillo, M.E.; Petrozzi-Helasvuo, V.; Aguilar-Luis, M.A.; Del Valle, L.J.; Cieza-Mora, E.; Bada, C.; Del Aguila, O.; et al. Identification of viral and bacterial etiologic agents of the pertussis-like syndrome in children under 5 years old hospitalized. BMC Infect. Dis. 2019, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Halperin, S.A.; Boucher, F.D.; Smith, B. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC). Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics 2004, 114, e96–e101. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Altunaiji, S.; Kukuruzovic, R.; Curtis, N.; Massie, J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst. Rev. 2007, 3, CD004404. [Google Scholar] [CrossRef]
- Shergis, J.L.; Wu, L.; Zhang, A.L.; Guo, X.; Lu, C.; Xue, C.C. Herbal medicine for adults with asthma: A systematic review. J. Asthma 2016, 53, 650–659. [Google Scholar] [CrossRef]
- Lee, B.; Kwon, C.Y.; Suh, H.W.; Kim, Y.J.; Kim, K.I.; Lee, B.J.; Lee, J.H. Herbal medicine for the treatment of chronic cough: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1230604. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Rennie, D.; Meade, M.; Cook, D. Users’ Guides to the Medical Literature: Essentials of Evidence Based Clinical Practice, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLBI). Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 January 2025).
- Mai, Y.C.; Lin, Q. Analysis of the curative effect of modified Sangbaipi decoction on pertussis-like syndrome in children. Chin. Pediatr. Integr. Tradit. West Med. 2021, 4, 362–365. [Google Scholar]
- Cui, J.; Wang, X.X. Clinical observation of modified Sangbai Pi decoction in treating pediatric pertussis syndrome in 29 cases. Chin. J. Ethnomed. Ethnopharm. 2015, 24, 98–99. [Google Scholar]
- Cui, Y.C. Clinical analysis of the effectiveness of Sangbai Pi decoction in treating pediatric pertussis syndrome. Pract. Clin. J. Integr. Tradit. Chin. West Med. 2019, 19, 97–98. [Google Scholar]
- Dong, C.H. Exploring the clinical efficacy of modified Sangbai Pi decoction in treating pediatric pertussis syndrome. Diabetes World 2021, 18, 32. [Google Scholar]
- Li, Q.Q.; Ge, G.L.; Han, X. Clinical observation of Xiao Chai Hu decoction combined with Xie Bai San in treating pediatric pertussis syndrome. Guangming J. Chin. Med. 2018, 20, 3027–3028. [Google Scholar]
- Liu, Y.H.; Xiang, H.J.; Fan, S.H. 25 cases of pediatric pertussis syndrome treated with a combination of Gan Yu Dun cough formulas and conventional Western medicine. Tradit. Chin. Med. Res. 2016, 29, 22–24. [Google Scholar]
- Lu, F.; Li, A.M.; Xu, S.S. The efficacy of modified reed stem decoction combined with Western medicine in treating whooping cough syndrome in children and its impact on moisture respiration lung function. Sichuan Tradit. Chin. Med. 2022, 40, 79–82. [Google Scholar]
- Ren, R.Y. Clinical experience with modified Sangbai Pi decoction in treating patients with pertussis-cough stage pertussis. Women’s Health Res 2017, 10, 52–53. [Google Scholar]
- Tang, Q.Q.; Li, L.F.; Guan, L.F.; Guan, M.C.; Hang, J.G. Observation of the effectiveness of Lvguo pills in auxiliary treatment for pediatric pertussis syndrome. Chin. J. Rural. Med. Pharm. 2020, 17, 17–18. [Google Scholar]
- Tao, M.M. Observation of clinical efficacy in treating whooping cough syndrome (phlegm-heat gel block syndrome). Anhui Univ. Tradit. Chin. Med. 2020, 10369, 1–57. [Google Scholar]
- Wang, X.S. Clinical observation of combined traditional Chinese and Western medicine in treating whooping cough syndrome. Pract. J. Tradit. Chin. Med. 2019, 35, 1090–1091. [Google Scholar]
