The Safety of Peripheral Nerve Blocks: The Role of Triple Monitoring in Regional Anaesthesia, a Comprehensive Review
Abstract
1. Introduction
2. Regional Anaesthesia
2.1. Selected Advantages and Disadvantages of Peripheral Nerve Blocks
2.2. Permanent Nerve Injury
2.3. Prevalence of Permanent Nerve Injury
- Careful planning of needle site entry to avoid vital structures such as vessels, pleura, viscera, or other nerve structures not being a target of intervention.
- Precise administration of LA close to the nerve structure, but outside the epineurium to reduce the total volume of LA and the risk of LAST.
- Avoidance of direct nerve injury by the needle and intraneural injection of LA.
2.4. Nerve Localization Techniques
2.5. Electrostimulation of Nerves
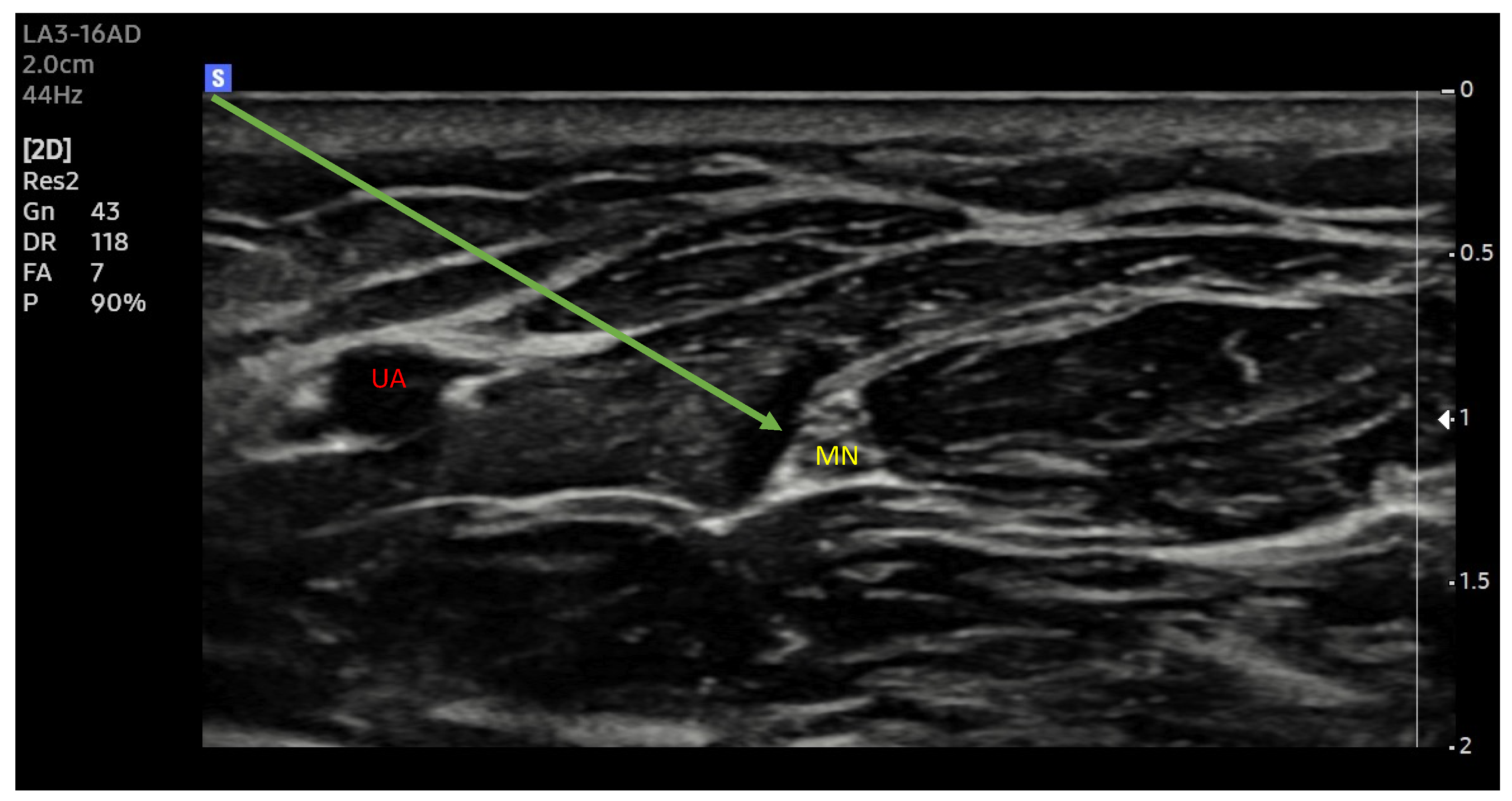
2.6. Ultrasonography
2.7. Injection Pressure Monitoring
2.8. Triple Monitoring
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russon, K.; Anaesthetist, C.; Harclerode, Z.; Anaesthetics, S.T. Peripheral Nerve Blocks “Getting Started” Anaesthesia Tutorial of the Week 134 18Th May 2009. Access 2009, 1–6. Available online: https://resources.wfsahq.org/atotw/peripheral-nerve-blocks-getting-started/ (accessed on 5 March 2024).
- El-Boghdadly, K.; Pawa, A.; Chin, K.J. Local anesthetic systemic toxicity: Current perspectives. Local Reg. Anesth. 2018, 11, 35–44. [Google Scholar] [CrossRef]
- Nerve Block Anesthesia—PubMed. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431109/ (accessed on 31 October 2023).
- Farley, A.; McLafferty, E.; Johnstone, C.; Hendry, C. Nervous system: Part 3. Nurs. Stand. 2014, 28, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Neal-Smith, G.; Hopley, E.; Gourbault, L.; Watts, D.T.; Abrahams, H.; Wilson, K.; Athanassoglou, V. General Versus Regional Anaesthesia for Lower Limb Arthroplasty and Associated Patient Satisfaction Levels: A Prospective Service Evaluation in the Oxford University Hospitals. Cureus 2021, 13, e17024. [Google Scholar] [CrossRef] [PubMed]
