Is Craniosacral Therapy Effective? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
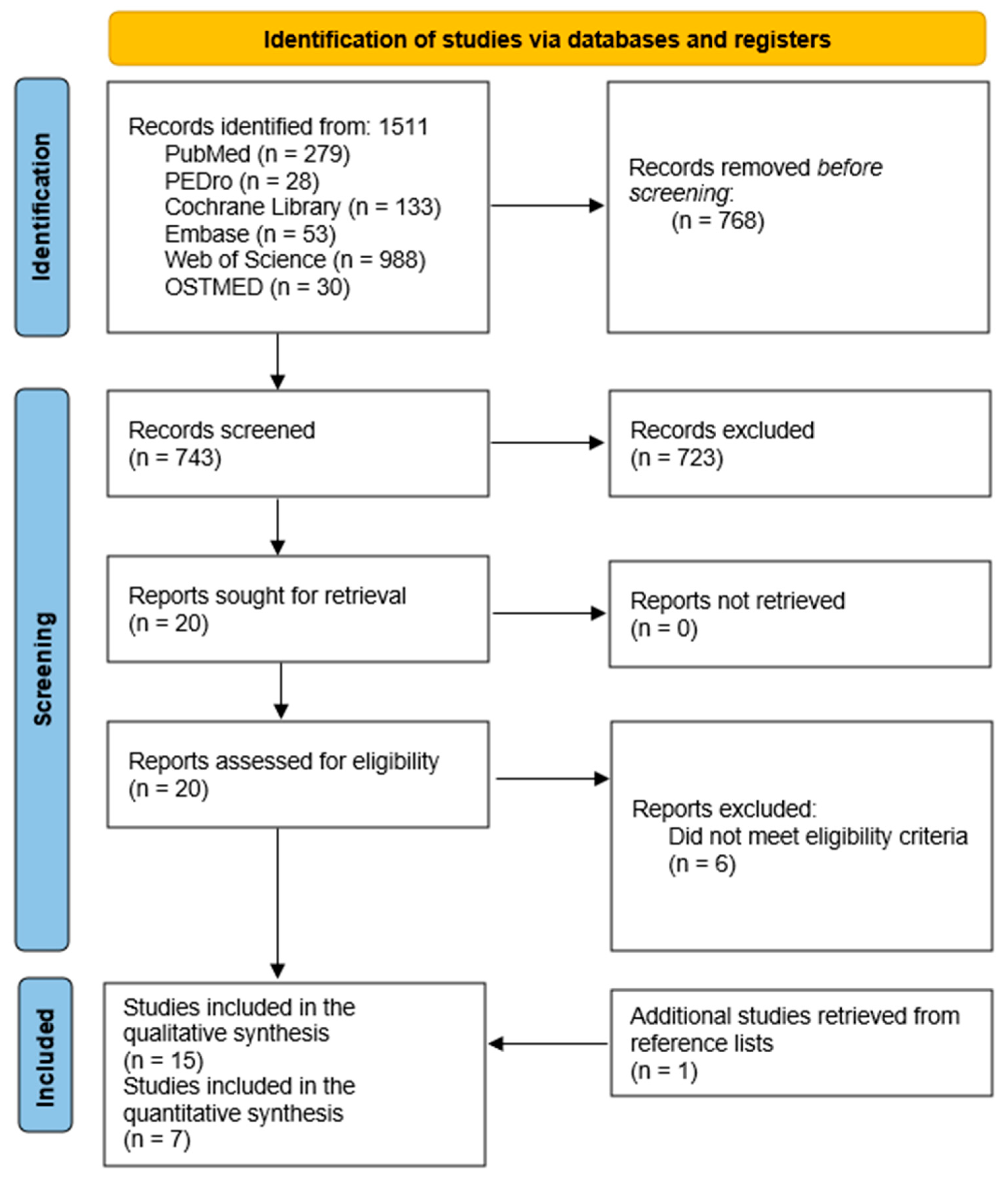
2.2. Search Strategy
2.3. Eligibility Criteria and Study Selection
2.4. Data Extraction
2.5. Methodological Quality, Risk of Bias, and Certainty of Evidence
2.6. Data Synthesis and Analysis
3. Results
3.1. Clinical Effectiveness on Musculoskeletal Conditions
3.2. Clinical Effectiveness for Non-Musculoskeletal Conditions
3.3. High- Versus Low-Quality Studies
3.4. Adverse Events
4. Discussion
4.1. Musculoskeletal Conditions
4.2. Non-Musculoskeletal Conditions
4.3. Implications for Clinical Practice
4.4. Limitations and Future Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Detailed Search Strategy According to the PRISMA Model
| PUBMED |
| Search strategy: (“osteopathic manipulation” [MeSH] OR “cranial mobilization” OR “craniosacral mobilization” OR “craniosacral manipulation” OR “cranial therapy” OR “craniosacral” OR “osteopathic cranial manipulative medicine” OR “cranial osteopathy” OR “craniosacral osteopathy” OR “cranial manipulation” OR “cranial field” OR “cranial osteopathic manipulative medicine” OR “osteopathic cranial manipulative medicine” OR “cranial manipulative medicine” OR “primary respiratory mechanism” OR “cranial rhythmic impulse” OR “fourth ventricular”) |
| Filter: clinical trial/randomized controlled trial |
| Data: 17 August 2023 |
| Studies retrieved: 279 |
| PEDRO |
| Search strategy: craniosacral |
| Data: 17 August 2023 |
| Studies retrieved: 21 |
| Search strategy: cranial osteopathy |
| Data: 17 August 2023 |
| Studies retrieved: 7 |
| Cochrane Library |
| Search strategy: (“osteopathic manipulation” OR “cranial mobilization” OR “craniosacral mobilization” OR “craniosacral manipulation” OR “cranial therapy” OR “craniosacral OR “osteopathic cranial manipulative medicine” OR “cranial osteopathy” OR “craniosacral osteopathy” OR “cranial manipulation” OR “cranial field” OR “cranial osteopathic manipulative medicine” OR “osteopathic cranial manipulative medicine” OR “cranial manipulative medicine” OR “primary respiratory mechanism” OR “cranial rhythmic impulse” OR “fourth ventricular”) |
| Data: 17 August 2023 |
| Studies retrieved: 133 |
| WOS |
| Search strategy: “osteopathic manipulation “ OR “cranial mobilization” OR “craniosacral mobilization” OR “craniosacral manipulation” OR “cranial therapy” OR “craniosacral” OR “osteopathic cranial manipulative medicine” OR “cranial osteopathy” OR “craniosacral osteopathy” OR “cranial manipulation” OR “cranial field” OR “cranial osteopathic manipulative medicine” OR “osteopathic cranial manipulative medicine” OR “cranial manipulative medicine” OR “primary respiratory mechanism” OR “cranial rhythmic impulse” OR “fourth ventricular” |
| Data: 17 August 2023 |
| Studies retrieved: 988 |
| OSTMED |