- Wang, S.Y. Observation of the effectiveness of a self-drafted cough suppression and phlegm transformation formula in whoop ing cough syndrome. Guangxi Tradit. Chin. Med. 2019, 42, 15–17. [Google Scholar]
- Wang, F.; Jin, X.H.; Zhang, Y.H. Clinical observation of lampshade leaf granules combined with cycloerythromycin in treating pediatric pertussis syndrome. Pract. J. Tradit. Chin. Med. 2021, 37, 227–228. [Google Scholar]
- Wang, H.; Zhang, T.T.; Duan, C.C.; Zhen, L.; Zhang, Z.P. Clinical study of Baikening granules combined with budesonide in treatment of pertussis syndrome in children. Drugs Clin. 2024, 39, 382–386. [Google Scholar]
- Yan, W.; Song, J.P.; Zhao, C.J. Clinical observation on 46 cases of pediatric pertussis syndrome with stage of spasmodic cough treated by modified Weijing Tang combined with erythromycin. J. Pediatr. Tradit. Chin. Med. 2019, 15, 52–55. [Google Scholar]
- Zhang, X.F. Analysis of the clinical characteristics and treatment of children with whooping cough syndrome. China Contin. Med. Educ. 2018, 10, 91–93. [Google Scholar]
- Zhang, B.F. Clinical observation of 60 cases of pertussis syndrome in children treated with mulberry pica. Contemp. Med. 2018, 24, 25–27. [Google Scholar]
- Zhang, X.F.; Jin, X.H.; Lu, F.X.; Li, H.J.; Zhang, Z.Y.; Feng, Y.; Deng, P.P.; Gao, X.H.; Yang, J.L. 70 children with pertussis treated with lampstand leaf granules in combination with Western medicine. Res. Integr. Tradit. Chin. West. Med. 2019, 4, 213–214. [Google Scholar]
- Zhang, Z.Y.; Jin, X.H.; Zhang, X.N.; Zhang, X.F. Clinical study of Dengtaiye Granules combined with cycloester erythromycin in treatment of pertussis-like syndrome. Drugs Clin. 2020, 12, 2365–2369. [Google Scholar]
- Zhang, X.F.; Lu, F.X.; Li, H.J.; Zhang, Z.Y.; Deng, P.P.; Gao, X.H.; Jin, X.H. Clinical study on treatment of pertussis-like synd rome with Dengtaiye Granules. Res. Integr. Tradit. Chin. West. Med. 2021, 1, 8–10. [Google Scholar]
- Zhang, H.G.; Zhu, S.; Zhu, Z.P.; Wang, X.L.; Tian, X.L.; Zhao, W.J. Effects of Jiawei Weijing Decoction on tidal breathing lung function and inflammatory factors in child patients with pertussis syndrome. Pharmacol. Clin. Chin. Mater. Med. 2022, 2, 199–202. [Google Scholar]
- Zhang, X.F.; Yu, Y.J.; Zhang, X.N. Effect of Xiao’er Feike Granules combined with erythromycin cyclocarbonate on pulmonary function status and cellular immunity in children with pertussis-like syndrome. Chin. Foreign Med. Res. 2023, 9, 1–4. [Google Scholar]
- Zhi, Y.L. Clinical observation on Dengtaiye Granule combined with Western medicine in the treatment of 60 children with pertussis-like syndrome. Chin. J. Ethnomed. Ethnopharm. 2021, 7, 91–93. [Google Scholar]
- Liu, Y.; Ai, T.; Fan, Y.; Xie, C.; Lou, R.; Zeng, X.; Wang, L.; Peng, Y.; Chen, M. Etiological distribution of pertussis-like syndro me in 756 children in Chengdu. Transl. Pediatr. 2021, 10, 984–989. [Google Scholar] [CrossRef]
- Wang, K.; Bettiol, S.; Thompson, M.J.; Roberts, N.W.; Perera, R.; Heneghan, C.J.; Harnden, A. Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst. Rev. 2014, 9, CD003257. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, S.; Cherry, J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 2005, 18, 326–382. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Calicó, I.; Manresa, J.M.; Andreu, A.; Moraga, F.; Valle, I. Microorganisms isolated in cases of pertussis-like synd rome. Enferm. Infecc. Microbiol. Clin. 2000, 18, 433–438. [Google Scholar]
- Mahmoudi, S.; Banar, M.; Pourakbari, B.; Alavi, H.S.; Eshaghi, H.; Ahari, A.A.; Mamishi, S. Identification of etiologic agents of the pertussis-like syndrome in children by real-time PCR method. Prague Med. Rep. 2018, 119, 61–69. [Google Scholar] [CrossRef]