- Kettner, S.C.; Willschke, H.; Marhofer, P. Does regional anaesthesia really improve outcome? Br. J. Anaesth. 2011, 107, i90–i95. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Kopp, S.L.; Burkle, C.M.; Duncan, C.M.; Jacob, A.K.; Erwin, P.J.; Murad, M.H.; Mantilla, C.B. Neuraxial vs general anaesthesia for total hip and total knee arthroplasty: A systematic review of comparative-effectiveness research. Br. J. Anaesth. 2016, 116, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Zhang, D.A. Prolonged Use of a Continuous Peripheral Nerve Block Catheter for Analgesia after Pediatric Foot and Ankle Surgery. Case Rep. Anesthesiol. 2021, 2021, 8026961. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.A. Complications of Regional Anesthesia: Nerve Injury and Peripheral Neural Blockade. J. Neurosurg. Anesthesiol. 2004, 16, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.S. Management of peripheral nerve injury. J. Clin. Orthop. Trauma 2019, 10, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Seddon, H.J. A classification of nerve injuries. Br. Med. J. 1942, 2, 237. [Google Scholar] [CrossRef]
- Liguori, G.A.; Zayas, V.M.; Chisholm, M.F. Transient Neurologic Symptoms after Spinal Anesthesia with Mepivacaine and Lidocaine. Anesthesiology 1998, 88, 619–623. [Google Scholar] [CrossRef]
- Torp, K.D.; Metheny, E.; Simon, L.V. Lidocaine Toxicity; StatPearls: Treasure Island, FL, USA, 2022; pp. 1–4. [Google Scholar]
- Slaven, S.E.; Dedeogullari, E.S.; Parks, N.L.; Sershon, R.A.; Fricka, K.B.; Hamilton, W.G. Spinal Anesthesia for Primary Hip and Knee Arthroplasty: Comparative Rates of Transient Neurological Symptoms and Urinary Retention Using Lidocaine, Mepivacaine, and Bupivacaine. J. Arthroplast. 2023, 38, S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Brull, R.; McCartney, C.J.L.; Chan, V.W.S.; El-Beheiry, H. Neurological complications after regional anesthesia: Contemporary estimates of risk. Anesth. Analg. 2007, 104, 965–974. [Google Scholar] [CrossRef]
- Pietraszek, P. Regional anaesthesia-induced peripheral nerve injury. Anaesthesiol. Intensive Ther. 2018, 50, 367–377. [Google Scholar] [CrossRef]
