Language and Communication Interventions in People with Alzheimer’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Eligibility Criteria
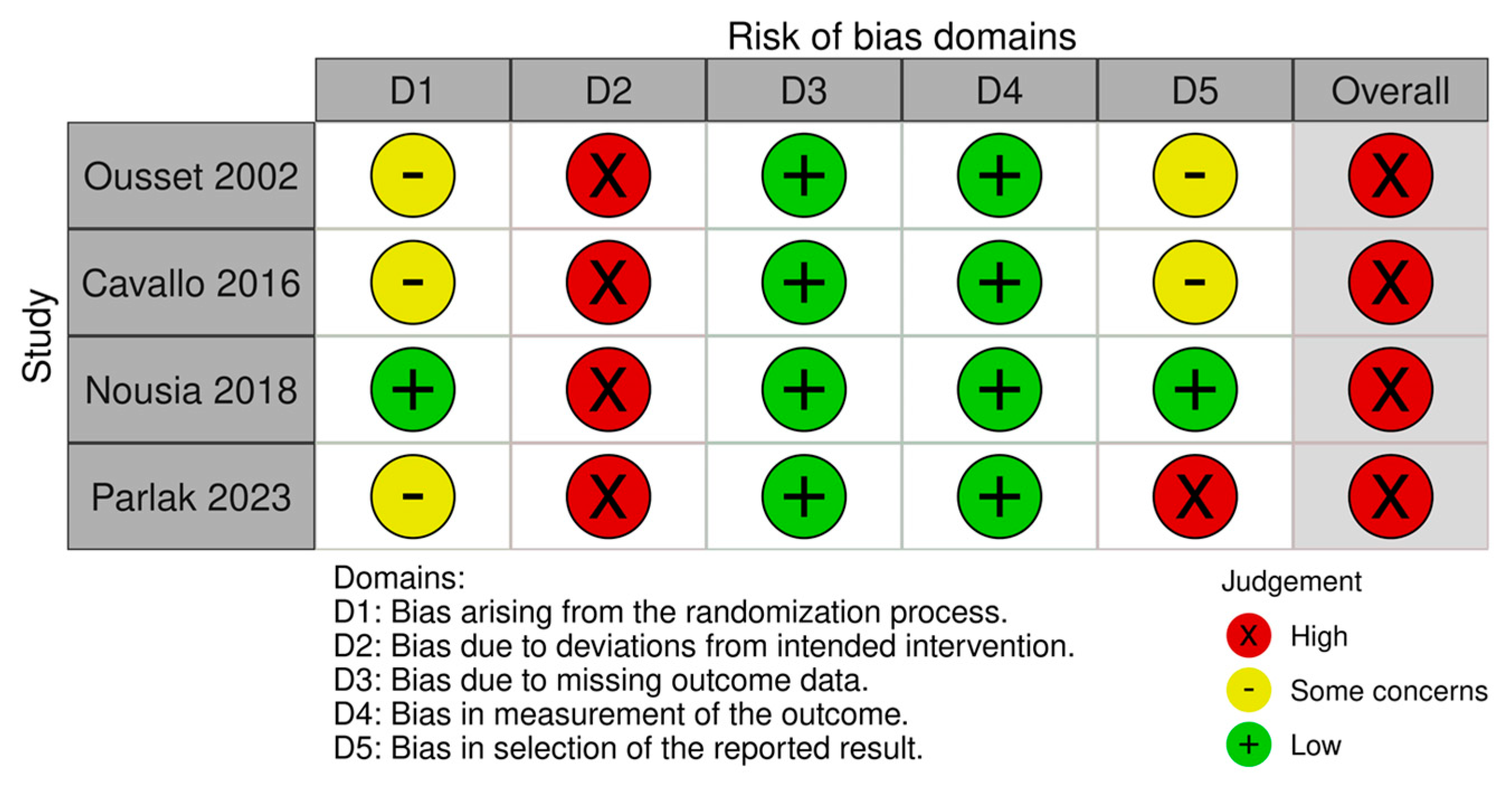
2.4. Risk of Bias Assessment Tool
3. Results
3.1. Study Characteristics
3.2. Patients’ Characteristics
3.3. Intervention Characteristics
3.4. Language and Cognitive Domains Targeted
3.5. Noncognitive Outcomes: Quality of Life Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. PRISMA Guidelines
| Section and Topic | Item # | Checklis Titem | Location WHere, Item Is Reported |
| Title | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 2, 3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 3, 4 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 4, 5 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 4, 5 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | 5 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 4, 5 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 4 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | NA |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | NA | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | 6 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | NA |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 2 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | NA | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | NA | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | NA | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | NA |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | 6 |
| Results | |||
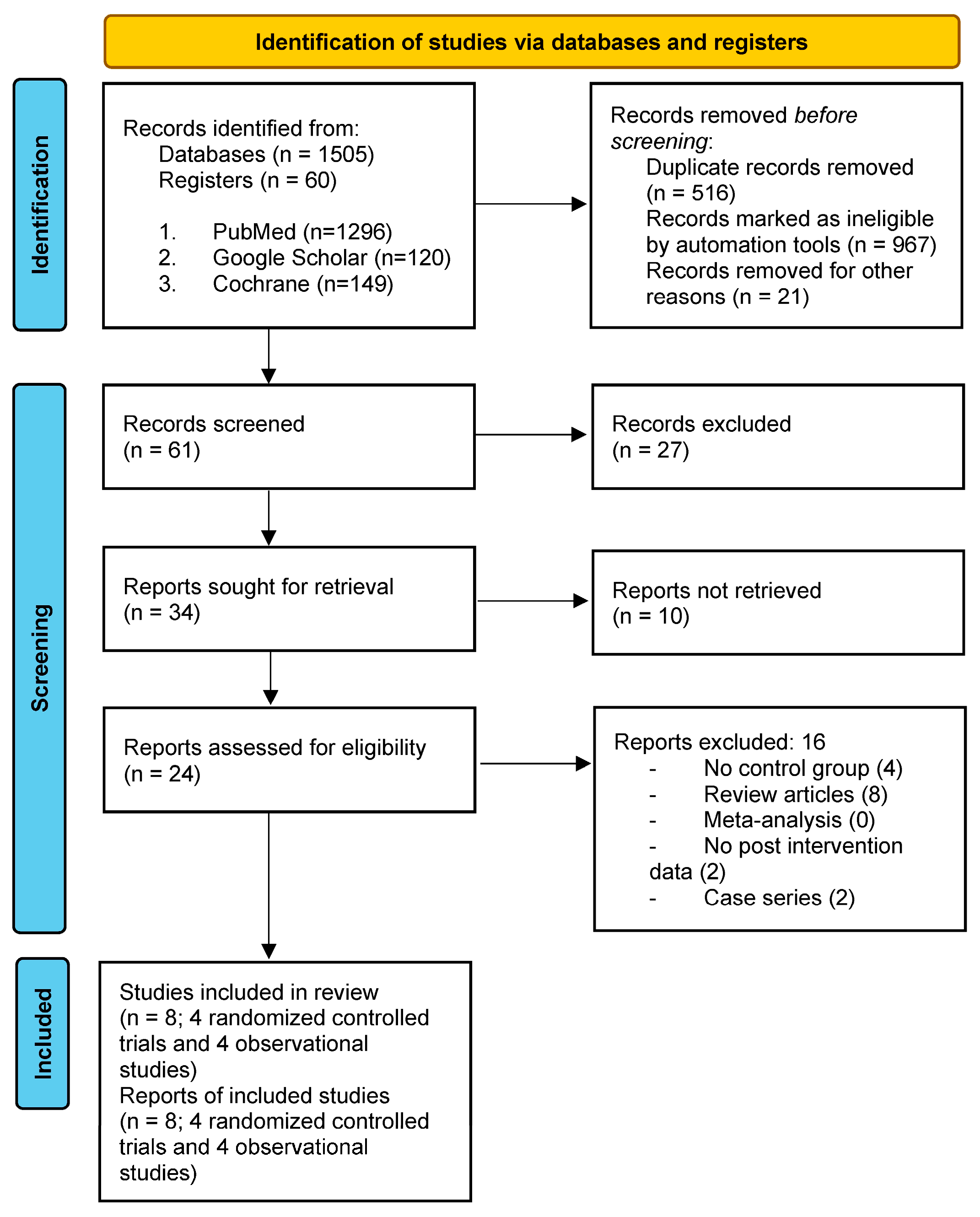
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 4, 5 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | NA | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 6, 7 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 6 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 10–19 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | NA |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | NA | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | NA | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | NA | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | NA |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | 15–19 |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 16 |
| 23b | Discuss any limitations of the evidence included in the review. | 16 | |
| 23c | Discuss any limitations of the review processes used. | 16 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 16 | |
| Other Information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | NA |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | NA | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | NA | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | 22 |
| Competing interests | 26 | Declare any competing interests of review authors. | 22 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | NA |
References
- Jack, C.R., Jr.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef]
- American Psychiatric Association and American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Vandenberghe, R.; Tournoy, J. Cognitive aging and Alzheimer’s disease. Postgrad. Med. J. 2005, 81, 343–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kempler, D. Neurocognitive Disorders in Aging; Sage Publications: Thousand Oaks, CA, USA, 2005. [Google Scholar]
- Lekeu, F.; Van der Linden, M.; Chicherio, C.; Collette, F.; Degueldre, C.; Franck, G.; Moonen, G.; Salmon, E. Brain Correlates of Performance in a Free/Cued Recall Task With Semantic Encoding in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2003, 17, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Taler, V.; Phillips, N.A. Language performance in Alzheimer’s disease and mild cognitive impairment: A comparative review. J. Clin. Exp. Neuropsychol. 2008, 30, 501–556. [Google Scholar] [CrossRef]