- Amsden, G.W. Erythromycin, clarithromycin, and azithromycin: Are the differences real? Clin. Ther. 1996, 18, 56–72. [Google Scholar] [CrossRef]
- Carter, B.L.; Woodhead, J.C.; Cole, K.J.; Milavetz, G. Gastrointestinal side effects with erythromycin preparations. Drug Intell. Clin. Pharm. 1987, 21, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y. Safety of azithromycin in pediatric infectious diseases: A clinical systematic review and meta-analysis. Transl. Pediatr. 2021, 10, 2594–2601. [Google Scholar] [CrossRef]
- Hansen, M.P.; Scott, A.M.; McCullough, A.; Thorning, S.; Aronson, J.K.; Beller, E.M.; Glasziou, P.P.; Hoffmann, T.C.; Clark, J.; Del Mar, C.B. Adverse events in people taking macrolide antibiotics versus placebo for any indication. Cochrane Database Syst. Rev. 2019, 1, CD011825. [Google Scholar] [CrossRef]
- Hon, K.L.E.; Leung, A.K.C. Medications and Recent Patents for Status Asthmaticus in Children. Recent. Pat. Inflamm. Allergy Drug Discov. 2017, 11, 12–21. [Google Scholar] [PubMed]
- Aryani, T.; Rahmawat, R.K.; Cintyadewi, N.P.; Puspitasari, A.D.; Rasyid, A.N.; Samirah. Patterns of bronchodilator therapy in asthmatic outpatients. J. Public. Health Afr. 2023, 14, 2533. [Google Scholar] [CrossRef]
- Koziol-White, C.; Johnstone, T.B.; Corpuz, M.L.; Cao, G.; Orfanos, S.; Parikh, V.; Deeney, B.; Tliba, O.; Ostrom, R.S.; Dainty, I.; et al. Budesonide enhances agonist-induced bronchodilation in human small airways by increasing cAMP production in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L345–L355. [Google Scholar] [CrossRef]
- Akhtar, A.; Abbas, S.A.; Zaidi, S.H.M.; Sohail, A.; Alam, M.I.; Raza, L. The Acute Effects of the Use of Salbutamol and Ipratropium on the Heart Rates of Patients With Obstructive Airway Disease. Cureus 2023, 15, e46409. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, M.; Seo, M.S.; Shin, J.Y. Risk of neuropsychiatric adverse events associated with montelukast use in children and adolescents: A population-based case-crossover study. BMJ Paediatr. Open 2024, 8, e002483. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.B. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Adv. Pediatr. 2006, 53, 101–110. [Google Scholar] [CrossRef]
- Shi, T.; Lin, J.; Liang, S.; Song, Y.; Zhao, X.; Xiao, M.; Ti, H. Sangbaipi decoction exerted in vitro and in vivo anti-influenza effect through inhibiting viral proteins. J. Ethnopharmacol. 2024, 331, 118258. [Google Scholar] [CrossRef]
- Seo, C.S.; Lim, H.S.; Jeong, S.J.; Ha, H.; Shin, H.K. HPLC-PDA analysis and anti-inflammatory effects of Mori Cortex Radicis. Nat. Prod. Commun. 2013, 8, 1443–1446. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J.; Jeong, S.J.; Lee, H.J.; Kim, S.H.; Park, E.J. Cortex Mori Radicis extract exerts antiasthmatic effects via enhancement of CD4+CD25+Foxp3+ regulatory T cells and inhibition of Th2 cytokines in a mouse asthma model. J. Ethnopharmacol. 2011, 138, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shergis, J.; Chen, X.; Yu, X.; Guo, X.; Zhang, A.L.; Lu, C.; Xue, C.C. Chinese herbal medicine (Weijing Decoction) combined with pharmacotherapy for the treatment of acute exacerbations of chronic obstructive pulmonary disease. Evid. Based Complement. Alternat Med. 2014, 2014, 257012. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Han, Z.X.; Cheng, X.; Zhang, F.L.; Zhang, J.Y.; Su, Z.J.; Li, B.P.; Jiang, Z.R.; Li, R.Z.; Xie, Y.; et al. Combinational study with network pharmacology, molecular docking, and preliminary experiments on exploring common mechanisms underlying the effects of Weijing Decoction on various pulmonary diseases. Heliyon 2023, 9, e15631. [Google Scholar] [CrossRef]