- Hebl, J.R.; Kopp, S.L.; Schroeder, D.R.; Horlocker, T.T. Neurologic complications after neuraxial anesthesia or analgesia in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesth. Analg. 2006, 103, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.B.; Brummett, C.M.; Welch, T.D.; Tremper, K.K.; Shanks, A.M.; Guglani, P.; Mashour, G.A. Perioperative peripheral nerve injuries: A retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology 2009, 111, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Barrington, M.J.; Watts, S.A.; Gledhill, S.R.; Thomas, R.D.; Said, S.A.; Snyder, G.L.; Tay, V.S.; Jamrozik, K. Preliminary results of the australasian regional anaesthesia collaboration: A prospective audit of more than 7000 peripheral nerve and plexus blocks for neurologic and other complications. Reg. Anesth. Pain Med. 2009, 34, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Orebaugh, S.L.; Williams, B.A.; Vallejo, M.; Kentor, M.L. Adverse outcomes associated with stimulator-based peripheral nerve blocks with versus without ultrasound visualization. Reg. Anesth. Pain Med. 2009, 34, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.M. Ultrasound-Guided Regional Anesthesia and Patient Safety. Reg. Anesth. Pain Med. 2010, 35, S59–S67. [Google Scholar] [CrossRef] [PubMed]
- Auroy, Y.; Benhamou, D.; Bargues, L.; Ecoffey, C.; Falissard, B.; Mercier, F.; Bouaziz, H.; Samii, K. Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service. Anesthesiology 2002, 97, 1274–1280. [Google Scholar] [CrossRef]
- Die Anaesthesierung des Plexus Brachialis bei Operationen an der Oberen Extremitat, Munchen Med. Available online: https://scholar.google.com/scholar?hl=pl&as_sdt=0%2C5&q=Die+Anasthesierung+des+plexus+brachialis+bei+operationen+an+der+oberen+extremitat&btnG= (accessed on 5 March 2024).
- Winnie, A.P.; Collins, V.J. The Subclavian Perivascular Technique of Brachial Plexus Anesthesia. Anesthesiology 1964, 25, 353–363. [Google Scholar] [CrossRef]
- Interscalene Brachial Plexus Block. Available online: https://oce-1ovid-1com-1000036a60081.han3.wum.edu.pl/article/00000539-197005000-00029/HTML (accessed on 5 March 2024).