| Search strategy: “craniosacral therapy” OR “cranial osteopathy” OR “osteopathy in the cranial field” OR “osteopathic cranial manipulative medicine” |
| Data: 17 August 2023 |
| Studies retrieved: 30 |
Appendix B. Synthesis of Quantitative Results and Certainty of Evidence
| Outcome | No. of Studies (Participants) | Risk of Bias | Inconsistency | Imprecision | Indirectness | Publication Bias | Pooled Effect Estimate | Certainty of Evidence |
| Headache disorders | ||||||||
| Pain intensity | 2 (110) | Very serious a | None | Serious c | None | Begg test: 0.29 Egger test: 0.01 | MD: −0.79 (−1.39, −0.20) | Very low |
| Headache impact | 2 (60) | Very serious a | None | Serious c | Serious d | No suspected | SMD: 0.02 (−0.44, 0.48) | Very low |
| Low back pain | ||||||||
| Pain intensity | 2 (123) | Very serious a | Very serious b | Serious c | None | No suspected | SMD: −1.68 (−3.89, 0.52) | Very low |
| Infant colic | ||||||||
| Crying time | 2 (82) | Very serious a | Very serious b | Serious c | Serious d | No suspected | MD: −1.78 (−4.01, 0.44) | Very low |
| Sleeping time | 2 (82) | Very serious a | Very serious b | Serious c | Serious d | No suspected | MD: 1.77 (−0.12, 3.66) | Very low |
| MD: mean difference; SMD: standardized mean difference. a Risk of bias was downgraded because more than 50% of the studies included presented fair or low methodological quality. b Inconsistency was downgraded because I2 was higher than 75%. c Imprecision was downgraded because the interventions were heterogeneous. d Indirectness was downgraded because the number of patients was <100. | ||||||||
References
- Ernst, E. Craniosacral Therapy: A Systematic Review of the Clinical Evidence. Focus Altern. Complement. Ther. 2012, 17, 197–201. [Google Scholar] [CrossRef]
- World Health Organization. Benchmarks for Training in Osteopathy; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Wray, J.; Edwards, V.; Wyatt, K. Parents’ Attitudes Toward the Use of Complementary Therapy by Their Children with Moderate or Severe Cerebral Palsy. J. Altern. Complement. Med. 2014, 20, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Rourai, S.; Cerritellii, F.; Esteves, J.E.; Verbeeck, J.; Dun, P.L.S.V. The Spanish Osteopathic Practitioners Estimates and RAtes (OPERA) Study: A Crosssectional Survey. PLoS ONE 2020, 15, e0234713. [Google Scholar] [CrossRef] [PubMed]
- Hestbaeck, L.; Hartvigsen, J.; Christensen, H.; Werner, V. Osteopathy and Chiropractic Treatment in Babies with Infantile Colic. Acta Paediatr. 2023, 112, 2239–2240. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Ménard, M.; Jacquot, E.; Marangelli, G.; Merdy, O.; Clouzeau, C.; Tavernier, P.; Verbeeck, J.; Vaucher, P.; Esteves, J.E.; et al. The Profile of French Osteopaths: A Cross-Sectional Survey. Int. J. Osteopat. Med. 2023, 49, 100672. [Google Scholar] [CrossRef]
