Mobile Apps for Patients with Peritoneal Dialysis: Systematic App Search and Evaluation
Abstract
1. Introduction
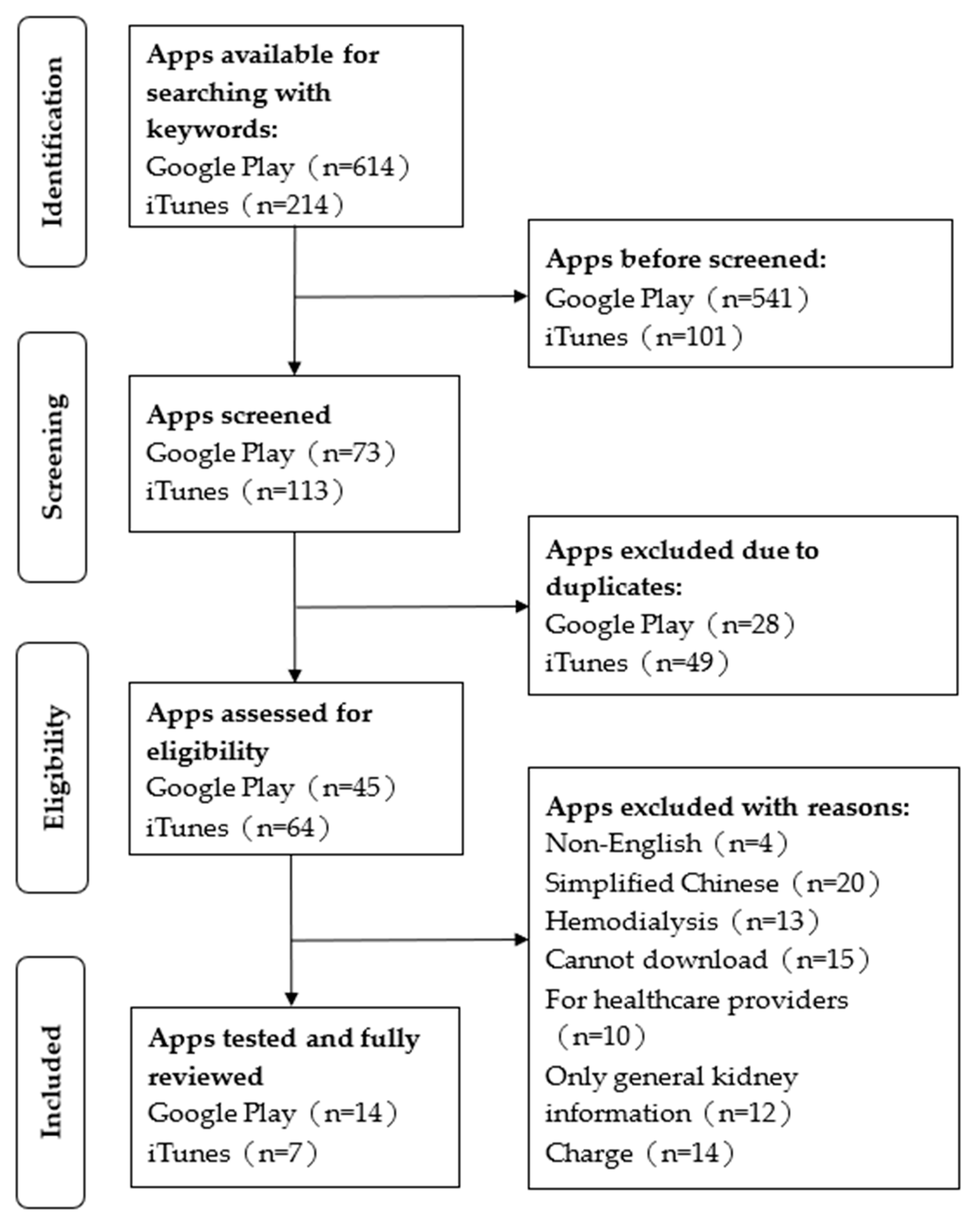
2. Materials and Methods
2.1. Study Design
2.2. App Search Strategy
2.3. Selection Criteria
2.4. Data Extraction
2.5. Measures of Rating Instrument
2.6. Comprehensive Self-Management of PD
2.7. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Privacy and Security Features
3.3. MARS App Quality Scores
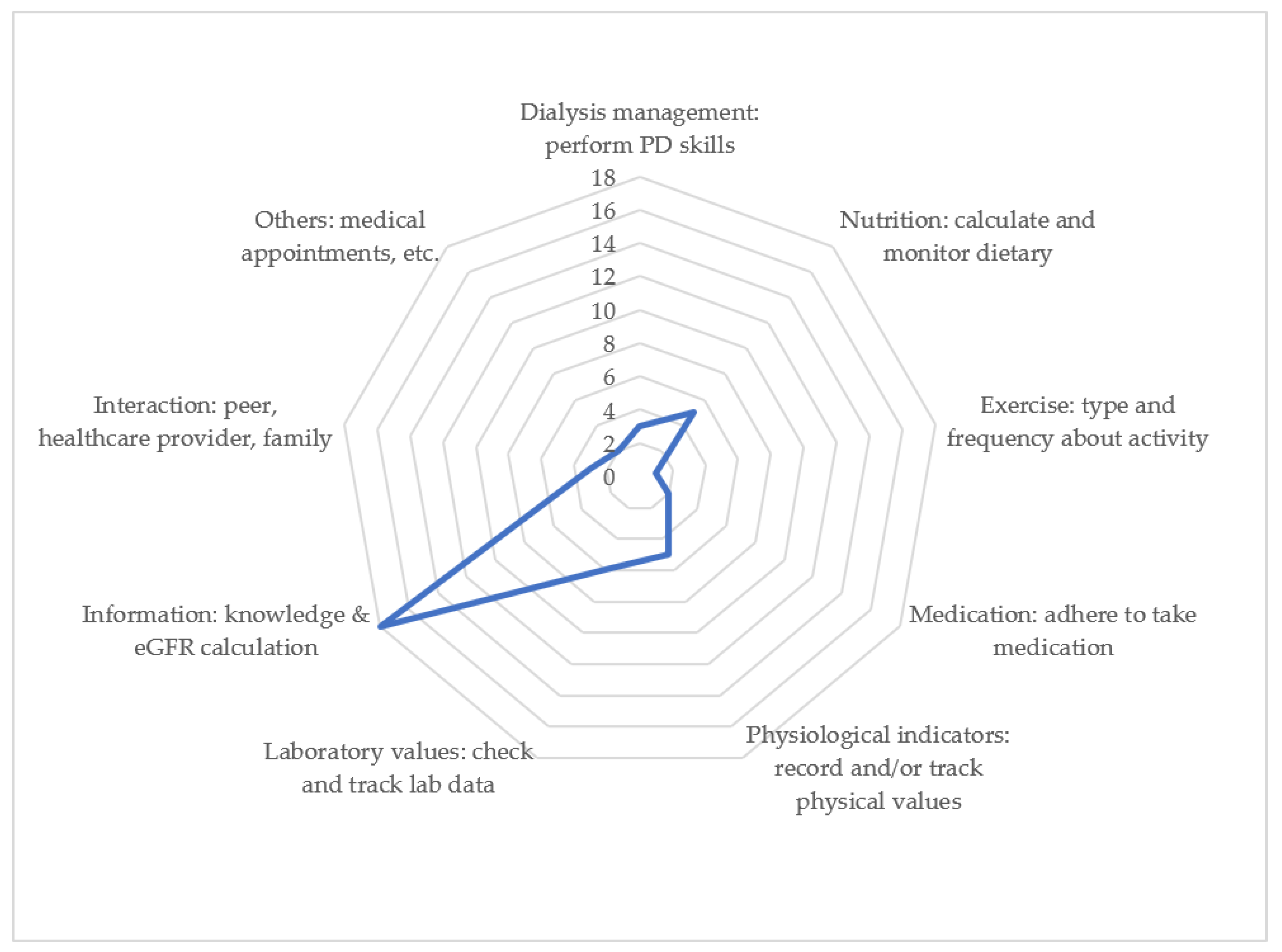
3.4. Comprehensive Self-Management of PD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, J.C.; Zhang, L.X. Prevalence and disease burden of chronic kidney disease. In Renal Fibrosis: Mechanisms and Therapies; Springer: Singapore, 2019; pp. 3–15. [Google Scholar]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.R. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- National Kidney Foundation. Treatment and Support; National Kidney Foundation: New York, NY, USA, 2023. [Google Scholar]
- Brown, E.A.; Blake, P.G.; Boudville, N.; Davies, S.; de Arteaga, J.; Dong, J.; Finkelstein, F.; Foo, M.; Hurst, H.; Johnson, D.W. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit. Dial. Int. 2020, 40, 244–253. [Google Scholar] [CrossRef]
- Cartwright, E.J.; ZSGoh, Z.; Foo, M.; Chan, C.M.; Htay, H.; Griva, K. eHealth interventions to support patients in delivering and managing peritoneal dialysis at home: A systematic review. Perit. Dial. Int. 2021, 41, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Chen, H.H.; Wu, M.J.; Hsu, B.G.; Tsai, J.C.; Kuo, C.C.; Lin, S.P.; Chen, T.H.; Sue, Y.M. Out-of-pocket costs and productivity losses in haemodialysis and peritoneal dialysis from a patient interview survey in Taiwan. BMJ Open 2019, 9, e023062. [Google Scholar] [CrossRef] [PubMed]
- Piraino, B. Innovations in Treatment Delivery, Risk of Peritonitis, and Patient Retention on Peritoneal Dialysis. Semin. Dial. 2017, 30, 158–163. [Google Scholar] [CrossRef]
- Hsu, C.C. Kidney Disease in Taiwan 2020; National Health Research Institutes & Taiwan Society of Nephrology: Zhunan, Taiwan, 2021. [Google Scholar]
- Karopadi, A.N.; Mason, G.; Rettore, E.; Ronco, C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol. Dial. Transplant. 2013, 28, 2553–2569. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, M.F.; Kalnicki, M.; Lindenauer, S. Valuable features in mobile health apps for patients and consumers: Content analysis of apps and user ratings. JMIR Mhealth Uhealth 2015, 3, e4283. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, L.; Jain, A.K.; Man, R.H.; Onabajo, N.; Desveaux, L.; Shaw, J.; Hensel, J.; Agarwal, P.; Saragosa, M.; Jamieson, T. Exploring the utility and scalability of a telehomecare intervention for patients with chronic kidney disease undergoing peritoneal dialysis—A study protocol. BMC Nephrol. 2017, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. mHealth: New Horizons for Health through Mobile Technologies; World Health Organization: Geneva, Switzerland, 2011.