- Brown, A.R. Peripheral nerve stimulator vs. paresthesia. Rev. Mex. Anestesiol. 2004, 27, 76–82. [Google Scholar]
- Selander, D.; Edshage, S.; Wolff, T. Paresthesiae or No Paresthesiae? Nerve Lesions after Axillary Blocks. Acta Anaesthesiol. Scand. 1979, 23, 27–33. [Google Scholar] [CrossRef]
- Andersson, A.; Åkeson, J.; Dahlin, L.B. Efficacy and safety of axillary brachial plexus block for operations on the hand. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2006, 40, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.M. Regional nerve block with the aid of a nerve stimulator. Anaesthesia 1972, 27, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.M.; Melton, M.S.; Grill, W.M.; Nielsen, K.C. Peripheral nerve stimulation in regional anesthesia. Reg. Anesth. Pain Med. 2012, 37, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, B.; Chelly, J.E. Current channeling: A theory of nerve stimulator failure. Anesth. Analg. 2003, 96, 1531–1532. [Google Scholar] [CrossRef]
- Robards, C.; Hadzic, A.; Somasundaram, L.; Iwata, T.; Gadsden, J.; Xu, D.; Sala-Blanch, X. Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth. Analg. 2009, 109, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Warman, P.; Nicholls, B. Ultrasound-guided nerve blocks: Efficacy and safety. Best Pract. Res. Clin. Anaesthesiol. 2009, 23, 313–326. [Google Scholar] [CrossRef]
- Liu, S.S.; Zayas, V.M.; Gordon, M.A.; Beathe, J.C.; Maalouf, D.B.; Paroli, L.; Liguori, G.A.; Ortiz, J.; Buschiazzo, V.; Ngeow, J.; et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesth. Analg. 2009, 109, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Sakura, S.; Yokokawa, N.; Tadenuma, S. Incidence and effects of unintentional intraneural injection during ultrasound-guided subgluteal sciatic nerve block. Reg. Anesth. Pain Med. 2012, 37, 289–293. [Google Scholar] [CrossRef]
- Liu, S.S.; Yadeau, J.T.; Shaw, P.M.; Wilfred, S.; Shetty, T.; Gordon, M. Incidence of unintentional intraneural injection and postoperative neurological complications with ultrasound-guided interscalene and supraclavicular nerve blocks. Anaesthesia 2011, 66, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Koscielniak-Nielsen, Z.J. Ultrasound-guided peripheral nerve blocks: What are the benefits? Acta Anaesthesiol. Scand. 2008, 52, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Saporito, A.; Quadri, C.; Capdevila, X. The ability of a real-time injection pressure monitoring system to discriminate between perineural and intraneural injection of the sciatic nerve in fresh cadavers. Anaesthesia 2018, 73, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Vermeylen, K.; Hermans, M.; Soetens, F.; Vereecke, E.; Steinfeldt, T.; Groen, G.; Hadzic, A.; Van De Velde, M. Opening injection pressure is higher in intraneural compared with perineural injections during simulated nerve blocks of the lower limb in fresh human cadavers. Reg. Anesth. Pain Med. 2017, 42, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Krol, A.; Vala, A.; Phylactides, L.; Szarko, M.; Reina, M.A.; De Andres, J. Injection pressure mapping of intraneural vs. perineural injections: Further lessons from cadaveric studies. Minerva Anestesiol. 2018, 84, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Krol, A.; Szarko, M.; Vala, A.; De Andres, J. Pressure monitoring of intraneural an perineural injections into the median, radial, and ulnar nerves; lessons from a cadaveric study. Anesthesiol. Pain Med. 2015, 5, 22723. [Google Scholar] [CrossRef]
- Gadsden, J.C.; Choi, J.J.; Lin, E.; Robinson, A. Opening injection pressure consistently detects needle-nerve contact during ultrasound-guided interscalene brachial plexus block. Anesthesiology 2014, 120, 1246–1253. [Google Scholar] [CrossRef]