- Kochhann, R.; Pereira, A.H.; Holz, M.R.; Chaves, M.L.; Fonseca, R.P. P2-303: Deficits in Unconstrained, Phonemic and Semantic Verbal Fluency in Healthy Elders, Mild Cognitive Impairment and Mild Alzheimer’s Disease Patients. Alzheimer’s Dement. 2016, 12, P751–P752. [Google Scholar] [CrossRef]
- Emery, V.O.B. Language Impairment in Dementia of the Alzheimer Type: A Hierarchical Decline? Int. J. Psychiatry Med. 2000, 30, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Altmann, L.J.P.; Kempler, D.; Andersen, E.S. Speech Errors in Alzheimer’s Disease: Reevaluating Morphosyntactic Preservation. J. Speech Lang. Hear. Res. 2001, 44, 1069–1082. [Google Scholar] [CrossRef]
- Kertesz, A. Language deterioration in dementia. In Dementia: Presentations, Differential Diagnosis, and Nosology, 2nd ed.; NetLibrary, Incorporated Distributor; Johns Hopkins University Press: Baltimore, MD, USA; Boulder, CO, USA, 2004; pp. 108–122. [Google Scholar]
- Johnson, A.F.; Jacobson, B.H. (Eds.) Medical Speech-Language Pathology: A Practitioner’s Guide, 3rd ed.; Updated; Thieme: New York, NY, USA, 2017. [Google Scholar]
- Macoir, J.; Turgeon, Y. Dementia and language. In The Encyclopedia of Language and Linguistics, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2006; pp. 423–430. [Google Scholar]
- Fisher, N.J.; Tierney, M.C.; Rourke, B.P.; Szalai, J.P. Verbal Fluency Patterns in Two Subgroups of Patients With Alzheimer’s Disease. Clin. Neuropsychol. 2004, 18, 122–131. [Google Scholar] [CrossRef]
- Fraser, K.C.; Meltzer, J.A.; Rudzicz, F. Linguistic Features Identify Alzheimer’s Disease in Narrative Speech. J. Alzheimer’s Dis. 2015, 49, 407–422. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Wang, H.; Sun, Y. Sentence comprehension in patients with dementia of the Alzheimer’s type. PeerJ 2019, 7, e8181. [Google Scholar] [CrossRef]
- Colombo, L.; Fonti, C.; Stracciari, A. Italian verb inflection in Alzheimer dementia. Neuropsychologia 2009, 47, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Manouilidou, C.; Roumpea, G.; Nousia, A.; Stavrakaki, S.; Nasios, G. Revisiting Aspect in Mild Cognitive Impairment and Alzheimer’s Disease: Evidence From Greek. Front. Commun. 2020, 5, 434106. [Google Scholar] [CrossRef]
- Chapin, K.; Clarke, N.; Garrard, P.; Hinzen, W. A finer-grained linguistic profile of Alzheimer’s disease and Mild Cognitive Impairment. J. Neurolinguist. 2022, 63, 101069. [Google Scholar] [CrossRef]
- Marková, J.; Horváthová, L.; Králová, M.; Cséfalvay, Z. Sentence comprehension in Slovak-speaking patients with Alzheimer’s disease: Sentence comprehension in Alzheimer’s disease. Int. J. Lang. Commun. Disord. 2017, 52, 456–468. [Google Scholar] [CrossRef]
- Varlokosta, S.; Fragkopoulou, K.; Arfani, D.; Manouilidou, C. Methodologies for assessing morphosyntactic ability in people with Alzheimer’s disease. Int. J. Lang. Commun. Disord. 2024, 59, 38–57. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, N.F.; Ivanova, M.V.; Baldo, J.V. What Do Language Disorders Reveal about Brain–Language Relationships? From Classic Models to Network Approaches. J. Int. Neuropsychol. Soc. 2017, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Brownsett, S.; Copland, D. Language and language disorders: Neuroscience to clinical practice. Pract. Neurol. 2019, 19, 380–388. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Woodward, M. Aspects of Communication in Alzheimer’s Disease: Clinical Features and Treatment Options. Int. Psychogeriatr. 2013, 25, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moreno, J.; Satorres, E.; Soria-Urios, G.; Meléndez, J.C. Cognitive Stimulation in Moderate Alzheimer’s Disease. J. Appl. Gerontol. 2022, 41, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Pei, J.; Zhan, Y.-J.; Cai, Y.-W. Overview of Meta-Analyses of Five Non-Pharmacological Interventions for Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 594432. [Google Scholar] [CrossRef] [PubMed]