- Doyle, N.; Murphy, M.; Brennan, L.; Waugh, A.; McCann, M.; Mellotte, G. The “Mikidney” smartphone app pilot study: Empowering patients with Chronic Kidney Disease. J. Ren. Care 2019, 45, 133–140. [Google Scholar] [CrossRef]
- Fan, K.; Zhao, Y. Mobile health technology: A novel tool in chronic disease management. Intell. Med. 2022, 2, 41–47. [Google Scholar] [CrossRef]
- Free, C.; Phillips, G.; Galli, L.; Watson, L.; Felix, L.; Edwards, P.; Patel, V.; Haines, A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med. 2013, 10, e1001362. [Google Scholar] [CrossRef]
- Siddique, A.B.; Krebs, M.; Alvarez, S.; Greenspan, I.; Patel, A.; Kinsolving, J.; Koizumi, N. Mobile apps for the care management of chronic kidney and end-stage renal diseases: Systematic search in app stores and evaluation. JMIR Mhealth Uhealth 2019, 7, e12604. [Google Scholar] [CrossRef]
- Yi, J.Y.; Kim, Y.; Cho, Y.M.; Kim, H. Self-management of chronic conditions using mHealth interventions in Korea: A systematic review. Healthc. Inform. Res. 2018, 24, 187–197. [Google Scholar] [CrossRef]
- Lee, Y.L.; Cui, Y.Y.; Tu, M.H.; Chen, Y.C.; Chang, P. Mobile health to maintain continuity of patient-centered care for chronic kidney disease: Content analysis of apps. JMIR Mhealth Uhealth 2018, 6, e10173. [Google Scholar] [CrossRef]
- World Health Organization. mhealth Use of Appropriate Digital Technologies for Public Health; World Health Organization: Geneva, Switzerland, 2018.
