Actuarial Analysis of Survival after Breast Cancer Diagnosis among Lithuanian Females
Abstract
1. Introduction
2. Aim and Reasearch Overview
3. Some Notations and Mathematical Preliminaries
3.1. Kaplan–Meier Estimates
3.2. Cox Proportional Hazard Model
3.2.1. Univariate and Multivariate Cox Model
3.2.2. Stratified Cox Model
4. Data and Their Limitations
- Exact date of cancer diagnosis.
- Circumstance of diagnosis: due to patient’s initiative, cancer awareness program, or death certificate.
- Stage at diagnosis.
- Date of last patient inspection (follow up date).
- Exact date of death, if known.
5. Main Results
5.1. Kaplan–Meier Estimates
5.2. Cox Models
5.2.1. Univariate Cox Model
5.2.2. Multivariate Cox Model
5.2.3. Stratified Cox Model
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. R Scripts Used for Calculations
- #Kaplan-Meier calculations
- data1 <- read_excel(“C:/Users/R/Krūties vėžys 1995-2016 m.xlsx”) #Read input data
- attach(data1)
- km1 <- survfit( Surv(Time, Death)~ 1, type=“kaplan-meier”)
- summary(km1)
- #Time– time since diagnosis until end of observations, in months.
- #Death=1 in case of death of patient; Death=0 otherwise.
- survplots <- list()
- survplots[[1]]<-ggsurvplot(km1,combine=TRUE, data1, title=’Visos pacientės’,
- legend.title = “All cases (patients)”, legend.labs = c(“Strata”), xlim = c(0,60),
- xlab=“Time since diagnosis, months”, ylab=“Survival probability”, conf.int = TRUE,
- ggtheme = theme_bw(), break.time.by = 12, surv.median.line = “hv”)
- #By stage
- km2 <- survfit( Surv(Time, Death)~ Stage, type=“kaplan-meier”)
- summary(km2)
- fit <- list(km1 = km1, km2=km2)
- survplots[[2]]<-ggsurvplot(km2, data1,title=’Kaplan - Meier survival function by stage at diagnosis’,
- legend.title = “Stage”, legend.labs = c(“1”, “2”, “3”, “4”), xlim = c(0,60),
- xlab=“Time since diagnosis, months”,ylab=“Survival probability”, conf.int = TRUE, pval = TRUE,
- risk.table.height = 0.4,ggtheme = theme_bw(),
- break.time.by = 1, surv.median.line = “hv”)
- #log-rank test
- surv_diff <- survdiff(Surv(Time, Death)~ Stage)
- surv_diff
- #By circumstance
- km3 <- survfit( Surv(Time, Death)~ Circumstance, type=“kaplan-meier”)
- summary(km3)
- fit <- list(km1 = km1, km3=km3)
- survplots[[3]]<-ggsurvplot(fit,combine=TRUE, data1,
- title=’Kaplan - Meier survival function by circumstance of diagnosis’,legend.title = “Circumstance”,
- legend.labs = c(“Strata”,“Patient initiative”, “Cancer awareness program”), xlim = c(0,60),
- xlab=“Time since diagnosis, months”,ylab=“Survival probability”,conf.int = TRUE,
- pval = TRUE, risk.table.height = 0.4,ggtheme = theme_bw(),
- break.time.by = 12, surv.median.line = “hv”)
- #log-rank test
- surv_diff <- survdiff(Surv(Time, Death)~ Circumstance)
- surv_diff
- #By period of diagnosis
- km4 <- survfit( Surv(Time, Death)~ Year, type=“kaplan-meier”)
- summary(km4)
- fit <- list(km1 = km1, km4=km4)
- survplots[[4]]<-ggsurvplot(fit, combine=TRUE,data1,
- title=’Kaplan - Meier survival function by period of diagnosis’, legend.title = “Metai”,
- legend.labs = c(“Strata”,“1995-2004”, “2005-2016”), xlim = c(0,60),
- xlab=“Time since diagnosis, months”,ylab=“Survival probability”,conf.int = TRUE,
- pval = TRUE, risk.table.height = 0.4,
- ggtheme = theme_bw(), break.time.by = 12, surv.median.line = “hv”)
- #log-rank test
- surv_diff <- survdiff(Surv(Time, Death)~ Year)
- surv_diff
- arrange_ggsurvplots(survplots, print = TRUE,ncol = 2, nrow = 2,
- risk.table.height = 0.3, title=’Kaplan-Meier estimates’)
- #Cox PH model
- #Univariate model
- res.cox1 <- coxph(Surv(Time, Death) ~ Stage)
- summary(res.cox1)
- res.zph1 <- cox.zph(res.cox1)
- res.cox2 <- coxph(Surv(Time, Death) ~ Circumstance)
- summary(res.cox2)
- res.zph2 <- cox.zph(res.cox2)
- res.cox3 <- coxph(Surv(Time, Death) ~ Year)
- summary(res.cox3)
- res.zph3 <- cox.zph(res.cox3)
- #Schoenfeld test
- plot(res.zph1)
- plot(res.zph2)
- plot(res.zph3)
- # Multivariate Cox model
- res.cox4 <- coxph(Surv(Time, Death) ~ Stage+Circumstance+Year)
- summary(res.cox4)
- res.zph4 <- cox.zph(res.cox4)
- #Stratified Cox Model
- res.cox5 <- coxph(Surv(Time, Death) ~ Circumstance+Year+strata(Stage))
- summary(res.cox5)
- res.zph5 <- cox.zph(res.cox5)
References
- American Cancer Society. Available online: https://www.cancer.org/cancer/types/breast-cancer/about.html (accessed on 5 July 2023).