- Zucchella, C.; Sinforiani, E.; Tamburin, S.; Federico, A.; Mantovani, E.; Bernini, S.; Casale, R.; Bartolo, M. The Multidisciplinary Approach to Alzheimer’s Disease and Dementia. A Narrative Review of Non-Pharmacological Treatment. Front. Neurol. 2018, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Carrion, C.; Folkvord, F.; Anastasiadou, D.; Aymerich, M. Cognitive Therapy for Dementia Patients: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2018, 46, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Soria, I.; Peralta-Marrupe, P.; Calatayud-Sanz, E.; Latorre, E. Efficacy of Cognitive Intervention Programs in Amnesic Mild Cognitive Impairment: A Systematic Review. Arch. Gerontol. Geriatr. 2021, 94, 104332. [Google Scholar] [CrossRef] [PubMed]
- Hopper, T.; Holland, A.; Rewega, M. Conversational Coaching: Treatment Outcomes and Future Directions. Aphasiology 2002, 16, 745–761. [Google Scholar] [CrossRef]
- Spector, A.; Orrell, M.; Hall, L. Systematic Review of Neuropsychological Outcomes in Dementia from Cognition-Based Psychological Interventions. Dement. Geriatr. Cogn. Disord. 2012, 34, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Yip, C.C.K.; Yu, E.C.S.; Man, D.W.K. Evaluation of a computer-assisted errorless learning-based memory training program for patients with early Alzheimer’s disease in Hong Kong: A pilot study. Clin. Interv. Aging 2013, 8, 623–633. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ousset, P.J.; Viallard, G.; Puel, M.; Celsis, P.; Démonet, J.F.; Cardebat, D. Lexical Therapy and Episodic Word Learning in Dementia of the Alzheimer Type. Brain Lang. 2002, 80, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, M.; Hunter, E.M.; Van Der Hiele, K.; Angilletta, C. Computerized Structured Cognitive Training in Patients Affected by Early-Stage Alzheimer’s Disease is Feasible and Effective: A Randomized Controlled Study. Arch. Clin. Neuropsychol. 2016, 31, 868–876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nousia, A.; Siokas, V.; Aretouli, E.; Messinis, L.; Aloizou, A.-M.; Martzoukou, M.; Karala, M.; Koumpoulis, C.; Nasios, G.; Dardiotis, E. Beneficial Effect of Multidomain Cognitive Training on the Neuropsychological Performance of Patients with Early-Stage Alzheimer’s Disease. Neural Plast. 2018, 2018, 2845176. [Google Scholar] [CrossRef] [PubMed]
- Parlak, M.M.; Köse, A.; Güç, M.; Munis, B. Development of Mobile Compatible Software for Cognitive–Communication Disorder in Individuals with Alzheimer’s Disease. Int. J. Lang. Commun. Disord. 2023, 59, 234–254. [Google Scholar] [CrossRef] [PubMed]
- Noonan, K.A.; Pryer, L.R.; Jones, R.W.; Burns, A.S.; Lambon Ralph, M.A. A Direct Comparison of Errorless and Errorful Therapy for Object Name Relearning in Alzheimer’s Disease. Neuropsychol. Rehabil. 2012, 22, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Spironelli, C.; Bergamaschi, S.; Mondini, S.; Villani, D.; Angrilli, A. Functional Plasticity in Alzheimer’s Disease: Effect of Cognitive Training on Language-Related ERP Components. Neuropsychologia 2013, 51, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, M.; Cerulla, N.; Chico, G.; Quintana, M.; Garolera, M. Comparison of Neuropsychological and Functional Outcomes in Alzheimer’s Disease Patients with Good or Bad Response to a Cognitive Stimulation Treatment: A Retrospective Analysis. Int. Psychogeriatr. 2016, 28, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Tripathi, M.; Pandey, R.; Dey, A.B.; Nehra, A. Development and Validation of Cognitive Training Intervention for Alzheimer’s Disease (CTI-AD): A Picture-Based Interventional Program. Dementia 2018, 19, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Grabher, B.J. Effects of Alzheimer Disease on Patients and Their Family. J. Nucl. Med. Technol. 2018, 46, 335–340. [Google Scholar] [CrossRef]