- Yang, Y.; Chen, H.; Qazi, H.A.; Morita, P.P. Intervention and Evaluation of Mobile Health (mHealth) Technologies in Chronic Dialysis Patient Management: A Scoping Review. JMIR Mhealth Uhealth 2020, 8, e15549. [Google Scholar] [CrossRef]
- Dey, V.; Jones, A.; Spalding, E.M. Telehealth: Acceptability, clinical interventions and quality of life in peritoneal dialysis. SAGE Open Med. 2016, 4, 2050312116670188. [Google Scholar] [CrossRef]
- Quach, S.; Benoit, A.; Oliveira, A.; Packham, T.L.; Goldstein, R.; Brooks, D. Features and characteristics of publicly available mHealth apps for self-management in chronic obstructive pulmonary disease. Digit. Health 2023, 9, 20552076231167007. [Google Scholar] [CrossRef]
- Appiah, B.; Kretchy, I.A.; Yoshikawa, A.; Asamoah-Akuoko, L.; France, C.R. Perceptions of a mobile phone-based approach to promote medication adherence: A cross-sectional application of the technology acceptance model. Explor. Res. Clin. Soc. Pharm. 2021, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. More Active People for a Healthier World Global Action Plan on Physical Activity 2018–2030; World Health Organization: Geneva, Switzerland, 2019.
- Biebuyck, G.K.; Neradova, A.; de Fijter, C.W.; Jakulj, L. Impact of telehealth interventions added to peritoneal dialysis-care: A systematic review. BMC Nephrol. 2022, 23, 292. [Google Scholar] [CrossRef] [PubMed]
- Lunney, M.; Lee, R.; Tang, K.; Wiebe, N.; Bello, A.K.; Thomas, C.; Rabi, D.; Tonelli, M.; James, M.T. Impact of telehealth interventions on processes and quality of care for patients with ESRD. Am. J. Kidney Dis. 2018, 72, 592–600. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; McCann, M.; Brady, A.M. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 5, CD011425. [Google Scholar] [CrossRef]
- Belisario, J.S.M.; Huckvale, K.; Greenfield, G.; Car, J.; Gunn, L.H. Smartphone and tablet self management apps for asthma. Cochrane Database Syst. Rev. 2013, 2013, CD010013. [Google Scholar] [CrossRef] [PubMed]
- Creber, R.M.M.; Maurer, M.S.; Reading, M.; Hiraldo, G.; Hickey, K.T.; Iribarren, S. Review and analysis of existing mobile phone apps to support heart failure symptom monitoring and self-care management using the Mobile Application Rating Scale (MARS). JMIR Mhealth Uhealth 2016, 4, e5882. [Google Scholar]
- Paganini, S.; Meier, E.; Terhorst, Y.; Wurst, R.; Hohberg, V.; Schultchen, D.; Strahler, J.; Wursthorn, M.; Baumeister, H.; Messner, E.-M. Stress Management Apps: Systematic Search and Multidimensional Assessment of Quality and Characteristics. JMIR Mhealth Uhealth 2023, 11, e42415. [Google Scholar] [CrossRef] [PubMed]
- Opipari-Arrigan, L.; Dykes, D.M.; Saeed, S.A.; Thakkar, S.; Burns, L.; Chini, B.A.; McPhail, G.L.; Eslick, I.; Margolis, P.A.; Kaplan, H.C. Technology-Enabled Health Care Collaboration in Pediatric Chronic Illness: Pre-Post Interventional Study for Feasibility, Acceptability, and Clinical Impact of an Electronic Health Record–Linked Platform for Patient-Clinician Partnership. JMIR Mhealth Uhealth 2020, 8, e11968. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Van Der Kleij, R.M.; van der Boog, P.J.; Chang, X.; Chavannes, N.H. Electronic health self-management interventions for patients with chronic kidney disease: Systematic review of quantitative and qualitative evidence. J. Med. Internet Res. 2019, 21, e12384. [Google Scholar] [CrossRef] [PubMed]