- van Dun, P.L.S.; Verbeeck, J.; Arcuri, L.; Esteves, J.E.; Cerritelli, F. The Profile of Belgian Osteopaths: A Cross-Sectional Survey. Healthcare 2022, 10, 2136. [Google Scholar] [CrossRef]
- Rui, J.S.; Nunes, A.; Esteves, J.E.; Cerritelli, F.; Verbeeck, J.; Lopes, S.; Paquete, M.; van Dun, P.L.S. The Portuguese Osteopathic Practitioners Estimates and RAtes (OPERA): A Cross-Sectional Survey. Int. J. Osteopat. Med. 2022, 43, 23–30. [Google Scholar] [CrossRef]
- van Dun, P.L.S.; Arcuri, L.; Verbeeck, J.; Esteves, J.E.; Cerritelli, F. The Austrian Osteopathic Practitioners Estimates and RAtes (OPERA): A Cross- Sectional Survey. PLoS ONE 2022, 17, e0278041. [Google Scholar] [CrossRef]
- Álvarez-Bustins, G.; López-Plaza, P.V.; Roura-Carvajal, S. Profile of Osteopathic Practice in Spain: Results from a Standardized Data Collection Study. BMC Complement. Altern. Med. 2018, 18, 129. [Google Scholar] [CrossRef]
- Leach, M.J.; Sundberg, T.; Fryer, G.; Austin, P.; Thomson, O.P.; Adams, J. An Investigation of Australian Osteopaths’ Attitudes, Skills and Utilisation of Evidence-Based Practice: A National Cross-Sectional Survey. BMC Health Serv. Res. 2019, 19, 498. [Google Scholar] [CrossRef]
- Hidalgo, D.F.; MacMillan, A.; Thomson, O.P. ‘It’s All Connected, so It All Matters’—The Fallacy of Osteopathic Anatomical Possibilism. Int. J. Osteopat. Med. 2024; in press. [Google Scholar] [CrossRef]
- King, H.H. Osteopathy in the Cranial Field. In Foundations of Osteopathic Medicine, 3rd ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012; pp. 728–748. [Google Scholar]
- Bordoni, B.; Escher, A.R. The Osteopath’s Imprint: Osteopathic Medicine Under the Nanoscopic Lens. Cureus 2023, 15, e33914. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Martin, C.W.; Bassett, K.; Kazanjian, A. A Systematic Review of Craniosacral Therapy: Biological Plausibility, Assessment Reliability and Clinical Effectiveness. Complement. Ther. Med. 1999, 7, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Ito, J.; Tokiguchi, S.; Furusawa, T. High-Resolution CT Findings in the Development of the Sphenooccipital Synchondrosis. Am. J. Neuroradiol. 1996, 17, 117–120. [Google Scholar] [PubMed]
- Downey, P.; Barbano, T.; Kapur-Wadhwa, R.; Sciote, J.; Siegel, M.; Mooney, M. Craniosacral Therapy: The Effects of Cranial Manipulation on Intracranial Pressure and Cranial Bone Movement. J. Orthop. Sports Phys. Ther. 2006, 36, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Guillaud, A.; Darbois, N.; Monvoisin, R.; Pinsault, N. Reliability of Diagnosis and Clinical Efficacy of Cranial Osteopathy: A Systematic Review. PLoS ONE 2016, 11, e0167823. [Google Scholar] [CrossRef]