- Kuca, K.; Maresova, P.; Klimova, B.; Valis, M.; Hort, J. Alzheimer’s disease and language impairments: Social intervention and medical treatment. Clin. Interv. Aging 2015, 10, 1401–1408. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef]
- Alzheimer’s Disease International. Alzheimer’s Disease International Website. Available online: https://www.alzint.org/ (accessed on 25 January 2024).
- Liampas, I.; Siokas, V.; Lyketsos, C.G.; Dardiotis, E. Associations between Neuropsychiatric Symptoms and Incident Alzheimer’s Dementia in Men Versus Women. J. Neurol. 2023, 270, 2069–2083. [Google Scholar] [CrossRef]
- Liampas, I.; Siokas, V.; Lyketsos, C.G.; Dardiotis, E. Cognitive Performance and Incident Alzheimer’s Dementia in Men Versus Women. J. Prev. Alzheimer’s Dis. 2023, 11, 162–170. [Google Scholar] [CrossRef]
- Morello, A.N.d.C.; Lima, T.M.; Brandão, L. Language and Communication Non-Pharmacological Interventions in Patients with Alzheimer’s Disease: A Systematic Review. Communication Intervention in Alzheimer. Dement. Neuropsychol. 2017, 11, 227–241. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Emergencias 2013, 2013, CD003260. [Google Scholar] [CrossRef]
- Kudlicka, A.; Martyr, A.; Bahar-Fuchs, A.; Sabates, J.; Woods, B.; Clare, L. Cognitive rehabilitation for people with mild to moderate dementia. Cochrane Database Syst. Rev. 2023, 2023, CD013388. [Google Scholar] [CrossRef]
- Hopper, T.; Bourgeois, M.; Pimentel, J.; Qualls, C.D.; Hickey, E.; Frymark, T.; Schooling, T. An Evidence-Based Systematic Review on Cognitive Interventions for Individuals With Dementia. Am. J. Speech-Lang. Pathol. 2013, 22, 126–145. [Google Scholar] [CrossRef]
- Krein, L.; Jeon, Y.-H.; Amberber, A.M.; Fethney, J. The Assessment of Language and Communication in Dementia: A Synthesis of Evidence. Am. J. Geriatr. Psychiatry 2019, 27, 363–377. [Google Scholar] [CrossRef]
- Bayles, K.; Tomoeda, C. ABCD: Arizona Battery for Communication Disorders of Dementia; Pro Education: Austin, TX, USA, 2005. [Google Scholar]
- Savage, S.; Hsieh, S.; Leslie, F.; Foxe, D.; Piguet, O.; Hodges, J.R. Distinguishing Subtypes in Primary Progressive Aphasia: Application of the Sydney Language Battery. Dement. Geriatr. Cogn. Disord. 2013, 35, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the Addenbrooke’s Cognitive Examination III in Frontotemporal Dementia and Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Groups | Participants per Group | Intervention |
|---|---|---|---|---|
| Ousset et al. (2002) [36] | France | AD 1 | 8 LT 2 group 8 OT 3 group | (LT 2) Naming sessions presented on a computer |
| Noonan et al. (2012) [40] | United Kingdtom | AD 1 | 8 | Naming sessions |
| Spitonelli et al. (2013) [41] | Italy | AD 1 | 11 | Tasks were presented by either paper and pencil or by a computer |
| Cavallo et al. (2016) [37] | Italy | AD 1 | 40 TG 4 40 CG 5 | Rehabilitative software Brainer1 |
| Martínez-Moreno et al. (2016) [42] | Spain | AD 1 | 60 | At the Outpatients Clinics in the Day Hospital (pen and paper tasks) |
| Nousia et al. (2018) [38] | Greece | AD 1 | 25 TG 4 25 CG 5 | 1st part = 30 min. computer-based intervention 2nd part = 30 min. exercises with paper and pencil |