- Lukkanalikitkul, E.; Kongpetch, S.; Chotmongkol, W.; Morley, M.G.; Anutrakulchai, S.; Srichan, C.; Thinkhamrop, B.; Chunghom, T.; Wiangnon, P.; Thinkhamrop, W. Optimization of the Chronic Kidney Disease–Peritoneal Dialysis App to Improve Care for Patients on Peritoneal Dialysis in Northeast Thailand: User-Centered Design Study. JMIR Form. Res. 2022, 6, e37291. [Google Scholar] [CrossRef]
- Bennett, P.N.; Bohm, C.; Harasemiw, O.; Brown, L.; Gabrys, I.; Jegatheesan, D.; Johnson, D.W.; Lambert, K.; Lightfoot, C.J.; MacRae, J. Physical activity and exercise in peritoneal dialysis: International Society for Peritoneal Dialysis and the Global Renal Exercise Network practice recommendations. Perit. Dial. Int. 2022, 42, 8–24. [Google Scholar] [CrossRef]
- Markossian, T.W.; Boyda, J.; Taylor, J.; Etingen, B.; Modave, F.; Price, R.; Kramer, H.J. A mobile app to support self-management of chronic kidney disease: Development study. JMIR Hum. Factors 2021, 8, e29197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Bao, J.; Setiawan, I.M.A.; Saptono, A.; Parmanto, B. The mHealth App Usability Questionnaire (MAUQ): Development and validation study. JMIR Mhealth Uhealth 2019, 7, e11500. [Google Scholar] [CrossRef]
- Gross, G.; Lull, C.; von Ahnen, J.; Olsavszky, V.; Knitza, J.; Schmieder, A.; Leipe, J. German Mobile Apps for Patients with Psoriatic Arthritis: Systematic App Search and Content Analysis. Health Policy Technol. 2022, 11, 100697. [Google Scholar] [CrossRef]
- Ryan, S.; Chasaide, N.N.; O’Hanrahan, S.; Corcoran, D.; Caulfield, B.; Argent, R. mHealth Apps for musculoskeletal rehabilitation: Systematic search in APP stores and content analysis. JMIR Rehabil. Assist. Technol. 2022, 9, e34355. [Google Scholar] [CrossRef] [PubMed]
- StatCounter. Mobile Operating System Market Share Worldwide. 2023. Available online: https://gs.statcounter.com/os-market-share/mobile/worldwide (accessed on 31 January 2024).
- Nguyen, A.; Eschiti, V.; Bui, T.C.; Nagykaldi, Z.; Dwyer, K. Mobile Health Interventions to Improve Health Behaviors and Healthcare Services among Vietnamese Individuals: A Systematic Review. Healthcare 2023, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Ronco, C.; Karopadi, A.N.; Rosner, M.H. Telemedicine and remote monitoring: Supporting the patient on peritoneal dialysis. Perit. Dial. Int. 2016, 36, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Heinsch, M.; Betts, D.; Booth, D.; Kay-Lambkin, F. Barriers and facilitators to the use of e-health by older adults: A scoping review. BMC Public Health 2021, 21, 1556. [Google Scholar] [CrossRef]
- Zhou, L.; Bao, J.; Watzlaf, V.; Parmanto, B. Barriers to and facilitators of the use of mobile health apps from a security perspective: Mixed-methods study. JMIR Mhealth Uhealth 2019, 7, e11223. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile app rating scale: A new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth 2015, 3, e3422. [Google Scholar] [CrossRef]