- Haller, H.; Lauche, R.; Sundberg, T.; Dobos, G.; Cramer, H. Craniosacral Therapy for Chronic Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Musculoskelet. Disord. 2019, 21, 1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Savović, J.; Page, M.; Elbers, R.; Sterne, J. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; Cochrane: Oxford, UK, 2023. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE Guidelines: 7. Rating the Quality of Evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Rind, D.; Devereaux, P.J.; Montori, V.M.; Freyschuss, B.; Vist, G.; et al. GRADE Guidelines 6. Rating the Quality of Evidence—Imprecision. J. Clin. Epidemiol. 2011, 64, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Deeks, J.; Altman, D. Chapter 16. Special Topics in Statistics. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Cochrane: Oxford, UK, 2011. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Cciences; Lawrence Erlbaum Associates Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 1–694. [Google Scholar] [CrossRef]
- Gesslbauer, C.; Vavti, N.; Keilani, M.; Mickel, M.; Crevenna, R. Effectiveness of Osteopathic Manipulative Treatment versus Osteopathy in the Cranial Field in Temporomandibular Disorders—A Pilot Study Cranial Field in Temporomandibular Disorders—A Pilot Study. Disabil. Rehabil. 2018, 40, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Białoszewski, D.; Bebelski, M.; Lewandowska, M.; Słupik, A. Przydatność Terapii Czaszkowo-Krzyżowej w Leczeniu Chorych z Niespecyficznymi Bólami Dolnego Odcinka Kręgosłupa. Doniesienie Wstępne. Ortop. Traumatol. Rehabil. 2014, 16, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, C.; Amiri, A.; Sarrafzadeh, J.; Dadgoo, M.; Jafari, H. Comparative Study of Muscle Energy Technique, Craniosacral Therapy, and Sensorimotor Training Effects on Postural Control in Patients with Nonspecific Chronic Low Back Pain. J. Fam. Med. Prim. Care 2020, 9, 978–984. [Google Scholar]
- Terrell, Z.T.; Moudy, S.C.; Hensel, K.L.; Patterson, R.M. Effects of Osteopathic Manipulative Treatment vs. Osteopathic Cranial Manipulative Medicine on Parkinsonian Gait. J. Osteopat. Med. 2022, 122, 243–251. [Google Scholar] [CrossRef]
- Mehl-Madrona, L.; Kligle, B.; Silverman, S.; Lynton, H.; Merrell, W. The Impact of Acupuncture and Craniosacral Therapy Interventions on Clinical Outcomes in Adults with Asthma. Explor. J. Sci. Health 2007, 3, 28–36. [Google Scholar] [CrossRef]
- Castro-Sánchez, A.M.; Matarán-Peñarrocha, G.A.; Sánchez-Labraca, N.; Manuel, J.; Granero-Molina, J.; Moreno-Lorenzo, C. A Randomized Controlled Trial Investigating the Effects of Craniosacral Therapy on Pain and Heart Rate Variability in Fibromyalgia Patients. Clin. Rehabil. 2011, 25, 25–35. [Google Scholar] [CrossRef]
- Hanten, W.P.; Olson, S.L.; Hodson, J.L.; Imler, V.L.; Knab, V.M.; Magee, J.L. The Effectiveness of CV-4 and Resting Position Techniques on Subjects with Tension-Type Headaches. J. Man. Manip. Ther. 1999, 7, 64–70. [Google Scholar] [CrossRef]