- Rao, K.S.; Sun, Y.; Hui, T.T.; Gong, Y.Q.; Zhu, L.P.; Guo, W. Research on the HPLC method for determining alkaline content induck feet leaf granules. J. Yunnan Univ. Tradit. Chin. Med. 2008, 3, 9–12. [Google Scholar]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2016, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Wang, G.F.; Xu, D.; Zhang, H.Y.; Cui, Y.L.; Wang, Q.S. Glycyrrhizin-mediated liver-targeted alginate nanogels delivers quercetin to relieve acute liver failure. Int. J. Biol. Macromol. 2021, 168, 93–104. [Google Scholar] [CrossRef]
- Li, F.; Liu, B.; Li, T.; Wu, Q.; Xu, Z.; Gu, Y.; Li, W.; Wang, P.; Ma, T.; Lei, H. Review of Constituents and Biological Activities of Triterpene Saponins from Glycyrrhizae radix et Rhizoma and Its Solubilization Characteristics. Molecules 2020, 25, 3904. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Wang, S.; Xiang, Z.; Li, X.; Wang, Q.; Dong, W.; Gao, P.; Dai, L. Phytochemistry and pharmacology of Armeniacae semen Amarum: A review. J. Ethnopharmacol. 2023, 8, 116265. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Kui, F.; Gao, F.; Niu, Y.; Li, W.; Zhang, Y.; Guo, Z.; Du, G. The mechanism and active compounds of Semen Armeniacae Amarum treating coronavirus disease 2019 based on network pharmacology and molecular docking. Food Nutr. Res. 2021, 65, 5623. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Zhang, B. Amygdalin Attenuates Airway Epithelium Apoptosis, Inflammation, and Epithelial-Mesenchymal Transition through Restraining the TLR4/NF-κB Signaling Pathway on LPS-Treated BEAS-2B Bronchial Epithelial Cells. Int. Arch. Allergy Immunol. 2021, 182, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Zhang, X.X.; Shen, P.; Huang, C.S.; Deng, H.F.; Zhou, L.; Yue, L.X.; Shen, B.Y.; Zhou, W.; Gao, Y. Pinelliae rhizoma alleviated acute lung injury induced by lipopolysaccharide via suppressing endoplasmic reticulum stress-mediated NLRP3 inflammasome. Front. Pharmacol. 2022, 13, 883865. [Google Scholar] [CrossRef] [PubMed]



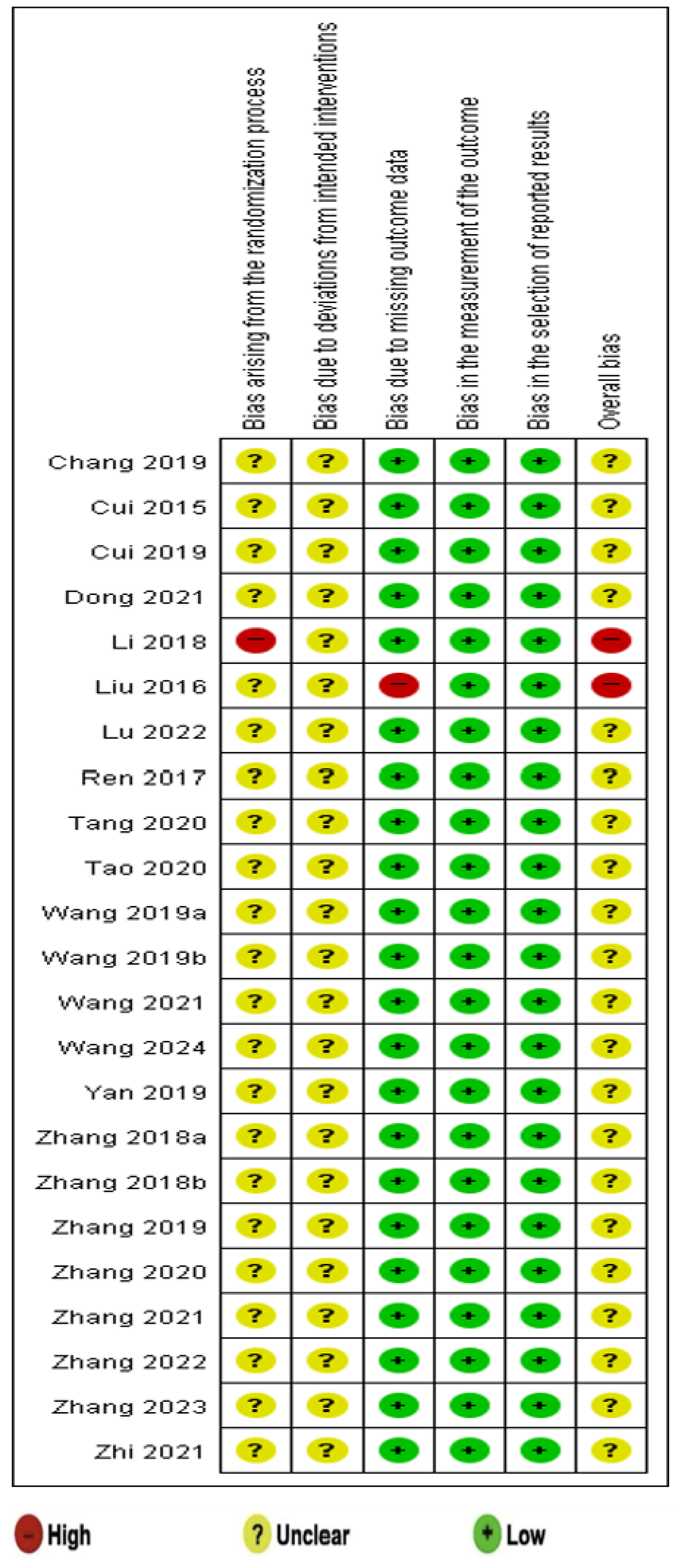
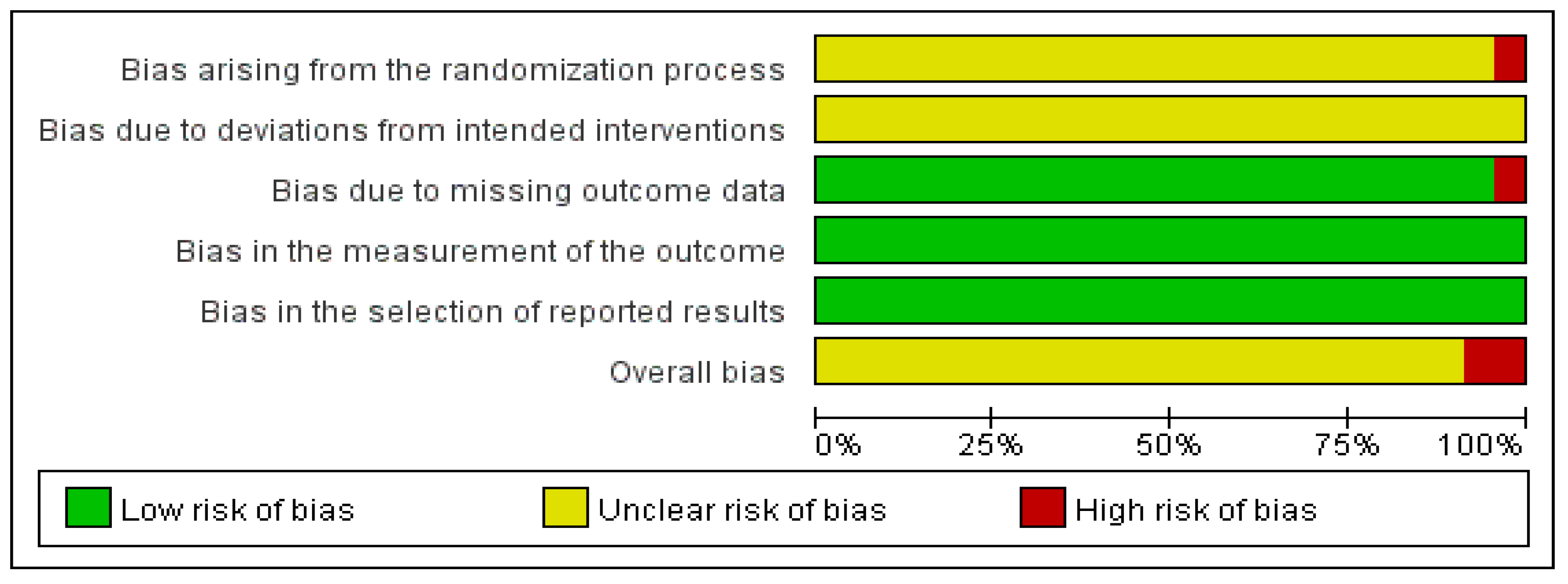
| First Author (Year) | Study Location | Sample Size (E/C) | Gender (M/F) | Age Distribution (Mean ± SD) | Duration of Disease (Mean ± SD) | Experimental Intervention (E) | Total Treatment Periods | Outcome Measurement |
|---|---|---|---|---|---|---|---|---|
| Control Intervention (C) | ||||||||
| Chang (2019) [18] | China | 40 (20/20) | E: 20 (12/8) C: 20 (11/9) | E: (3.42 ± 0.53) yr C: (3.35 ± 0.49) yr | E: (15.24 ± 2.61) d C: (15.18 ± 2.56) d | (C) + HM | 10–12 d | (1)(2)(3)(4)(5) (6) |
| Azithromycin | ||||||||
| Cui (2015) [19] | China | 58 (29/29) | E: 29 (16/13) C: 29 (15/14) | E: (2.3 ± 1.8) yr C: (2.6 ± 1.8) yr | (8.75± 2.1) d | (C) + HM | 2 wk | (1)(4)(5)(6) |
| Erythromycin | ||||||||
| Cui (2019) [20] | China | 80 (40/40) | E: 40 (23/17) C: 40 (21/19) | E: (3.5 ± 0.6) yr C: (3.7 ± 0.3) yr | E: (15.2 ± 2.6) d C: (15.6 ± 2.5) d | (C) + HM | 2 wk | (1)(2)(4)(6) |
| Erythromycin | ||||||||
| Dong (2021) [21] | China | 100 (50/50) | E: 50 (28/22) C: 50 (30/20) | E: (3.0 ± 1.8) yr C: (2.9 ± 1.66) yr | NR | (C) + HM | NR | (1)(5)(6) |
| Erythromycin | ||||||||
| Li (2018) [22] | China | 68 (34/34) | NR | NR | NR | (C) + HM | NR | (1)(4)(6) |
| Erythromycin + CT | ||||||||
| Liu (2016) [23] | China | 50 (25/25) | E: 25 (15/10) C: 25 (13/12) | E: (5.32 ± 1.68) yr C: (5.78 ± 1.21) yr | E: (10.8± 4.6) d C: (11.3± 5.3) d | (C) + HM | 2 wk | (2)(4)(7) |