- The International Agency for Research on Cancer of the World Health Organization. Available online: https://www.iarc.who.int/news-events/current-and-future-burden-of-breast-cancer-global-statistics-for-2020-and-2040/ (accessed on 5 July 2023).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Institute of Hygiene. Causes of Death Registry. Available online: https://www.hi.lt/en/mortality-in-lithuania.html (accessed on 4 August 2023). (In Lithuanian).
- Narod, S.A.; Giannakeas, V.; Sopik, V. Time to death in breast cancer patients as an indicator of treatment response. Breast Cancer Res. Treat. 2018, 172, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.; Gao, H.; Yasui, Y.; Dabbs, K.; Winget, M. Survival in stage I–III breast cancer patiens by surgical treatment in a publicly funded health care system. Ann. Oncol. 2015, 26, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Buzdar, A.U.; Smith, T.L.; Kau, S.W.; Yang, Y.; Hortobagyi, G.N. Is breast cancer survival improving? Cancer 2004, 100, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Meier, P. Estimation of a distribution function from incomplete observations. J. Appl. Probab. 1975, 12, 67–87. [Google Scholar] [CrossRef]
- Meier, P.; Karrison, T.; Chapell, R.; Xie, H. The price of Kaplan-Meier. J. Am. Stat. Assoc. 2004, 99, 890–896. [Google Scholar] [CrossRef]
- Staudt, Y.; Wagner, J. Factors driving duration to croos-selling in non-life insurance: New empirical evidence from Switzerland. Risks 2022, 10, 187. [Google Scholar] [CrossRef]
- Tholkage, S.; Zheng, Q.; Kulasekera, K.G. Conditional Kaplan-Meier estimator with functional covariates for time-to-event data. Stats 2022, 5, 1113–1129. [Google Scholar] [CrossRef]
- Nemes, S. Asymptotic relative efficiency of parametric and nonparametric survival estimators. Stats 2023, 6, 1147–1159. [Google Scholar] [CrossRef]
- Arsyad, R.; Thamrin, S.A.; Jaya, A.K. Extended Cox model for breast cancer survival data using Bayesian approach: A case study. J. Phys. Conf. Ser. 2019, 1341, 092013. [Google Scholar] [CrossRef]
- Lin, R.H.; Lin, C.S.; Chuang, C.L.; Kujabi, B.K.; Chen, Y.C. Breast cancer survival analysis model. Appl. Sci. 2022, 12, 1971. [Google Scholar] [CrossRef]
- Pereira, L.C.; Silva, S.J.; Fidelis, C.R.; Brito, A.L.; Xavier Júnior, S.F.A.; Andrade, L.S.S.; Oliveira, M.E.C.; Oliveira, T.A. Cox model and decision trees: An application to breast cancer data. Rev. Panam. Salud. Publica 2022, 46, e17. [Google Scholar] [CrossRef] [PubMed]
- Bustan, M.N.; Arman; Aidid, M.K.; Gobel, F.A.; Syamsidar. Cox proportional hazard survival analysis to inpatient breast cancer cases. J. Phys. Conf. Ser. 2018, 1028, 012230. [Google Scholar] [CrossRef]
- Putter, H.; Sasako, M.; Hartgrink, H.H.; Velde, C.J.H.; Houwelingen, J.C. Long-term survival with non-proportional hazards: Results from the dutch gastric cancer trial. Stat. Med. 2005, 24, 2807–2821. [Google Scholar] [CrossRef] [PubMed]
- Akezaki, Y.; Nakata, E.; Kikuuchi, M.; Sugihara, S.; Katayama, H.; Hamada, M.; Ozaki, T. Association between overall survival and activities of daily living in patients with spinal bone metastases. Healthcare 2022, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, J.; Budach, W.; Nestle-Krämling, C.; Wollandt, S.; Tomaskovich, B.; Corradini, S.; Bölke, E.; Krug, D.; Fehm, T.; Ruckhäberle, E.; et al. Predictive factors of long-term survival after neoadjuvant radiotherapy and chemotherapy in high-risk breast cancer. Cancers 2022, 14, 4031. [Google Scholar] [CrossRef] [PubMed]