- Matarán-Peñarrocha, G.A.; Castro-Sánchez, A.; Carballo-García, G.; Moreno-Lorenzo, C.; Parrón-Carreño, T.; Onieva-Zafra, M.D. Influence of Craniosacral Therapy on Anxiety, Depression and Quality of Life in Patients with Fibromyalgia. Evid.-Based Complement. Altern. Med. 2011, 2011, 178769. [Google Scholar] [CrossRef] [PubMed]
- Amrovabady, S.; Pishyareh, Z.; Esteki, M.; Haghgoo, H. Effect of Craniosacral Therapy on Students’ Symptoms of Attention Deficit Hyperactivity Disorder. Iran. Rehabil. J. 2013, 11, 27–33. [Google Scholar]
- Arnadottir, T.S.; Sigurdardottir, A.K. Is Craniosacral Therapy Effective for Migraine? Tested with HIT-6 Questionnaire. Complement. Ther. Clin. Pract. 2013, 19, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gómez, E.; Inglés, M.; Aguilar-Rodríguez, M.; Mollà-Casanova, S.; Sempere-Rubio, N.; Serra-Añó, P.; Espí-López, G.V. Effect of a Craniosacral Therapy Protocol in People with Migraine: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Mazreati, N.; Rahemi, Z.; Aghajami, M.; Ajorpaz, N.M.; Miansehsaz, E. Effect of Craniosacral Therapy on the Intensity of Chronic Back Pain of Nurses: A Randomized Controlled Trial. Nurs. Pract. Today 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Castejón-Castejón, M.; Murcia-González, M.A.; Todri, J.; Lena, O.; Chillón-Martínez, R. Treatment of Infant Colic with Craniosacral Therapy. A Randomized Controlled Trial. Complement. Ther. Med. 2022, 71, 102885. [Google Scholar] [CrossRef] [PubMed]
- Hayden, C.; Mullinger, B. A Preliminary Assessment of the Impact of Cranial Osteopathy for the Relief of Infantile Colic. Complement. Ther. Clin. Pract. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Raith, W.; Marschik, P.B.; Sommer, C.; Maurer-Fellbaum, U.; Amhofer, C.; Avian, A.; Soral, S.; Müller, W.; Einspieler, C.; Urlesberger, B. General Movements in Preterm Infants Undergoing Craniosacral Therapy: A Randomised Controlled Pilot-Trial. BMC Complement. Altern. Med. 2015, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Senapati, A. Effectiveness of Combined Approach of Craniosacral Therapy (CST) and Sensory-Integration Therapy (SIT) on Reducing Features in Children with Autism. Indian J. Occup. Ther. 2015, 47, 3–8. [Google Scholar]
- Wyatt, K.; Edwards, V.; Franck, L.; Britten, N.; Creanor, S.; Maddick, A.; Logan, S. Cranial Osteopathy for Children with Cerebral Palsy: A Randomised Controlled Trial. Arch. Dis. Child. 2011, 96, 505–512. [Google Scholar] [CrossRef]
- Elden, H.; Ostgaard, H.; Glantz, A.; Marciniak, P.; Linner, A.-C.; Fagevik Olsen, M. Effects of Craniosacral Therapy as Adjunct to Standard Treatment for Pelvic Girdle Pain in Pregnant Women: A Multicenter, Single Blind, Randomized Controlled Trial. Acta Obstet. Gynecol. Scand. 2013, 92, 775–782. [Google Scholar] [CrossRef]
- Sandhouse, M.E.; Shechtman, D.; Sorkin, R.; Drowos, J.L.; Caban-Martinez, A.J.; Patterson, M.M.; Shallo-Hoffmann, J.; Hardigan, P.; Snyder, A. Effect of Osteopathy in the Cranial Field on Visual Function—A Pilot Study. J. Am. Acad. Orthop. Asoc. 2010, 110, 239–243. [Google Scholar]