| CT | ||||||||
| Lu (2022) [24] | China | 110 (55/55) | E: 55 (28/27) C: 55 (30/25) | E: (2.46 ± 0.71) yr C: (2.23 ± 0.65) yr | E: (14.27 ± 1.21) d C: (14.23 ± 1.13) d | (C) + HM | 2 wk | (1)(2)(3)(8) |
| Erythromycin + Sodium Ascorbate | ||||||||
| Ren (2017) [25] | China | 94 (47/47) | E: 47 (27/20) C: 47 (28/19) | E: (2.5 ± 1.2) yr C: (2.6 ± 1.3) yr | E: (8.9 ± 1.8) d C: (8.8 ± 1.9) d | (C) + HM | 3 d | (1)(4)(5)(6)(8) |
| Erythromycin | ||||||||
| Tang (2020) [26] | China | 60 (30/30) | E: 30 (16/14) C: 30 (17/13) | E: (2.4 ± 0.8) yr C: (2.5 ± 0.7) yr | E: (5.2 ± 1.7) d C: (5.3 ± 1.8) d | (C) + HM | 2 wk | (1) |
| Erythromycin + Budesonide, Ipratropium Bromide, Albuterol Sulfate Solution | ||||||||
| Tao (2020) [27] | China | 60 (30/30) | E: 30 (18/12) C: 30 (19/11) | NR | NR | (C) + HM | 2 wk | (1)(2)(8)(9) |
| Azithromycin | ||||||||
| Wang (2019a) [28] | China | 91 (46/45) | E: 46 (25/21) C: 45 (23/22) | E: (1.55 ± 0.58) yr C: (1.63 ± 0.54) yr | E: (19.28 ± 2.79) d C: (20.16 ± 2.33) d | (C) + HM | 2 wk | (1)(4) |
| Erythromycin | ||||||||
| Wang (2019b) [29] | China | 83 (41/42) | E: 41 (22/19) C: 42 (21/21) | E: (10.01 ± 1.39) m C: (9.89 ± 1.53) m | E: (33.69 ± 5.13) d C: (35.01 ± 6.03) d | (C) + HM | 2 wk | (1)(4)(6)(8) |
| Erythromycin | ||||||||
| Wang (2021) [30] | China | 86 (43/43) | E: 43 (28/15) C: 43 (26/17) | E: (1.55 ± 0.58) yr C: (1.63 ± 0.54) yr | E: (35.69 ± 1.25) d C: (35.71 ± 6.03) d | (C) + HM | 2 wk | (1)(4) |
| Erythromycin + CT | ||||||||
| Wang (2024) [31] | China | 98 (49/49) | E: 49 (28/15) C: 49 (26/17) | E: (3.14 ± 0.89) yr C: (3.22 ± 0.81) yr | E: (10.36 ± 3.18) d C: (10.18 ± 2.42) d | (C) + HM | 10 d | (1)(2)(3) |
| Budesonide | ||||||||
| Yan (2019) [32] | China | 92 (46/46) | E: 46 (30/16) C: 46 (29/17) | E: (2.47 ± 0.53) yr C: (2.56 ± 0.46) yr | E: (12.50 ± 2.81) d C: (12.91 ± 2.30) d | (C) + HM | 2 wk | (1)(4) |
| Erythromycin | ||||||||
| Zhang (2018a) [33] | China | 64 (32/32) | E: 32 (18/14) C: 32 (19/13) | NR | NR | (C) + HM | 10 d | (1) |
| Roxithromycin | ||||||||
| Zhang (2018b) [34] | China | 60 (30/30) | E: 30 (15/15) C: 30 (16/14) | E: (3.4 ± 0.8) yr C: (3.6 ± 0.7) yr | E: (15.5 ± 2.5) d C: (15.0 ± 2.7) d | (C) + HM | 2 wk | (1)(4)(5)(6) |
| Erythromycin | ||||||||
| Zhang (2019) [35] | China | 140 (70/70) | E: 70 (39/31) C: 70 (37/33) | E: (5.62 ± 1.30) yr C: (5.59 ± 1.29) yr | NR | (C) + HM | 2 wk | (1)(4) |
| Erythromycin | ||||||||
| Zhang (2020) [36] | China | 120 (60/60) | E: 60 (35/25) C: 60 (32/28) | E: (6.26 ± 1.33) yr C: (6.09 ± 1.42) yr | E: (14.31 ± 3.42) d C: (14.70 ± 3.05) d | (C) + HM | 2 wk | (1)(2)(4)(6)(10) |
| Erythromycin + CT | ||||||||
| Zhang (2021) [37] | China | 94 (47/47) | E: 47 (21/26) C: 47 (23/24) | E: (3.33 ± 1.39) yr C: (3.28 ± 1.42) yr | E: (13.24 ± 1.91) d C: (13.45 ± 1.86) d | (C) + HM | 2 wk | (1)(4)(10) |
| Erythromycin + Feilike HeJi, Montelukast | ||||||||
| Zhang (2022) [38] | China | 84 (42/42) | E: 42 (25/17) C: 42 (23/19) | E: (4.06 ± 1.32) yr C: (4.37 ± 1.27) yr | E: (13.19 ± 2.91) d C: (13.52 ± 2.86) d | (C) + HM | 2 wk | (1)(2)(3)(8) |
| Erythromycin | ||||||||
| Zhang (2023) [39] | China | 86 (43/43) | E: 43 (22/21) C: 43 (23/20) | E: (1.47 ± 0.33) yr C: (1.43 ± 0.35)yr | E: (15.48 ± 3.22) d C: (15.84 ± 3.38) d | (C) + HM | 2 wk | (1)(3)(10) |
| Erythromycin | ||||||||
| Zhi (2021) [40] | China | 120 (60/60) | E: 60 (31/29) C: 60 (30/30) | E: (5.62 ± 1.32) yr C: (5.73 ± 1.30) yr | E: (10 ± 3.0) d C: (10 ± 3.2) d | (C) + HM | 2 wk | (1)(2)(5) |
| Erythromycin |
| First Author (Year) | Prescription /Composition | Dosage (Day) | Frequency (Day) |
|---|---|---|---|