- Rim, C.H.; Lee, W.J.; Musaev, B.; Volichevich, T.Y.; Pozlitdinovich, Z.Y.; Lee, H.Y.; Nigmatovich, T.M.; Rim, J.S. Comparison of breast cancer and cervical cancer in Uzbekistan and Korea: The first report of the Uzbekistan-Korea onkology consortium. Medicina 2022, 58, 1428. [Google Scholar] [CrossRef]
- Gwak, H.; Woo, S.S.; Oh, S.J.; Kim, J.Y.; Shin, H.C.; Youn, H.J.; Chun, J.W.; Lee, D.; Kim, S.H. A comparison of the prognostic effects of fine needle aspiration and core needle biopsy in patients with breast cancer: A nationwide multicenter prospective registry. Cancers 2023, 15, 4638. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Liu, X.; Liu, Y.; Zhang, J. Prognoses of patients with hormone receptor-positive and human epidermal growth factor receptor 2-negative breast cancer receiving neoadjuvant chemotherapy before surgery: A restrospective analysis. Cancers 2023, 15, 1157. [Google Scholar] [CrossRef]
- Korobeinikova, E.; Ugenskiene, R.; Insodaite, R.; Rudzianskas, V.; Gudaitiene, J.; Juozaityte, E. The role of functional polymorphisms in oxidative stress-related genes on early-stage breast cancer survival. Int. J. Biol. Markers 2021, 36, 14. [Google Scholar] [CrossRef]
- Elsakov, P.; Ostapenko, V.; Luksyte, A.; Smailyte, G. Similar 10-Year survival in breast cancer patients of Lithuania and Poland with common BRCA1 founder mutations. Breast Cancer Manag. 2019, 8, BMT13. [Google Scholar] [CrossRef]
- Ivanauskienė, R.; Gedminaitė, J.; Juozaitytė, E.; Vanagas, G.; Šimoliūnienė, R.; Padaiga, Ž. Survival of women with breast cancer in Kaunas region, Lithuania. Medicina 2012, 48, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Dafni, U.; Tsourti, Z.; Alatsathianos, I. Breast cancer statistics in the European Union: Incidence and survival across European countries. Breast Care 2019, 14, 344. [Google Scholar] [CrossRef]
- Salimbajevs, T.; Irmejs, A.; Miklasevics, E.; Nakazawa-Miklasevica, M.; Subatniece, S. Breast Cancer Patients Survival Rates at Pauls Stradiņš Clinical University Hospital; Collection of Scientific Papers: Research Articles in Medicine & Pharmacy; Internal Medicine, Surgery, Medical Basic Sciences, Stomatology, Pharmacy; Rīga Stradiņš University: Rīga, Latvia, 2014; pp. 23–28. [Google Scholar]
- Skučaitė, A.; Puvačiauskienė, A.; Puišys, R.; Šiaulys, J. Actuarial analysis of survival among breast cancer patients in Lithuania. Healthcare 2021, 9, 383. [Google Scholar] [CrossRef]
- Macdonald, A.S.; Richards, S.J.; Currie, I.D. The Kaplan-Meier estimator. In Modelling Mortality with Actuarial Applications; Daykin, C., Macdonald, A., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 134–140. [Google Scholar]
- London, D. Survival Models and Their Estimation; ACTEX Publications: Winsted, CT, USA, 1988. [Google Scholar]
- Kleinbaum, D.G.; Klein, M. Survival Analysis. A Self Learning Text, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 45–73. [Google Scholar]
- CRAN—Package Survival. Available online: https://cran.r-project.org/web/packages/survival/index.html (accessed on 26 May 2022).