- Haller, H.; Lauche, R.; Cramer, H.; Rampp, T.; Saha, F.J.; Ostermann, T.; Dobos, G. Craniosacral Therapy for the Treatment of Chronic Neck Pain: A Randomized Sham-Controlled Trial. Clin. J. Pain 2016, 32, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sánchez, A.M.; Lara-Palomo, I.C.; Matarán-Peñarrocha, G.A.; Saavedra-Hernández, M.; Pérez-Mármol, J.M.; Aguilar-Ferrándiz, M.E. Benefits of Craniosacral Therapy in Patients with Chronic Low Back Pain: A Randomized Controlled Trial. J. Altern. Complement. Med. 2016, 22, 650–657. [Google Scholar] [CrossRef]
- Young, I.A.; Dunning, J.; Butts, R.; Cleland, J.A.; Fernández-de-las-Peñas, C. Psychometric Properties of the Numeric Pain Rating Scale and Neck Disability Index in Patients with Cervicogenic Headache. Cephalalgia 2019, 39, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric Properties of the Neck Disability Index and Numeric Pain Rating Scale in Patients with Mechanical Neck Pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef]
- Ostelo, R.W.J.G.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; De Vet, H.C. Interpreting Change Scores for Pain and Functional Status in Low Back Pain: Towards International Consensus Regarding Minimal Important Change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ogollah, R.; Bishop, A.; Lewis, M.; Grotle, M.; Foster, N.E. Responsiveness and Minimal Important Change for Pain and Disability Outcome Measures in Pregnancy-Related Low Back and Pelvic Girdle Pain. Phys. Ther. 2019, 99, 1551–1561. [Google Scholar] [CrossRef]
- Frahm Olsen, M.; Bjerre, E.; Hansen, M.D.; Tendal, B.; Hilden, J.; Hróbjartsson, A. Minimum Clinically Important Differences in Chronic Pain Vary Considerably by Baseline Pain and Methodological Factors: Systematic Review of Empirical Studies. J. Clin. Epidemiol. 2018, 101, 87–106.e2. [Google Scholar] [CrossRef]
- Castien, R.F.; Blankenstein, A.H.; Windt, D.A.V.D.; Dekker, J. Minimal Clinically Important Change on the Headache Impact Test-6 Questionnaire in Patients with Chronic Tension-Type Headache. Cephalalgia 2012, 32, 710–714. [Google Scholar] [CrossRef]
- Pradela, J.; Bevilaqua-Grossi, D.; Chaves, T.C.; Dach, F.; Carvalho, G.F. Measurement Properties of the Headache Impact Test (HIT-6TM Brazil) in Primary and Secondary Headaches. Headache 2021, 61, 527–535. [Google Scholar] [CrossRef]
- Macdelilld, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; Mcalpine, C.; Goldsmith, C.H. Measurement Properties of the Neck Disability Index: A Systematic Review. J. Orthop. Sports Phys. Ther. 2009, 39, 400–416. [Google Scholar] [CrossRef]