| Bajpai et al. (2020) [43] | India | AD 1 | 15 | AD 1: tasks with a trained caregiver |
| Parlak et al. (2023) [39] | Turkey | AD 1 | 16 TG 4 16 CG 5 | Computer-supported application (software) at home |
| Study | N 1 | AD 2 Type | Diagnostic Criteria | CDR 3 | GDS 4 Mean (±SD) | Gender (Males/Total) | Age in Years [Mean (SD 5)] | Education in Years [Mean (SD 5)] | Pharmacological Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Ousset et al. (2002) [36] | 8 AD 2 (LT 6) | Probable AD 2 | NINCDS-ADRDA 8 | - | - | 5/8 | 67.7 ± 12.9 | - | Cholinergic medication |
| 8 AD 2 (OT 7) | 3/8 | 73.8 ± 7.5 | - | ||||||
| Noonan et al. (2012) [40] | 8 AD 2 | Probable AD 2 | NINCDS-ADRDA 8 | - | - | - | - | - | - |
| Spironelli et al. (2013) [41] | 11 AD 2 | Mild-to-moderate AD 2 | NINCDS-ADRDA 8 | - | - | 2/11 | 78.18 (±4.99) Range = 70–88 | 7.54 (±3.59) | Anticolinesterasic drugs |
| Cavallo et al. (2016) [37] | 40 AD 2 (TG 9) | Early stage probable AD 2 | NINCDS-ADRDA 8 | - | - | 13/40 | 76.50 ± 2.88 | 8.53 ± 3.00 | Acetylcholinesterase inhibitors (36/40) |
| 40 AD 2 (CG 10) | 16/40 | 76.33 ± 3.83 | 8.12 ± 2.79 | Acetylcholinesterase inhibitors (38/40) | |||||
| Martínez-Morenoetal. (2016) [42] | 60 AD 2 | Probable AD 2 (mild stage) | NINCDS-ADRDA 8 | - | Mild stage | 25/60 | 75 ± 6.35 Range = 58–92 | Type 1 11 = 37 (62%) Type 2 12 = 13 (21%) Type 3 13 = 7 (12%) Type 4 14 = 1 (2%) Type 5 15 = 0 (0%) Type 6 16 = 2 (3%) | No ChEIs 17 = 27 (45%) ChEIs 17 = 33 (55%) |
| Nousia et al. (2018) [38] | 25AD 2 (TG 9) | Mild (early stage) AD 2 | NINCDS-ADRDA 8 | 1 | 2.40 (±1.61) | 9/25 | 76.24 (±5.14) | 8.08 (±3.01) | - |
| 25 AD 2 (CG 10) | 3.28 (±2.30) | 5/25 | 76.32 (±5.38) | 8.92 (±2.83) | - | ||||
| Bajpai et al. (2020) [43] | 15 AD 2 | Early AD 2 | NINCDS 8 | 1 | ≤8 | 9/15 | 60–69: 4/15 (26.7%) 70–79: 7/15 (46.7%) 80–89: 4/15 (26.7%) | 0–5: 0/15 (0.0%) 6–9: 1/15 (6.7%) 10–12: 5/15 (33.3%) ≥13: 9/15 (60.0%) | - |
| Parlak et al. (2023) [39] | 16 AD 2 (TG 9) | 6 mild, 6 moderate, 4 severe | DSM-5 18 & NIA-AA 19 | - | - | 7/16 | 75.00 ± 6.38 | 3.19 ± 2.90 | Acetylcholinesterase inhibitors for at least 3 months |
| 16 AD 2 (CG 10) | 6 mild, 6 moderate, 4 severe | 6/16 | 74.63 ± 6.60 | 3.19 ± 2.31 |
| Study | Other Cognitive Domains | Language Domains | Duration of Sessions | Quality of Life |
|---|---|---|---|---|
| Ousset et al. (2002) [36] | - | Lexical Therapy (naming sessions) | 5 months—45 min/session (i) 8 sessions (one session per week) (ii) 2 weeks off (iii) 8 sessions (one session per week) | - |
| Noonan et al. (2012) [40] | - | Name relearning | 10 sessions (participants were seen twice a week over a period of 5 weeks), each lasting between 40 and 60 min | - |
| Spironelli et al. (2013) [41] | Spatial and temporal orientation, attention, memory, logic reasoning, praxis and arithmetic skill | Language | 2 h/day and 4 days/week for 5 weeks + daily living activities (answering a phone call and remembering the message, or reading the newspaper and commenting the news of the day) | The experience of working together encouraged the sense of responsibility of patients with higher cognitive functioning for supporting those with more severe deficits when all participants carried out different everyday activities and tasks |
| Cavallo et al. (2016) [37] | Memory, attention, executive function | Language | Three 30 min sessions per week, for 12 weeks | Τhe comparison of patients’ scores on the HADS 1 did not show any statistically significant difference (anxiety: patients’ score = 7.65 ± 2.41, controls’ score = 7.57 ± 1.33; depression: patients’ score = 6.42 ± 2.21, controls’ score = 6.35 ± 2.21) |