- CRAN—Package Survminer. Available online: https://cran.r-project.org/web/packages/survminer/index.html (accessed on 26 May 2022).
- Macdonald, A.S.; Richards, S.J.; Currie, I.D. Example: A proportional hazard model. In Modelling Mortality with Actuarial Applications; Daykin, C., Macdonald, A., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 122–124. [Google Scholar]
- Macdonald, A.S.; Richards, S.J.; Currie, I.D. The Cox Model. In Modelling Mortality with Actuarial Applications; Daykin, C., Macdonald, A., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 124–125. [Google Scholar]
- Lee, T.L.; Wang, J.W. Statistical Methods for Survival Data Analysis, 4th ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A. The Breslow estimator of the nonparametric baseline survivor function in Cox’s regression model some heuristics. Epidemiology 2008, 19, 101–102. [Google Scholar] [CrossRef]
- National Health Insurance Fund under the Ministry of Health. Available online: https://ligoniukasa.lrv.lt/lt/veiklos-sritys/informacija-gyventojams/ligu-prevencijos-programos (accessed on 5 December 2023).
- Steponavičienė, L.; Briedienė, R.; Vansevičiūtė-Petkevičienė, R.; Gudavičienė-Petkevičienė, D.; Vincerževskienė, I. Breast cancer screening program in Lithuania: Trends in breast cancer mortality before and during the introduction of the mammography screening program. Acta Med. Litu. 2020, 7, 61–69. [Google Scholar] [CrossRef]