- Carrasco-Uribarren, A.; Mamud-Meroni, L.; Tarcaya, G.E.; Jiménez-Del-Barrio, S.; Cabanillas-Barea, S.; Ceballos-Laita, L. Clinical Effectiveness of Craniosacral Therapy in Patients with Headache Disorders: A Systematic Review and Meta-Analysis. Pain Manag. Nurs. 2024, 25, e21–e28. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, A.; von Hauenschild, P. A Systematic Review to Evaluate the Clinical Benefits of Craniosacral Therapy. Complement. Ther. Med. 2012, 20, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.B.; Samuel, O.C.; Li, Z.; Chen, W.; Sui, H.J. Effectiveness of Craniosacral Therapy in the Human Suboccipital Region on Hamstring Muscle: A Meta-Analysis Based on Current Evidence. Medicine 2023, 102, E32744. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas-Barea, S.; Jiménez-del-Barrio, S.; Carrasco-Uribarren, A.; Ortega-Martínez, A.; Pérez-Guillén, S.; Ceballos-Laita, L. Systematic Review and Meta-Analysis Showed That Complementary and Alternative Medicines Were Not Effective for Infantile Colic. Acta Paediatr. Int. J. Paediatr. 2023, 112, 1378–1388. [Google Scholar] [CrossRef]
- Posadzki, P.; Kyaw, B.M.; Dziedzic, A.; Ernst, E. Osteopathic Manipulative Treatment for Pediatric Conditions: An Update of Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4455. [Google Scholar] [CrossRef]
- Ernst, E.; Smith, K. More Harm Than Good? The Moral Maze of Complementary and Alternative Medicine; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
| Participants | Intervention | Outcome (Tool) | Main Results | PEDro Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (Year) | Mean Age (SD) | Diagnosis | CST Group | Control Group | Session Duration | Frequency (Sessions/Week) | Total Number of Sessions | |||
| Musculoskeletal disorders | ||||||||||
| Headache disorders | ||||||||||
| Hanten et al., 1999 A [34] | 36 (12) | TTH | CST (n = 20) | Resting position (n = 20) | 10 m | 1 s/w | 1 |
| ND ND | 4 |
| Hanten et al., 1999 B [34] | 36 (12) | TTH | CST (n = 20) | Control (n = 20) | 10 m | 1 s/w | 1 |
| ↑ Pain ↑ Impact | 4 |
| Arnadottir et al., 2013 [37] | 37.6 (9.3) | Migraine | CST (n = 10) | Control (n = 10) | NR | 1.5 s/w | 6 |
| ND | 5 |
| Muñoz-Gómez et al., 2022 [38] | CST: 40.92 (7.95) CG: 37.64 (9.42) | Migraine | CST (n = 25) | Sham intervention (n = 25) | 45 m | 1 s/w | 8 |
| ↑ Pain ↑ Severity | 6 |
| Neck pain | ||||||||||
| Haller et al., 2016 [47] | CST: 44.2 (9.7) CG: 45.0 (10.5) | CNP | CST (n = 27) | Sham intervention (n = 27) | 45 m | 1 s/w | 8 |
| ↑ Pain ↑ Disability | 8 |
| Low back pain | ||||||||||
| Castro-Sánchez et al., 2011 [33] | CST: 50 (11) CG: 53 (9) | CLBP | CST (n = 32) | Control (n = 32) | 50 m | 1 s/w | 10 |
| ↑ Pain ND | 7 |
| Mazreati et al., 2021 [39] | CST: 34.28 (3.28) CG: 33.11 (3.20) | CLBP | CST (n = 30) | Control (n = 29) | 30–45 m | NR | 8 |
| ↑ Pain | 6 |
| Pelvic girdle pain | ||||||||||
| Elden et al., 2013 [45] | CST: 30.6 (3.9) CG: 31.3 (4.3) | Pregnant women with pelvic girdle pain | CST + standard care (n = 55) | Standard care (n = 57) | 45 m | 1 s/w | 3 |