| Chang (2019) [18] | Sangbaipi decoction Mori Cortex Radicis 10 g, Pinelliae Rhizoma 10 g, Armeniacae Semen 10 g, Lepidii seu Descurainiae Semen 10 g, Stemonae Radix 10 g, Perillae Fructus 6 g, Scutellariae Radix 6 g, Glycyrrhizae Radix et Rhizoma 6 g, Ilicis pubescentis Radix 15 g, Meretricis Concha 15 g, Ficus hirta vahl. 15 g, Tribuli Fructus 5 g, Paridis Rhizoma 8 g, Aristolochiae Fructus 3 g | 0–1 yr: 30–100 mL 1–6 yr: 100–200 mL | 2 times |
| Cui (2015) [19] | Sangbaipi decoction Mori Cortex Radicis 10 g, Pinelliae Rhizoma 10 g, Armeniacae Semen 10 g, Fritillariae Thunbergii Bulbus 10 g, Perillae Fructus 10 g, Persicae Semen 10 g, Lepidii seu Descurainiae Semen 10 g, Scutellariae Radix 6 g, Glycyrrhizae Radix et Rhizoma 6 g | <1 yr: 30–100 mL 1–6 yr: 100–200 mL | 1 time |
| Cui (2019) [20] | Sangbaipi decoction Scutellariae Radix 6 g, Persicae Semen 10 g, Perillae Fructus 10 g, Mori Cortex Radicis 10 g, Lepidii seu Descurainiae Semen 10 g, Fritillariae Thunbergii Bulbus 10 g, Pinelliae Rhizoma 10 g, Armeniacae Semen 10 g, Glycyrrhizae Radix et Rhizoma 6 g | 200 mL | 1 time |
| Dong (2021) [21] | Sangbaipi decoction Persicae Semen 10 g, Mori Cortex Radicis 10 g, Fritillariae Thunbergii Bulbus 10 g, Armeniacae Semen 10 g, Pinelliae Rhizoma 10 g, Lepidii seu Descurainiae Semen 10 g, Perillae Fructus 10 g, Glycyrrhizae Radix et Rhizoma 6 g, Scutellariae Radix 6 g | <1 yr: 30–100 mL 1–6 yr: 100–200 mL | 1 time |
| Li (2018) [22] | Sosiho decoction and Sabaek powder Bupleuri Radix 9 g, Scutellariae Radix 6 g, Pinelliae Rhizoma 9 g, Zingiberis Rhizoma Recens 3 g, Mori Cortex Radicis 10 g, Lycii Radicis Cortex 10 g, Armeniacae Semen 10 g, Fritillariae Cirrhosae Bulbus 10 g, Lepidii seu Descurainiae Semen 10 g, Batryticatus Bombyx 10 g, Lumbricus 10 g, Cicadidae Periostracum 10 g, Glycyrrhizae Radix et Rhizoma 6 g | NR | NR |
| Liu (2016) [23] | Canyu dunke prescription Schizonepetae Spica 3 g, Saposhnikoviae Radix 3 g, Platycodonis Radix 6 g, Armeniacae Semen 5 g, Asteris Radix et Rhizoma 6 g, Stemonae Radix 5 g, Poria 3 g, Farfarae Flos 6 g, Lonicerae Flos 6 g, Fritillariae Cirrhosae Bulbus 3 g, Evodiae Fructus 3 g, Pinelliae Rhizoma 3 g, Codonopsis Pilosulae Radix 5 g, Glycyrrhizae Radix et Rhizoma 3 g | NR | NR |
| Lu (2022) [24] | Jiawei weijing decoction Phragmitis Rhizoma 15 g, Persicae Semen 10 g, Coicis Semen 5 g, Benincasae Semen 5 g, Lonicerae Flos 5 g, Forsythiae Fructus 5 g, Glycyrrhizae Radix et Rhizoma 6 g | NR | >4 yr: 2 times <4 yr: NR |
| Ren (2017) [25] | Sangbaipi decoction Glycyrrhizae Radix et Rhizoma 6 g, Scutellariae Radix 6 g, Mori Cortex Radicis 10 g, Lepidii seu Descurainiae Semen 10 g, Pinelliae Rhizoma 10 g, Perillae Fructus 10 g, Fritillariae Thunbergii Bulbus 10 g, Armeniacae Semen 10 g, Persicae Semen 10 g | <1 yr: 30–100 mL 1–6 yr: 100–200 mL | 2 times |
| Tang (2020) [26] | Lusika pill * (Beijing Tongrentang Co., Ltd. (Beijing, China) Tongrentang Pharmaceutical Factory) Armeniacae Semen, Gypsum Fibrosum, Glycyrrhizae Radix et Rhizoma, Asiasari Radix et Rhizoma, Perillae Fructus, Brassicae Semen, Arctii Fructus, Trichosanthis Pericarpium, Belamcandae Rhizoma, Indigo Pulverata Levis, Meretricis Concha, Trichosanthis Radix, Gardeniae Fructus, Artificial Bovis Calculus, Ephedrae Herba, Glycyrrhizae Radix et Rhizoma | 3 g | 2 times |
| Tao (2020) [27] | Zhenhai jingyan prescription (New Green Pharmaceutical Co., Ltd. (Sichuan, China)) Mori Cortex Radicis 8 g, Lycii Radicis Cortex 8 g, Aurantii Fructus Immaturus 6 g, Armeniacae Semen 8 g, Eriobotryae Folium 10 g, Stemonae Radix 10 g, Scorpio 1 g Fritillariae Thunbergii Bulbus 8 g, Belamcandae Rhizoma 10 g, Lumbricus 6 g, Arisaematis Rhizoma 3 g, | 60–100 mL | 2 times |