- Messner, E.-M.; Sturm, N.; Terhorst, Y.; Sander, L.B.; Schultchen, D.; Portenhauser, A.; Schmidbaur, S.; Stach, M.; Klaus, J.; Baumeister, H. Mobile Apps for the Management of Gastrointestinal Diseases: Systematic Search and Evaluation within App Stores. J. Med. Internet Res. 2022, 24, e37497. [Google Scholar] [CrossRef]
- Bardus, M.; van Beurden, S.B.; Smith, J.R.; Abraham, C. A review and content analysis of engagement, functionality, aesthetics, information quality, and change techniques in the most popular commercial apps for weight management. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 35. [Google Scholar] [CrossRef]
- Hee Ko, K.K.; Kim, S.K.; Lee, Y.; Lee, J.Y.; Stoyanov, S.R. Validation of a Korean version of mobile app rating scale (MARS) for apps targeting disease management. Health Inform. J. 2022, 28, 14604582221091975. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.; Siek, K.A.; Chaudry, B.; Jones, J.; Astroth, K.; Welch, J.L. An offline mobile nutrition monitoring intervention for varying-literacy patients receiving hemodialysis: A pilot study examining usage and usability. J. Am. Med. Inform. Assoc. 2012, 19, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Miró, J.; Llorens-Vernet, P. Assessing the quality of mobile health-related apps: Interrater reliability study of two guides. JMIR Mhealth Uhealth 2021, 9, e26471. [Google Scholar] [CrossRef] [PubMed]
| App Name/Platform | Registration Requirements | Privacy | Security | Language | Rating Star | Download Times | Version | Last Update | Developer | Function |
|---|---|---|---|---|---|---|---|---|---|---|
| eGFR calculator /G & A | N/A | N/A | unknown | English | 3.1 | 100,000+ | N/A | 10/11/2021 | National Kidney Foundation | Knowledge Calculation |
| Dialysis calculator /G | N/A | N/A | unknown | English | 4.4 | 10,000+ | N/A | 18/01/2021 | Rodrigo Sepúlveda Palamara | Calculation |
| My Kidney friend /G | N/A | Yes | encryption | English | N/A | 1000+ | N/A | 12/09/2022 | Anastasiia Elci | Record |
| eGFR calculator pro /G | N/A | Shares information | encryption | English | 5.0 | 100,000+ | N/A | 10/08/2021 | iMedical Apps | Calculation |
| kidney graphs result for kidney | N/A | Shares information | encryption | English | N/A | 50+ | N/A | 10/08/2021 | Torches Inc. | Record |
| Kidney Guide by Taipei Veterans General Hospital /G | Identity card number + password | Shares information to NHIA (Taiwan) | encryption | Chinese | N/A | 100+ | N/A | 27/07/2022 | Taipei Veterans General Hospital | Knowledge Record Tracking Reminder Interaction |
| Mizu- Your CKD companion /G & A | email + password | Shares information | encryption | English | N/A | 5000+ | N/A | 27/10/2023 | Carealytix | Knowledge Record Tracking Reminder Interaction |
| eGFR Calculator (CKD-EPI) /G | N/A | Yes | unknown | English | N/A | 10,000+ | N/A | 13/11/2021 | MDApp+ | Calculation |
| Kidney renal disease diet help /G | N/A | Shares ID | encryption | English | N/A | 10,000+ | N/A | 21/06/2023 | Data Recovery Software by RecoveryBull.com | Knowledge |
| RenalSense: Kidney Care App /G | N/A | Yes | encryption | English | N/A | 50+ | N/A | 10/11/2023 | Loop Systems | Knowledge Record |
| Chronic Kidney Disease /G | N/A | Yes | encryption | English | N/A | 100+ | N/A | 08/04/2023 | DevoDreamTeam | Knowledge |