- Roberto, D.; Quadri, C.; Capdevila, X.; Saporito, A. Identification of interfascial plane using injection pressure monitoring at the needle tip during ultrasound-guided TAP block in cadavers. Reg. Anesth. Pain Med. 2023, 48, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Bodhey, A.; Nair, A.; Seelam, S. SAFIRA pump: A novel device for fixed injection pressure and to control local anesthetic injection during peripheral nerve block. J. Anaesthesiol. Clin. Pharmacol. 2023, 39, 146. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y. Development of guidance techniques for regional anesthesia: Past, present and future. J. Pain Res. 2021, 14, 1631–1641. [Google Scholar] [CrossRef]
- Neal, J.M.; Wedel, D.J. Editorial: Ultrasound guidance and peripheral nerve injury: Is our vision as sharp as we think it is? Reg. Anesth. Pain Med. 2010, 35, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Brull, R.; Hadzic, A.; Reina, M.A.; Barrington, M.J. Pathophysiology and Etiology of Nerve Injury Following Peripheral Nerve Blockade. Reg. Anesth. Pain Med. 2015, 40, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, T.; Eider, J.; Nimphius, W.; Wiesmann, T.; De Andres, J.; Müller, H.H.; Wulf, H.; Steinfeldt, T. Dual guidance improves needle tip placement for peripheral nerve blocks in a porcine model. Acta Anaesthesiol. Scand. 2012, 56, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, G.; Strumia, A.; Costa, F.; Rizzo, S.; Del Buono, R.; Remore, L.M.; Bruno, F.; Agrò, F.E. Triple monitoring may avoid intraneural injection during interscalene brachial plexus block for arthroscopic shoulder surgery: A prospective preliminary study. J. Clin. Med. 2021, 10, 781. [Google Scholar] [CrossRef]
- Krol, A.; De Andres, J. Plexus and peripheral nerve block anaesthesia—A step beyond ultrasound or full circle? Rev. Esp. Anestesiol. Reanim. 2016, 63, 129–134. [Google Scholar] [CrossRef]




| Potential Complications with any Peripheral Nerve Block |
|---|
| Block failure |
| Intravascular injection |
| Local Anaesthetic Toxicity |
| Nerve damage: temporary or permanent |
| Injury secondary to numbness or weakness |
| Infection |
| Seddon’s Classification | |
|---|---|
| neurapraxia | Compression of the nerve without breaking its continuity. Nerve conduction is impaired. Symptoms disappear after a few to a few dozen days. |
| axonotmesis | The nerve is intact, but the axons are damaged. Complete loss of the nerve function occurs. Nerves can regenerate, but this can take up to several dozen months. |
| neurotmesis | Transection of the nerve causes its paralysis. No rapid regeneration is possible. Surgical intervention is necessary. |
| Sunderland’s Classification | |
|---|---|
| grade I | Lack of conduction due to nerve compression Corresponds to Seddon’s neurapraxia |
| grade II | Breaking of the axon without nerve damage Corresponds to Seddon’s axonotmesis |
| grade III | Damage of the endoneurium, without changes in the epi- and perineurium Return of function depends on endoneuronal fibrosis |
| grade IV | Damage to all sheaths apart from the epineurium Enlargement of the nerve may occur |
| grade V | Complete severance or disruption of the nerve Corresponds to Seddon’s neurotmesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśnicki, M.; Król, A.; Kosson, D.; Kołacz, M. The Safety of Peripheral Nerve Blocks: The Role of Triple Monitoring in Regional Anaesthesia, a Comprehensive Review. Healthcare 2024, 12, 769. https://doi.org/10.3390/healthcare12070769
Paśnicki M, Król A, Kosson D, Kołacz M. The Safety of Peripheral Nerve Blocks: The Role of Triple Monitoring in Regional Anaesthesia, a Comprehensive Review. Healthcare. 2024; 12(7):769. https://doi.org/10.3390/healthcare12070769
Chicago/Turabian StylePaśnicki, Marek, Andrzej Król, Dariusz Kosson, and Marcin Kołacz. 2024. "The Safety of Peripheral Nerve Blocks: The Role of Triple Monitoring in Regional Anaesthesia, a Comprehensive Review" Healthcare 12, no. 7: 769. https://doi.org/10.3390/healthcare12070769
APA StylePaśnicki, M., Król, A., Kosson, D., & Kołacz, M. (2024). The Safety of Peripheral Nerve Blocks: The Role of Triple Monitoring in Regional Anaesthesia, a Comprehensive Review. Healthcare, 12(7), 769. https://doi.org/10.3390/healthcare12070769