| Wang (2019a) [28] | Chunggan sapye decoction Bupleuri Radix 10 g, Cicadidae Periostracum 6 g, Lumbricus 3 g, Scutellariae Radix 6 g, Pinelliae Rhizoma 6 g, Armeniacae Semen 9 g, Fritillariae Thunbergii Bulbus 6 g, Lepidii seu Descurainiae Semen 9 g, Batryticatus Bombyx 6 g, Zingiberis Rhizoma Recens 3 g, Mori Cortex Radicis 10 g, Lycii Radicis Cortex 10 g | 0.5 pack | 2 times |
| Wang (2019b) [29] | Zhenhai hwadam prescription Batryticatus Bombyx 9 g, Gastrodiae Rhizoma 9 g, Aconiti Koreani Tuber 6 g, Aconiti Lateralis Radix Preparata 3 g, Aucklandiae Radix 6 g, Scorpio 3 g, Syzygii Flos 3 g, Citri Unshius Pericarpium 6 g, Pinelliae Tuber 3 g, Glycyrrhizae Radix et Rhizoma 3 g | <2 m: 20 mL 2–5 m: 30 mL 6–8 m: 40 mL 9–12 m: 50 mL >12 m: 60 mL | 2 times |
| Wang (2021) [30] | Dengtai ye granules * (Yunnan Botanical Pharmaceutical Co., Ltd. (Kunming, China), Z53020312) Folium Wrightiae Laevis | 30 g | 3 times |
| Wang (2024) [31] | Baikening granules * (Guangdong sencee pharmaceutical Co., Ltd. (Guangzhou, China), Z20050140) Ginkgonis Semen, Indigo Pulverata Levis, Fritillariae Thunbergii Bulbus | ≤1 yr: 3 pack 1–3 yr: 4.5 pack ≥3 yr: 6 pack | 3 times |
| Yan (2019) [32] | Jiawei weijing decoction Phragmitis Rhizoma 15 g, Stemonae Radix 10 g, Paeoniae Radix 10 g, Persicae Semen 10 g, Coicis Semen 6 g, Benincasae Semen 6 g, Arisaematis Rhizoma 3 g, Armeniacae Semen 6 g, Imperatae Rhizoma 6 g, Perillae Fructus 6 g, Scorpio 2 g, Peucedani Radix 6 g, Plantaginis Semen 6 g, Glycyrrhizae Radix et Rhizoma 3 g | <1 yr: 2/3 pack 1–3 yr: 1 pack 3–5 yr: 4/3 pack ≥5 yr: 2 pack | 2 times |
| Zhang (2018a) [33] | Heron cough pill * Ephedrae Herba, Asiasari Radix et Rhizoma, Arctii Fructus, Gypsum Fibrosum, Trichosanthis Radix, Gardeniae Fructus, Indigo Pulverata Levis, Glycyrrhizae Radix et Rhizoma, Armeniacae Semen | 6–12 m: 1.5 g 1–3 yr: 3.0 g | 2 times |
| Zhang (2018b) [34] | Sangbaipi decoction Persicae Semen 10 g, Mori Cortex Radicis 10 g, Fritillariae Thunbergii Bulbus 10 g, Pinelliae Rhizoma 10 g, Lepidii seu Descurainiae Semen 10 g, Perillae Fructus 10 g, Glycyrrhizae Radix et Rhizoma 6 g, Scutellariae Radix 6 g | <1 yr: 30–100 mL 1–6 yr: 100–200 mL | 1 time |
| Zhang (2019) [35] | Dengtai ye granules * (Yunnan Botanical Pharmaceutical Co., Ltd. (Kunming, China) Z53020312) Folium Wrightiae Laevis | 30 g | 3 times |
| Zhang (2020) [36] | Dengtai ye granules * (Yunnan Botanical Pharmaceutical Co., Ltd. (Kunming, China), Z53020312) Folium Wrightiae Laevis | 30 g | 3 times |
| Zhang (2021) [37] | Dengtai ye granules * (Yunnan Botanical Pharmaceutical Co., Ltd. (Kunming, China), Z53020312) Folium Wrightiae Laevis | <2 yr: 1 pack 2–5 yr: 3/2 pack >5 yr: 3 pack | 3 times |
| Zhang (2022) [38] | Jiawei weijing decoction Imperatae Rhizoma 30 g, Phragmitis Rhizoma 15 g, Stemonae Radix 10 g, Paeoniae Radix 10 g, Persicae Semen 10 g, Coicis Semen 6 g, Benincasae Semen 6 g, Armeniacae Semen 6 g, Perillae Fructus 6 g, Peucedani Radix 6 g, Plantaginis Semen 6 g, Pinelliae Tuber 3 g, Glycyrrhizae Radix et Rhizoma 3 g, Scorpio 2 g | NR | 2 times |
| Zhang (2023) [39] | Xiaoer Feike granules * (Changchun People’s Pharmaceutical Group Co., Ltd. (Changchun, China), Z20027415) Ginseng radix et rhizoma, Poria, Atractylodis Macrocephalae rhizoma, Citri Unshius Pericarpium, Rhei radix et rhizome, Lycii Radicis Cortex, Glehniae radix, Glycyrrhizae Radix et Rhizoma, Artemisiae annuae herba, Ophiopogonis radix, Cinnamomic ramulus, Zingiberis Rhizoma Recens, Aconiti radix, Trichosanthis Fructus, Farfarae flos, Asteris radix et rhizoma, Mori Cortex Radicis, Astragali radix, Lycii fructus, Arisaema cum Bile, Galli gigerii endothelium corneum, Trionycis carapax | <1 yr: 6 g 1–2 yr: 9 g | 3 times |