| Martínez-Moreno et al. (2016) [42] | Reality orientation, memory, executive functions, activities of daily living training | Language (1. discussing actual information of interest, 2. tasks involving reading, oral, and written comprehension and writing, 3. communication between participants) | 1 year group program (10–12 patients per group) of two to three weekly sessions (mean 115 sessions/year) of cognitive stimulation and occupational therapy of 2 or 3 h each | Functional capacity in the follow-up after the treatment showed that Responders had a better performance of IADL 2 |
| Nousia et al. (2018) [38] | Episodic and delayed memory, attention, processing speed, and executive function | Morphology, syntax, semantics, naming, verbal fluency, and word recall | 15 weeks 2 days/week 60 min/session +extra cognitive and language tasks for practice at home, in a weekly basis | The training group had verbal positive feedback on daily activities and functional communication |
| Bajpai et al. (2020) [43] | Memory (picture recognition task) Attention (spot the differences task) | Verbal learning task | 3 tasks per day (30–45 min) for 8 weeks | - |
| Parlak et al. (2023) [39] | Orientation, reminiscence, executive functions, short-term memory, attention and visual spatial functions, communication board | Language (functional expressions, naming and and showing what is said) | 1 h each day for 3 days a week (app sections) and 30 min. each day for 2 days (reminiscence section) Total 5 days/week for 7 weeks | Statements from patients’ caregivers regarding better functioning in everyday life |
| Study | Outcome Measures | p Value | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Ousset et al. (2002) [36] | LT group (mean ± SD) | OT group (mean ± SD) | ||||||
| Naming Hits | Pre | Post | Pre | Post | ||||
| Narrative LT items | 31.6 ± 3.7 | 33.6 ± 3.1 | Narrative LT items | 32.5 ± 2.8 | 31.4 ± 2.9 | |||
| LT items | 30.1 ± 3.2 | 31.7 ± 4 | LT i tems | 28.1 ± 4 | 28.9 ± 3.5 | |||
| External items | 22.9 ± 6.1 | 24.6 ± 8 | External items | 23.7 ± 3.5 | 21.9 ± 4.9 | |||
| Naming Errors | Pre | Post | Pre | Post | ||||
| Absence of production | 14.7 ± 8.1 | 13.6 ± 8.3 | Absence of production | 16.7 ± 7 | 20.5 ± 7.3 | |||
| Semantic errors | 14.6 ± 5.1 | 9.9 ± 4.8 | Semantic errors | 11.7 ± 5.1 | 12.4 ± 4.6 | |||
| Perceptual errors | 6 ± 4.3 | 6.5 ± 7.3 | Perceptual errors | 6.6 ± 4.1 | 5.1 ± 3.8 | |||
| Noonan et al. (2012) [40] | Week 1 post-therapy | Week 5 post-therapy | ||||||
| Picture version of the Pyramids and Palm Trees Test | r = 0.81, p = 0.01 | |||||||
| Boston Naming Test | r = 0.67, p = 0.071 | |||||||
| 100-item naming test | r = 0.67, p = 0.066 | r = 0.68, p = 0.062 | ||||||
| 64-item word-picture matching task | r = 0.65, p = 0.076 | |||||||
| Forward digit span | r = 0.69, p = 0.057 | |||||||
| Camden Recognition Memory for Faces Test | r = 0.62, p = 0.09 | |||||||
| Elevator Counting with Distraction | r = –0.7, p = 0.077 | |||||||
| Spironelli et al. (2013) [41] | Pre-treatment | Post-treatment | ||||||
| MMSE | 22.09 ± 0.58 | 21.73 ± 0.69 | ||||||
| MODA | 76.26 ± 1.85 | 77.62 ± 1.98 | ||||||
| ENB-2: M. I. -10 s | 2.45 | 2.82 | ||||||
| ENB-2: S.R.-I. | 4.64 | 5.09 | ||||||
| ENB-2: Abs. | 3.45 | 4.18 | 0.05 | |||||
| ENB-2: Flu. | 7.64 | 8.40 | ||||||
| ENB-2: Over. Figure | 17.09 | 17.36 | ||||||
| RTs for LF words | 921.02 ± 56.94 ms | 1062.85 ± 67.34 ms | ||||||
| RTs for HF words | 845.69 ± 48.54 ms | 973.00 ± 59.80 ms | ||||||
| RTs | 1008.83 ± 73.17 ms | 757.88 ± 48.78 ms | <0.1 | |||||
| Cavallo et al. (2016) [37] | TG Pre (mean ± SD) | TG 6-month follow up (mean ± SD) | CG Pre (mean ± SD) | CG 6-month follow up (mean ± SD) | ||||