| Stage at Diagnosis | Circumstance of Diagnosis | Period of Diagnosis | ||
|---|---|---|---|---|
| At Patient’s Initiative | Participation in CAP | 1995–2004 | 2005–2016 | |
| 1st | 6594 | 531 | 1913 | 5212 |
| 2nd | 11,576 | 376 | 5364 | 6588 |
| 3rd | 5858 | 111 | 2771 | 3198 |
| 4th | 3026 | 35 | 1730 | 1331 |
| Total | 27,054 | 1053 | 11,778 | 16,329 |
| Hazards | Cases Observed (%) | Deaths Observed (%) | Average Survival Time * | Median Survival Time ** |
|---|---|---|---|---|
| All cases (patients) | 28,107 (100%) | 9249 (100%) | 48.347 (1.152) | - |
| Stage at diagnosis | ||||
| 1st | 7125 (25.35%) | 675 (7.30%) | 57.431 (0.123) | - |
| 2nd | 11,952 (42.52%) | 2788 (30.14%) | 53.180 (0.134) | - |
| 3rd | 5969 (21.24%) | 3155 (34.11%) | 41.613 (0.271) | 54 (52–57) |
| 4th | 3061 (10.89%) | 2631 (28.45%) | 21.468 (0.376) | 14 (13–15) |
| Circumstance of diagnosis | ||||
| Patient’s initiative | 27,054 (96.25%) | 9072 (98.09%) | 48.093 (0.118) | - |
| Cancer awareness program | 1053 (3.75%) | 177 (1.91%) | 54.891 (0.417) | - |
| Period of diagnosis | ||||
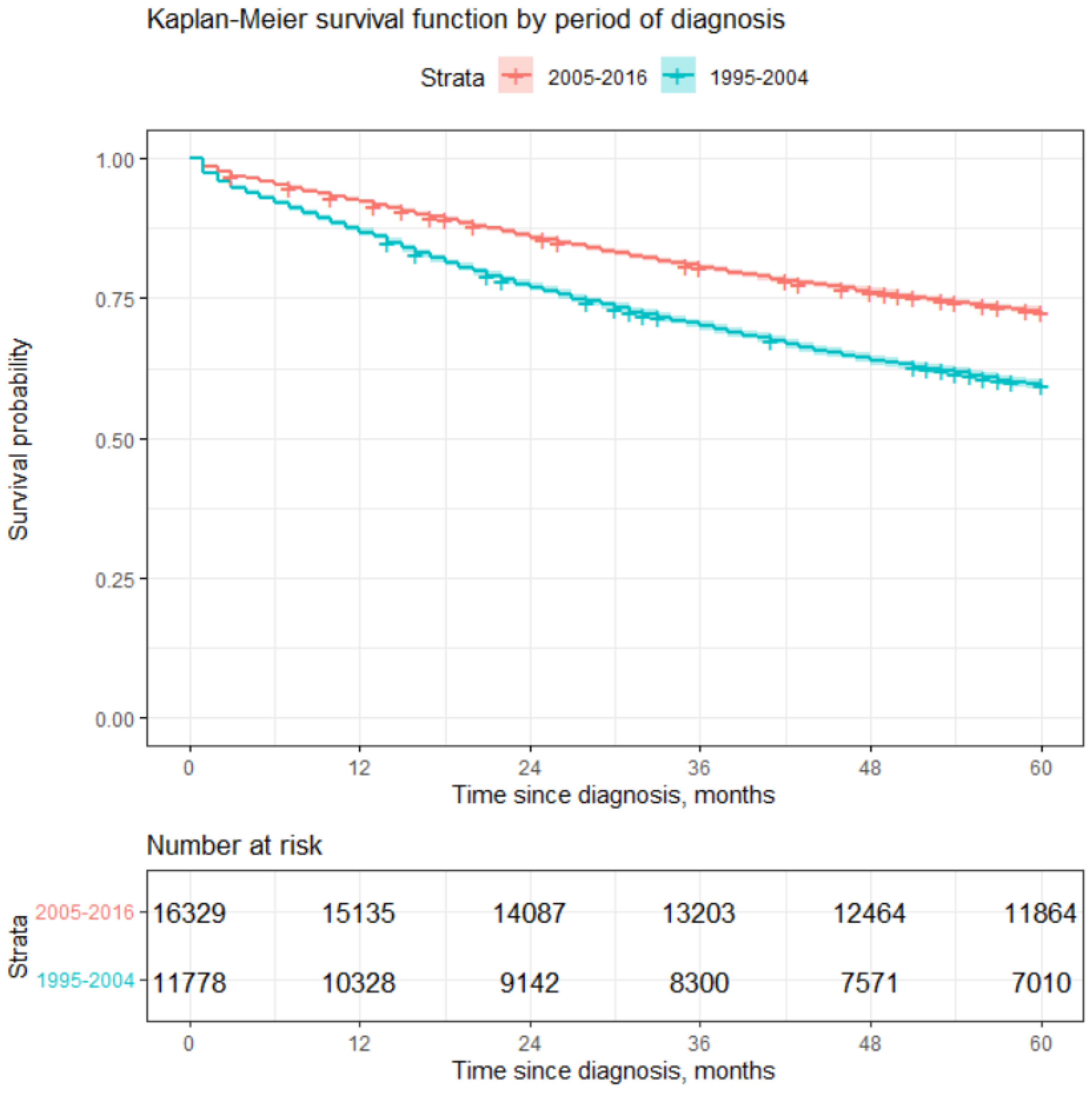
| 1995–2004 | 11,778 (41.90%) | 4774 (51.62%) | 45.291 (0.192) | - |
| 2005–2016 | 16,329 (58.10%) | 4475 (48.38%) | 50.553 (0.139) | - |
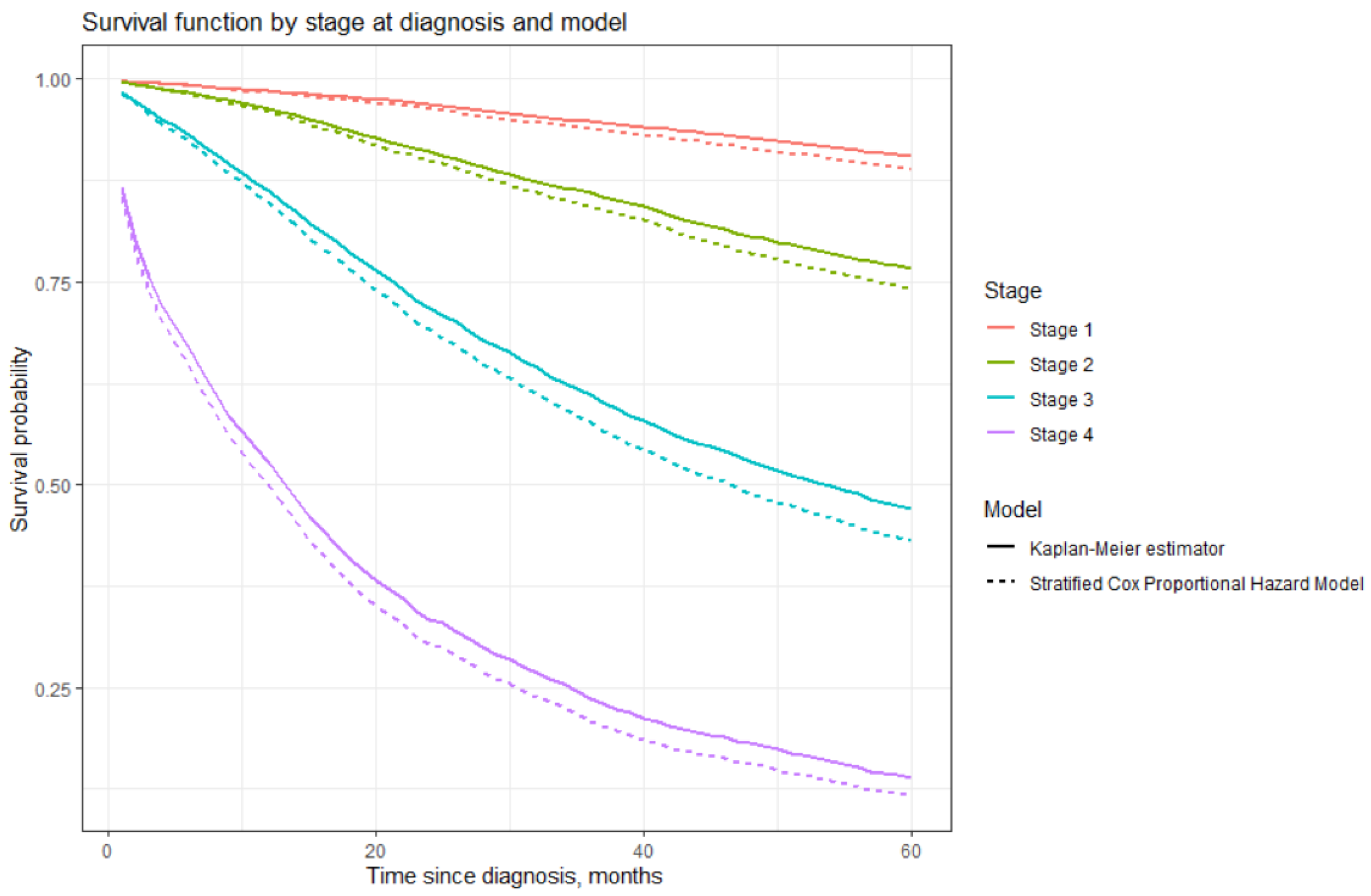
| Time Since Diagnosis in Years | Stage at Diagnosis | |||
|---|---|---|---|---|
| 1st * | 2nd | 3rd | 4th | |
| 1 | 98.582% (0.142%) | 96.318% (0.179%) | 86.212% (0.518%) | 52.630% (1.715%) |