| Zhi (2021) [40] | Dengtai ye granules * (Yunnan Botanical Pharmaceutical Co., Ltd. (Kunming, China), Z53020312) Folium Wrightiae Laevis | NR | 3 times |
| Outcomes | Subgroup | No. Participants (Studies) | Anticipated Absolute Effects (95% CI) | Relative Effect (95% CI) | Heterogeneity (I2) | Quality of Evidence |
|---|---|---|---|---|---|---|
| Risk with Intervention Group | ||||||
| TER | Total | 1888 (22 RCTs) | 159 more per 1000 (128 more to 191 more) | RR 1.20 (1.16 to 1.24) | 0 | ⨁⨁⨁◯ Moderate a, |
| Disappearance time of main symptom (spastic cough) (day) | Total | 815 (9 RCTs) | MD 3.31 lower (3.51 lower to 3.11 lower) | - | 94 | ⨁⨁⨁◯ Moderate a, |
| DY | 560 (5 RCTs) | MD 3.16 lower (3.4 lower to 2.93 lower) | - | 96 | ⨁⨁⨁◯ Moderate a, | |
| WJ | 92 (1 RCT) | MD 5.3 lower (6.17 lower to 4.43 lower) | - | Not applicable | ⨁⨁◯◯ Low a,b | |
| Sosiho decoction and Sabaek powder | 68 (1 RCT) | MD 2.98 lower (3.77 lower to 2.18 lower) | - | Not applicable | ⨁◯◯◯ Very Low a,b | |
| Canyu dunke prescription | 50 (1 RCT) | MD 5.08 lower (6.06 lower to 4.1 lower) | - | Not applicable | ⨁◯◯◯ Very Low a,b | |
| Zhenhai hwadam prescription | 45 (1 RCT) | MD 2.58 lower (3.34 lower to 1.82 lower) | - | Not applicable | ⨁⨁◯◯ Low a,b | |
| Recovery time of blood routine to normal range (day) | Total | 472 (6 RCTs) | MD 2.79 lower (3.06 lower to 2.52 lower) | - | 87 | ⨁⨁⨁◯ Moderate a |
| SB | 352 (5 RCTs) | MD 2.58 lower (2.9 lower to 2.27 lower) | - | 88 | ⨁⨁◯◯ Low a,b | |
| DY | 120 (1 RCT) | MD 3.28 lower (3.77 lower to 2.79 lower) | Not applicable | ⨁⨁◯◯ Low a,b | ||
| Hospitalization time (day) | Total | 703 (9 RCTs) | MD 2.61 lower (2.85 lower to 2.38 lower) | - | 83 | ⨁⨁⨁◯ Moderate a |
| SB | 432 (6 RCTs) | MD 3.07 lower (3.37 lower to 2.78 lower) | - | 39 | ⨁⨁⨁◯ Moderate a | |
| DY | 120 (1 RCT) | MD 3.73 lower (4.88 lower to 2.58 lower) | - | Not applicable | ⨁⨁◯◯ Low a,b | |
| Zhenhai hwadam prescription | 83 (1 RCT) | MD 1.73 lower (2.25 lower to 1.21 lower) | - | Not applicable | ⨁⨁◯◯ Low a,b | |
| Sosiho decoction and Sabaek powder | 68 (1 RCT) | MD 1.21 lower (1.9 lower to 0.51 lower) | - | Not applicable | ⨁◯◯◯ Very Low a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-U.; Shim, Y.-S.; Kim, E.-J.; Min, S.Y. Efficacy and Safety of Combined Treatment with Traditional Herbal Medicine and Western Medicine for Children with Pertussis-like Syndrome: Systematic Review and Meta-Analysis. Healthcare 2025, 13, 1131. https://doi.org/10.3390/healthcare13101131
Choi J-U, Shim Y-S, Kim E-J, Min SY. Efficacy and Safety of Combined Treatment with Traditional Herbal Medicine and Western Medicine for Children with Pertussis-like Syndrome: Systematic Review and Meta-Analysis. Healthcare. 2025; 13(10):1131. https://doi.org/10.3390/healthcare13101131
Chicago/Turabian StyleChoi, Ji-U, Young-Shin Shim, Eun-Jin Kim, and Sang Yeon Min. 2025. "Efficacy and Safety of Combined Treatment with Traditional Herbal Medicine and Western Medicine for Children with Pertussis-like Syndrome: Systematic Review and Meta-Analysis" Healthcare 13, no. 10: 1131. https://doi.org/10.3390/healthcare13101131
APA StyleChoi, J.-U., Shim, Y.-S., Kim, E.-J., & Min, S. Y. (2025). Efficacy and Safety of Combined Treatment with Traditional Herbal Medicine and Western Medicine for Children with Pertussis-like Syndrome: Systematic Review and Meta-Analysis. Healthcare, 13(10), 1131. https://doi.org/10.3390/healthcare13101131