| Kidney Failure Risk Equation /G | N/A | N/A | unknown | English | N/A | 100+ | N/A | 23/08/2021 | M. Parmar | Calculation |
| Kidney Care Community /G | N/A | Collects and shares information | encryption | English | N/A | 100+ | N/A | 26/10/2021 | Fresenius Medical Care North America | Knowledge Interaction |
| eGFR calculators pro /G | N/A | Collects information | encryption | English | N/A | 100,000+ | N/A | 19/11/2021 | iMedical Apps | Knowledge Calculation |
| PeriBuddy-managing peritonea /G & A | email + password | Collects information | encryption | English Chinese | N/A | N/A | 0.9.16 | 3 years ago | Outsource ESD | Tracking Interaction (Share information with others) |
| Renal dialysis /A | email + password | Collects information | unknown | English | 4.3 | N/A | 0.9.16 | 3 years ago | Coding Minds, Inc. | Record |
| Low Phosphorus Foods /A | N/A | N/A | unknown | English | N/A | N/A | 1.3.1 | 3 years ago | Nasir Hussain | Record Check the phosphorus content of food |
| Our Journey with PD /A | N/A | N/A | unknown | English | N/A | N/A | 2.1 | 3 years ago | Phoenix Children’s Hospital, Inc. | Knowledge |
| Kidney Diet Friendly Recipes /G & A | N/A | Collects information | Not guarantee | English | 4.4 | 1000+ | 3.1.0 | 1 week ago | Prestige Worldwide Apps LLC | Knowledge Check the phosphorus content of food |
| eGFR calculators pro /A | N/A | Traces and collects information | unknown | English | N/A | N/A | 3.3 | 2 years ago | Putu Angga Risky Raharja | Calculation |
| GFR Easycalc /G & A | N/A | Yes | encryption | English | N/A | 10+ | N/A | N/A | Louis Janssens | Calculation |
| App Name | Engagement | Functionality | Aesthetics | Information | Subjective Quality | App Specificity | Overall |
|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | |
| eGFR calculators | 3.20 | 5.00 | 4.50 | 4.19 | 4.50 | 3.83 | 4.20 |
| Dialysis calculator | 3.20 | 5.00 | 4.50 | 4.20 | 4.50 | 3.83 | 4.20 |
| My Kidney friend | 2.80 | 5.00 | 4.50 | 3.89 | 4.13 | 3.33 | 3.94 |
| eGFR calculator pro | 2.80 | 5.00 | 4.50 | 3.78 | 2.75 | 4.08 | 3.82 |
| kidney graphy result for kidney | 2.90 | 5.00 | 4.50 | 3.83 | 4.13 | 3.00 | 3.89 |
| Kidney Guide by Taipei Veterans General Hospital | 3.60 | 5.00 | 5.00 | 4.67 | 5.00 | 4.92 | 4.70 |
| Mizu- Your CKD companion | 4.00 | 5.00 | 5.00 | 4.76 | 5.00 | 5.00 | 4.79 |
| eGFR Calculator (CKD-EPI) | 2.80 | 5.00 | 4.33 | 3.52 | 3.25 | 2.50 | 3.57 |
| kidney renal disease diet help | 2.80 | 4.00 | 4.33 | 2.99 | 1.25 | 3.00 | 3.06 |
| Renal Sense: Kidney Care App | 2.80 | 4.00 | 4.33 | 2.83 | 1.25 | 2.50 | 2.95 |
| Chronic Kidney Disease | 2.40 | 3.75 | 3.00 | 2.28 | 1.00 | 1.50 | 2.32 |
| Kidney Failure Risk Equation | 2.40 | 3.75 | 3.00 | 2.34 | 1.00 | 1.50 | 2.33 |
| Kidney Care Community | 2.80 | 4.50 | 4.00 | 3.78 | 3.13 | 4.33 | 3.76 |
| eGFR calculator pro | 2.80 | 5.00 | 3.83 | 3.68 | 3.75 | 3.00 | 3.68 |
| Peri Buddy-managing peritonea | 3.00 | 5.00 | 4.33 | 3.77 | 3.63 | 3.00 | 3.79 |
| Renal dialysis | 3.10 | 5.00 | 4.33 | 4.04 | 4.25 | 4.00 | 4.12 |
| Low Phosphorus Foods | 3.50 | 5.00 | 4.33 | 4.31 | 3.75 | 5.00 | 4.31 |
| Our Journey with PD | 3.00 | 4.50 | 4.83 | 4.17 | 4.00 | 4.83 | 4.22 |