| MMSE | 22.65 ± 1.74 | 22.32 ± 0.97 | 23.05 ± 2.44 | 22.64 ± 0.96 | ||||
| DSF | 4.85 ± 1.60 | 5.95 ± 1.80 | 5.20 ± 1.85 | 5.18 ± 1.82 | ||||
| DSB | 3.20 ± 1.26 | 5.78 ± 1.44 | 4.10 ± 0.63 | 4.02 ± 0.88 | ||||
| Two-syllables word test | 4.80 ± 1.72 | 6.14 ± 1.42 | 6.00 ± 2.15 | 5.05 ± 2.15 | ||||
| RBMT (standardized profile score) | 8.60 ± 1.12 | 8.60 ± 1.12 | 8.80 ± 1.36 | 8.80 ± 1.36 | ||||
| RBMT (story immediate) | 6.72 ± 1.09 | 8.72 ± 1.24 | 7.04 ± 1.66 | 6.00 ± 1.41 | ||||
| RBMT (story delayed) | 5.35 ± 1.73 | 6.35 ± 1.73 | 6.52 ± 1.66 | 4.52 ± 1.44 | ||||
| GNT | 21.95 ± 2.57 | 22.04 ± 2.53 | 22.15 ± 2.17 | 22.18 ± 2.27 | ||||
| Token test | 30.30 ± 2.42 | 32.30 ± 2.42 | 30.69 ± 2.10 | 27.69 ± 2.10 | ||||
| VOSP (object decision) | 18.20 ± 0.72 | 18.25 ± 0.93 | 18.42 ± 0.81 | 18.45 ± 0.81 | ||||
| VOSP (position discrimination) | 19.22 ± 0.70 | 19.15 ± 0.74 | 19.29 ± 0.72 | 19.22 ± 0.70 | ||||
| VOSP (number location) | 8.87 ± 0.69 | 8.85 ± 0.58 | 9.00 ± 0.68 | 9.02 ± 0.62 | ||||
| Verbal fluency (letters) | 35.88 ± 2.66 | 36.57 ± 2.46 | 36.52 ± 2.45 | 37.35 ± 2.26 | ||||
| Verbal fluency (category) | 17.10 ± 1.88 | 16.27 ± 1.71 | 17.27 ± 1.76 | 15.95 ± 1.60 | ||||
| Hayling test (overall score) | 5.82 ± 1.24 | 5.42 ± 0.98 | 5.95 ± 1.15 | 5.37 ± 0.86 | ||||
| Brixton test | 4.95 ± 0.85 | 5.95 ± 1.34 | 5.22 ± 1.32 | 3.82 ± 1.65 | ||||
| Martínez-Moreno et al. (2016) [42] | R-Pre (mean ± SD) | NR-Pre (mean ± SD) | R-Post (mean ± SD) | NR-Post (mean ± SD) | ||||
| Person orientation | 55.76 ± 15.31 | 49.04 ± 18.92 | 0.16 | 54.55 ± 16.71 | 46.66 ± 18.18 | 0.086 | ||
| Space orientation | 46.52 ± 17.02 | 41.76 ± 18.16 | 0.33 | 58.29 ± 13.99 | 52.28 ± 19.67 | 0.18 | ||
| Time orientation | 32.03 ± 16.25 | 36 ± 19.04 | 0.41 | 40.52 ± 20.26 | 28.31 ± 18.40 | 0.018 | ||
| DSF | 49.87 ± 10.12 | 43.64 ± 8.7 | 0.015 | 47.06 ± 9.38 | 43.81 ± 8.06 | 0.17 | ||
| DSB | 46.97 ± 8.99 | 40.52 ± 10.99 | 0.018 | 46.17 ± 8.89 | 43.73 ± 9.9 | 0.34 | ||
| List learning | 27.65 ± 10.86 | 24.48 ± 9.9 | 0.28 | 29.24 ± 11.38 | 26.72 ± 11.86 | 0.45 | ||
| Story memory | 24.44 ± 10.47 | 19.04 ± 8.23 | 0.048 | 23.16 ± 12.13 | 19.72 ± 9.37 | 0.27 | ||
| List learning free recall | 19.64 ± 13.31 | 19.08 ± 13.68 | 0.88 | 16.32 ± 13.23 | 17.26 ± 14.33 | 0.81 | ||
| List learning recognition | 19.04 ± 9.45 | 15.88 ± 8.92 | 0.23 | 21.16 ± 14.63 | 19.44 ± 11.93 | 0.65 | ||
| Story free recall | 14.96 ± 7.58 | 16.20 ± 8.82 | 0.60 | 17 ± 9.48 | 16.04 ± 8.89 | 0.72 | ||
| Figure free recall | 27.96 ± 13.68 | 22.20 ± 12.92 | 0.13 | 25.76 ± 15.65 | 22.04 ± 14.88 | 0.40 | ||
| Visuoverbal naming | 41.76 ± 16.71 | 31.81 ± 15.95 | 0.027 | 39.48 ± 14.82 | 31.13 ± 15.07 | 0.056 | ||
| Constructional praxis | 47 ± 16.31 | 40.75 ± 17.45 | 0.20 | 47.96 ± 14.64 | 40.42 ± 17.45 | 0.11 | ||
| Category evocation | 37.59 ± 9.99 | 32.12 ± 9.78 | 0.1 | 36.79 ± 10.65 | 30.81 ± 10.96 | 0.045 | ||
| MMSE | 22.84 ± 3.37 | 22.79 ± 4.4 | 0.96 | 25.23 ± 3.22 | 20 ± 4.38 | 0.001 | ||
| BI | 95.97 ± 3.96 | 93.97 ± 7.24 | 0.19 | 92.58 ± 7.29 | 89.66 ± 9.06 | 0.17 | ||
| IADL | 5.13 ± 1.67 | 4.52 ± 1.92 | 0.19 | 4.68 ± 1.49 | 3.72 ± 1.96 | 0.038 | ||
| Nousia et al. (2018) [38] | TG—pre | TG—post | p value | CG—pre | CG—post | p value | p value (TG post- CG post) | |
| Recall | 17.44 ± 3.66 | 18.16 ± 3.48 | 0.151 | 16.60 ± 3.26 | 16.20 ± 2.45 | 0.33 | 0.887 | |
| Delayed memory | 0.16 ± 0.37 | 1.20 ± 1.08 | ≤0.001 | 0.40 ± 0.50 | 0.12 ± 0.33 | 0.08 | ≤0.001 | |
| Word recognition | 18.08 ± 1.32 | 18.68 ± 1.28 | 0.028 | 18.40 ± 1.25 | 17.96 ± 1.48 | 0.20 | 0.008 | |
| BNT | 11.84 ± 1.57 | 13.40 ± 1.04 | ≤0.001 | 11.64 ± 1.32 | 11.40 ± 1.30 | 0.22 | ≤0.001 | |