| 2 | 96.841% (0.214%) | 91.003% (0.288%) | 71.832% (0.811%) | 33.420% (2.551%) |
| 3 | 94.804% (0.277%) | 85.935% (0.370%) | 61.117% (1.033%) | 23.733% (3.241%) |
| 4 | 92.767% (0.324%) | 80.635% (0.488%) | 52.865% (0.646%) | 18.153% (3.842%) |
| 5 | 90.517% (0.384%) | 76.654% (0.505%) | 47.092% (1.373%) | 13.984% (4.489%) |
| Stage at Diagnosis | Current Study | Lithuania 1995–2012 * | Latvia 2005 ** | CONCORD-3 Study *** |
|---|---|---|---|---|
| 1st | 90.52% | 90.22% | 88% | - **** |
| 2nd | 76.65% | 75.68% | 74% | - |
| 3rd | 47.09% | 45.43% | 43% | - |
| 4th | 13.98% | 13.85% | 4% | - |
| Overall | 67% | - | - | 73% |
| Time Since Diagnosis | Circumstance of Diagnosis | |
|---|---|---|
| On Patient’s Initiative * | CAP in Years | |
| 1 | 89.7% (0.185%) | 96.5% (0.567%) |
| 2 | 81.7% (0.235%) | 92.8% (0.798%) |
| 3 | 75.6% (0.261%) | 89.5% (0.943%) |
| 4 | 70.4% (0.278%) | 86.6% (1.050%) |
| 5 | 66.4% (0.287%) | 83.2% (1.153%) |
| Stage at Diagnosis | Examined at Patients Initiative | Examined during CAPs | ||
|---|---|---|---|---|
| Number of Cases | Percentage of Cases | Number of Cases | Percentage of Cases | |
| 1 | 6594 | 24% | 531 | 50% |
| 2 | 11,576 | 43% | 376 | 36% |
| 3 | 5858 | 22% | 111 | 11% |
| 4 | 3026 | 11% | 35 | 3% |
| Total | 27,054 | 100% | 1053 | 100% |
| Time Since Diagnosis in Years | Period of Diagnosis | |
|---|---|---|
| 1995–2004 * | 2005–2016 | |
| 1 | 86.8% (0.311%) | 92.3% (0.2092%) |