| Kidney Diet Friendly Recipes | 2.30 | 4.50 | 4.00 | 3.41 | 2.75 | 3.08 | 3.34 |
| eGFR calculator pro | 2.80 | 5.00 | 4.50 | 3.78 | 2.75 | 4.08 | 3.82 |
| GFR Easycalc | 2.80 | 5.00 | 4.50 | 3.78 | 2.75 | 4.08 | 3.82 |
| Mean (SD) | 2.94 (0.40) | 4.71 (0.46) | 4.29 (0.52) | 3.71 (0.65) | 3.26 (1.27) | 3.54 (1.04) | 3.74 (0.65) |
| App Name | Dialysis Management: Perform PD Skills, Record the Volume of Fluid, Prevent Peritonitis | Nutrition Management: Calculate and Monitor Nutrition/Diet and Food Phosphorus Content | Exercise Management: Type, Frequency, and Amount of Activity | Medication Management: Taking Medication Regularly | Physiological Indicators: Record and/or Trace Blood Pressure and Body Weight, Especially Pre-Dialysis and Post-Dialysis | Laboratory Values: Check and Track Lab Data, Such as Creatinine and/or Blood Sugar Levels | Information: Knowledge and eGFR Calculation | Interaction: Peer, HCP, Family | Others: Medical Appointments, Social Resources, Quality of Life | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| eGFR calculators | ✓ | 1 | ||||||||
| Dialysis calculator | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | ||||
| My Kidney friend | ✓ | ✓ | 2 | |||||||
| eGFR calculators pro | ✓ | 1 | ||||||||
| kidney graphy result for kidney | ✓ | ✓ | 2 | |||||||
| Kidney Guide by Taipei Veterans General Hospital | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |
| Mizu- Your CKD companion | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||
| eGFR Calculator (CKD-EPI) | ✓ | 1 | ||||||||
| kidney renal disease diet help | ✓ | 1 | ||||||||
| RenalSense: Kidney Care App | ✓ | ✓ | 2 | |||||||
| Chronic Kidney Disease | ✓ | 1 | ||||||||
| Kidney Failure Risk Equation | ✓ | 1 | ||||||||
| Kidney Care Community | ✓ | ✓ | 2 | |||||||
| eGFR calculators pro | ✓ | 1 | ||||||||
| PeriBuddy-managing peritonea | ✓ | ✓ | ✓ | 3 | ||||||
| Renal dialysis | ✓ | ✓ | ✓ | 3 | ||||||
| Low Phosphorus Foods | ✓ | ✓ | 2 | |||||||
| Our Journey with PD | ✓ | 1 | ||||||||
| Kidney Diet Friendly Recipes | ✓ | ✓ | 2 | |||||||
| eGFR calculators pro | ✓ | 1 | ||||||||
| GFR Easycalc | ✓ | 1 | ||||||||
| Total score | 4 | 5 | 1 | 2 | 7 | 6 | 18 | 3 | 2 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, S.-M.; Wang, M.-L.; Fang, Y.-W.; Lin, M.-L.; Chen, S.-F. Mobile Apps for Patients with Peritoneal Dialysis: Systematic App Search and Evaluation. Healthcare 2024, 12, 719. https://doi.org/10.3390/healthcare12070719
Chao S-M, Wang M-L, Fang Y-W, Lin M-L, Chen S-F. Mobile Apps for Patients with Peritoneal Dialysis: Systematic App Search and Evaluation. Healthcare. 2024; 12(7):719. https://doi.org/10.3390/healthcare12070719
Chicago/Turabian StyleChao, Shu-Mei, Ming-Ling Wang, Yu-Wen Fang, Mei-Ling Lin, and Shu-Fen Chen. 2024. "Mobile Apps for Patients with Peritoneal Dialysis: Systematic App Search and Evaluation" Healthcare 12, no. 7: 719. https://doi.org/10.3390/healthcare12070719
APA StyleChao, S.-M., Wang, M.-L., Fang, Y.-W., Lin, M.-L., & Chen, S.-F. (2024). Mobile Apps for Patients with Peritoneal Dialysis: Systematic App Search and Evaluation. Healthcare, 12(7), 719. https://doi.org/10.3390/healthcare12070719