| SF | 22.12 ± 6.05 | 28.16 ± 6.08 | ≤0.001 | 23.36 ± 7.44 | 22.16 ± 6.31 | 0.13 | ≤0.001 | |
| CDT | 8.96 ± 2.22 | 10.28 ± 2.59 | 0.01 | 9.72 ± 1.93 | 9.52 ± 1.36 | 0.24 | ≤0.001 | |
| DSF | 5.48 ± 0.71 | 6.60 ± 1.35 | ≤0.001 | 5.04 ± 0.93 | 4.88 ± 1.13 | 0.35 | ≤0.001 | |
| DSB | 3.68 ± 0.75 | 4.32 ± 0.75 | 0.001 | 3.36 ± 0.81 | 3.32 ± 0.98 | 0.80 | 0.004 | |
| TMT A | 177.24 ± 45.88 | 151.80 ± 39.48 | ≤0.001 | 177.56 ± 56.02 | 210.16 ± 66.58 | 0.01 | ≤0.001 | |
| TMT B | 300 ± 00.00 | 290.60 ± 24.67 | 0.017 | 297.84 ± 10.80 | 299.00 ± 5.00 | 0.32 | 0.003 | |
| Week 1 | Week 8 | |||||||
| Bajpai et al. (2020) [43] | Memory | 48.5 ± 22.9 s | 60.5 ± 21.8 s | |||||
| Attention | 216.6 ± 78.2 s | 286.8 ± 87.0 s | ||||||
| Language | 211.8 ± 68.4 s | 270.4 ± 104.9 s | ||||||
| Parlak et al. (2023) [39] | TG pre (mean ± SD) | TG post (mean ± SD) | p value (TG pre-TG post) | CG pre (mean ± SD) | CG post (mean ± SD) | p value (GG pre-CG post) | p value (TG -CG post) | |
| MMSE | ||||||||
| Orientation | 5.19 ± 1.97 | 6.81 ± 2.48 | <0.001 | 4.56 ± 2.15 | 4.25 ± 2.11 | 0.096 | 0.004 | |
| Registration | 2.50 ± 0.89 | 2.81 ± 0.54 | 0.136 | 2.38 ± 1.08 | 2.69 ± 0.60 | 0.173 | 0.542 | |
| Attention and Calculation | 1.81 ± 1.90 | 2.50 ± 2.36 | 0.007 | 2.75 ± 1.98 | 2.25 ± 1.94 | 0.015 | 0.747 | |
| Recall | 0.20 ± 0.41 | 0.20 ± 0.41 | 1.000 | 0.25 ± 0.57 | 0.13 ± 0.50 | 0.164 | 0.654 | |
| Language | 5.69 ± 2.024 | 7.25 ± 1.06 | 0.001 | 6.19 ± 1.37 | 6.00 ± 1.50 | 0.383 | 0.011 | |
| Total | 15.38 ± 5.80 | 19.56 ± 5.76 | <0.001 | 16.13 ± 5.65 | 15.19 ± 5.46 | 0.055 | 0.035 | |
| LATA | ||||||||
| Speech fluency | 23.69 ± 5.33 | 26.06 ± 4.69 | <0.001 | 24.38 ± 7.38 | 22.81 ± 7.54 | 0.001 | 0.154 | |
| Auditory comprehension | 43.81 ± 17.10 | 52.81 ± 14.88 | <0.001 | 44.88 ± 13.58 | 44.31 ± 11.97 | 0.771 | 0.085 | |
| Repetition | 13.38 ± 4.74 | 16.00 ± 4.14 | 0.001 | 14.81 ± 4.94 | 13.56 ± 3.03 | 0.083 | 0.067 | |
| Naming | 34.44 ± 11.62 | 37.38 ± 9.59 | 0.009 | 33.25 ± 8.50 | 32.63 ± 7.88 | 0.574 | 0.136 | |
| Reading | 31.36 ± 13.60 | 34.73 ± 13.52 | 0.007 | 32.91 ± 14.50 | 30.82 ± 15.05 | 0.006 | 0.529 | |
| Grammar | 12.25 ± 4.83 | 15.06 ± 5.06 | <0.001 | 11.56 ± 5.27 | 10.31 ± 3.96 | 0.091 | 0.006 | |
| Word actions | 15.50 ± 5.06 | 17.69 ± 4.42 | <0.001 | 16.00 ± 4.39 | 15.13 ± 3.96 | 0.084 | 0.095 | |
| Writing | 22.73 ± 16.52 | 25.73 ± 15.08 | 0.081 | 25.00 ± 14.58 | 24.73 ± 14.67 | 0.341 | 0.876 | |
| Total | 198.63 ± 76.11 | 223.18 ± 70.43 | 0.001 | 211.72 ± 68.83 | 203.54 ± 58.59 | 0.210 | 0.485 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, N.K.; Nousia, A.; Georgopoulou, E.-N.; Martzoukou, M.; Liampas, I.; Dardiotis, E.; Nasios, G. Language and Communication Interventions in People with Alzheimer’s Disease: A Systematic Review. Healthcare 2024, 12, 741. https://doi.org/10.3390/healthcare12070741
Dimitriou NK, Nousia A, Georgopoulou E-N, Martzoukou M, Liampas I, Dardiotis E, Nasios G. Language and Communication Interventions in People with Alzheimer’s Disease: A Systematic Review. Healthcare. 2024; 12(7):741. https://doi.org/10.3390/healthcare12070741
Chicago/Turabian StyleDimitriou, Nefeli K., Anastasia Nousia, Eleni-Nefeli Georgopoulou, Maria Martzoukou, Ioannis Liampas, Efthimios Dardiotis, and Grigorios Nasios. 2024. "Language and Communication Interventions in People with Alzheimer’s Disease: A Systematic Review" Healthcare 12, no. 7: 741. https://doi.org/10.3390/healthcare12070741
APA StyleDimitriou, N. K., Nousia, A., Georgopoulou, E.-N., Martzoukou, M., Liampas, I., Dardiotis, E., & Nasios, G. (2024). Language and Communication Interventions in People with Alzheimer’s Disease: A Systematic Review. Healthcare, 12(7), 741. https://doi.org/10.3390/healthcare12070741