| 2 | 77.0% (0.388%) | 85.9% (0.2726%) |
| 3 | 70.1% (0.422%) | 80.5% (0.3101%) |
| 4 | 63.9% (0.443%) | 76.2% (0.3336%) |
| 5 | 59.4% (0.453%) | 72.6% (0.3493%) |
| Stage at Diagnosis | Period of Diagnosis: 1995–2004 | Period of Diagnosis: 2005–2016 | ||
|---|---|---|---|---|
| Number of Cases | Percentage of Cases | Number of Cases | Percentage of Cases | |
| 1 | 1913 | 16% | 5212 | 32% |
| 2 | 5364 | 46% | 6588 | 40% |
| 3 | 2771 | 24% | 3198 | 20% |
| 4 | 1730 | 15% | 1331 | 8% |
| Total | 11,778 | 100% | 16,329 | 100% |
| Covariate: | Regression Coefficient * | Hazard Rate ** | p *** |
|---|---|---|---|
| Stage: | |||
| 1st | 1 | ||
| 2nd | 0.975 (0.043) | 2.651 (2.437–2.884) | <0.001 |
| 3rd | 2.030 (0.043) | 7.617 (7.009–8.278) | <0.001 |
| 4th | 3.15914 (0.044) | 23.550 (21.626–25.646) | <0.001 |
| Circumstance | |||
| Patients initiative | 1 | ||
| CAP | −0.810 (0.076) | 0.445 (0.384–0.516) | <0.001 |
| Period of diagnosis | |||
| 1995–2004 | 1 | ||
| 2005–2016 | −0.490 (0.021) | 0.612 (0.588–0.638) | <0.001 |
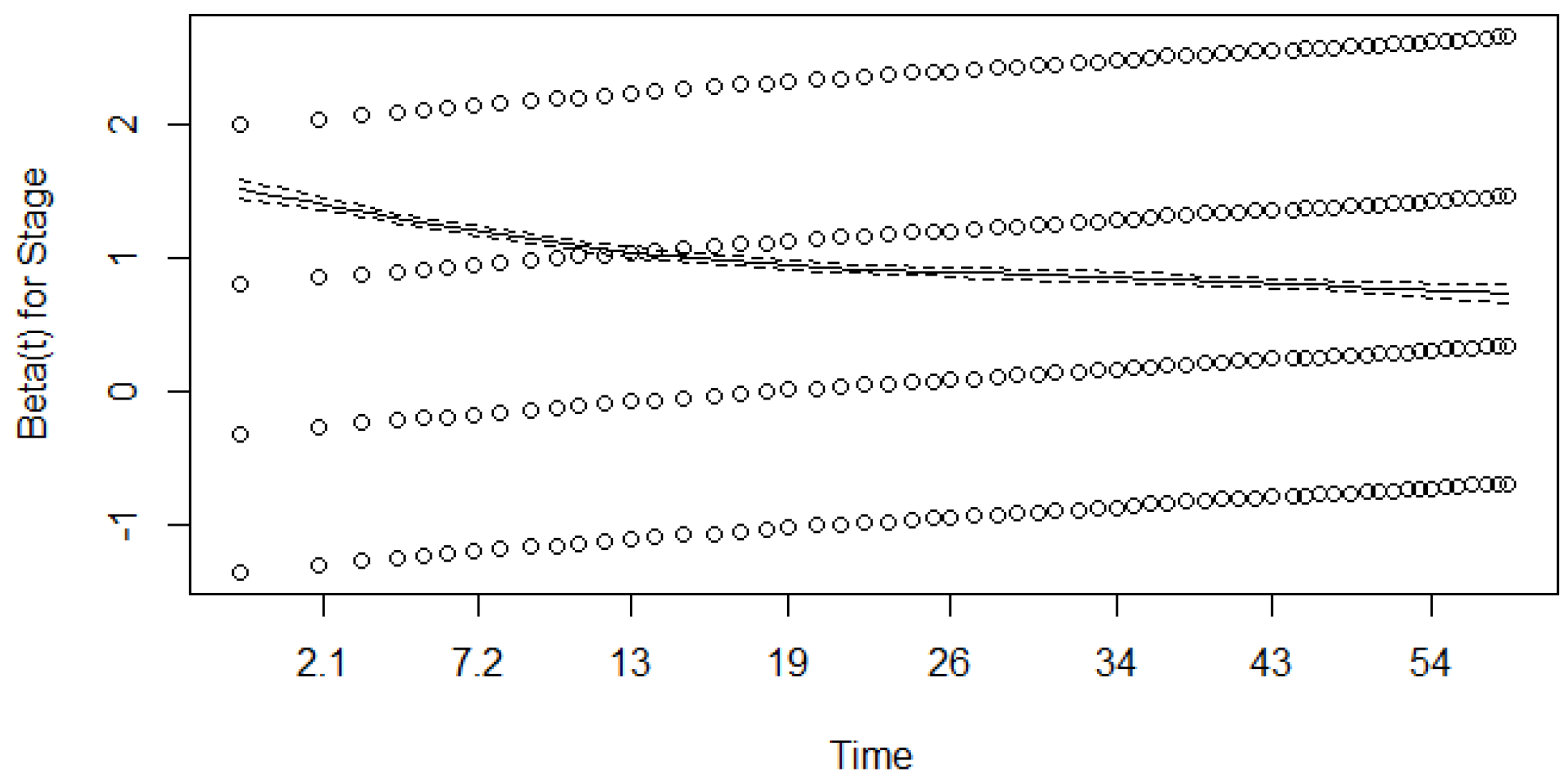
| Covariate | Statistics | Degrees of Freedom | p Value |
|---|---|---|---|
| Stage | 415 | 3 | <0.001 |
| Circumstance | 8.5 | 1 | 0.0036 |
| Period | 6.5 | 1 | 0.011 |
| Statistics | Degrees of Freedom | p Value | |
|---|---|---|---|
| Stage | 414.61 | 3 | <0.001 |
| Circumstance | 4.92 | 1 | 0.027 |
| Period | 1.49 | 1 | 0.223 |
| Overall | 417.60 | 5 | <0.001 |
| Statistics | Degrees of Freedom | p Value | |
|---|---|---|---|
| Circumstance | 1.08 | 1 | 0.30 |
| Period | 1.62 | 1 | 0.20 |
| Overall | 2.89 | 2 | 0.24 |
| Covariate | Regression Coefficient * | Hazard Ratio ** | p *** |
|---|---|---|---|
| Circumstance | |||
| Patients’ initiative | 1 | ||
| CAP | −0.271 (0.076) | 0.763 (0.657–0.886) | <0.001 |
| Period of diagnosis | |||
| 1995–2004 | 1 | ||
| 2005–2016 | −0.221 (0.021) | 0.802 (0.769–0.836) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levickytė, J.; Skučaitė, A.; Šiaulys, J.; Puišys, R.; Vincerževskienė, I. Actuarial Analysis of Survival after Breast Cancer Diagnosis among Lithuanian Females. Healthcare 2024, 12, 746. https://doi.org/10.3390/healthcare12070746
Levickytė J, Skučaitė A, Šiaulys J, Puišys R, Vincerževskienė I. Actuarial Analysis of Survival after Breast Cancer Diagnosis among Lithuanian Females. Healthcare. 2024; 12(7):746. https://doi.org/10.3390/healthcare12070746
Chicago/Turabian StyleLevickytė, Justina, Aldona Skučaitė, Jonas Šiaulys, Rokas Puišys, and Ieva Vincerževskienė. 2024. "Actuarial Analysis of Survival after Breast Cancer Diagnosis among Lithuanian Females" Healthcare 12, no. 7: 746. https://doi.org/10.3390/healthcare12070746
APA StyleLevickytė, J., Skučaitė, A., Šiaulys, J., Puišys, R., & Vincerževskienė, I. (2024). Actuarial Analysis of Survival after Breast Cancer Diagnosis among Lithuanian Females. Healthcare, 12(7), 746. https://doi.org/10.3390/healthcare12070746