| ↑ Morning pain ND ND ND | 8 |
| Fibromyalgia | ||||||||||
| Matarán-Peñarrocha et al., 2011 [35] | CST: 48.25 (13.34) CG: 52.26 (10.98) | Fibromyalgia | CST (n = 43) | Sham intervention (n = 41) | 60 m | 2 s/w | 50 |
| ↑ Pain | 4 |
| Non-musculoskeletal conditions | ||||||||||
| Infantile colic | ||||||||||
| Castejón-Castejón et al., 2022 [40] | CST: 39.14 (20.15) days CG: 33.69 (15.14) days | Infantile colic | CST (n = 29) | Control (n = 25) | 30–40 m | 1 s/w | 1 to 3 |
| ↑ Crying ↑ Sleeping | 6 |
| Hayden et al., 2006 [41] | CST:46.4 (5.4) days CG: 44.5 (5.0) days | Infantile colic | CST (n = 14) | Control (n = 14) | 30 m | 1 s/w | 4 |
| ↑ Crying ↑ Sleeping | 5 |
| Preterm infants | ||||||||||
| Raith et al., 2016 [42] | CST: 28 (25–33) weeks CG: 30 (27–33) weeks | Preterm infants | CST (n = 12) | Control (n = 13) | NR | 2 s/w | 6 |
| ND | 5 |
| Autism | ||||||||||
| Mishra and Senapati 2015 [43] | CST: 3–10 CG: 3–10 | Children with autism | CST + standard care (n = 10) | Standard care (n = 10) | 60 m | 5 s/w | 40 |
| ↑ Autism evaluation | 5 |
| Hyperactivity disorder | ||||||||||
| Amrovabady et al., 2013 [36] | CST: 9.5 CG: 9.9 | Attention deficit hyperactivity disorder | CST + standard care (n = 12) | Standard care (n = 12) | 30 m | 2 s/w | 15 |
| ↑ Symptoms ↑ Behaviour | 3 |
| Cerebral palsy | ||||||||||
| Wyatt et al., 2011 [44] | CST: 8.0 (5–12) CG: 7.6 (5–12) | Cerebral palsy | CST (n = 62) | Control (n = 67) | NR | 1 s/month | 6 |
| ND ND ND | 6 |
| Visual function | ||||||||||
| Sandhouse et al., 2010 [46] | 24.38 (3.03) | Patients with myopia, hyperopia, or astigmatism | CST (n = 15) | Sham intervention (n = 14) | 5 m | 1 s/w | 1 |
| ND ND ND ↑ right pupillary size ND ND | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceballos-Laita, L.; Ernst, E.; Carrasco-Uribarren, A.; Cabanillas-Barea, S.; Esteban-Pérez, J.; Jiménez-del-Barrio, S. Is Craniosacral Therapy Effective? A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 679. https://doi.org/10.3390/healthcare12060679
Ceballos-Laita L, Ernst E, Carrasco-Uribarren A, Cabanillas-Barea S, Esteban-Pérez J, Jiménez-del-Barrio S. Is Craniosacral Therapy Effective? A Systematic Review and Meta-Analysis. Healthcare. 2024; 12(6):679. https://doi.org/10.3390/healthcare12060679
Chicago/Turabian StyleCeballos-Laita, Luis, Edzard Ernst, Andoni Carrasco-Uribarren, Sara Cabanillas-Barea, Jaime Esteban-Pérez, and Sandra Jiménez-del-Barrio. 2024. "Is Craniosacral Therapy Effective? A Systematic Review and Meta-Analysis" Healthcare 12, no. 6: 679. https://doi.org/10.3390/healthcare12060679
APA StyleCeballos-Laita, L., Ernst, E., Carrasco-Uribarren, A., Cabanillas-Barea, S., Esteban-Pérez, J., & Jiménez-del-Barrio, S. (2024). Is Craniosacral Therapy Effective? A Systematic Review and Meta-Analysis. Healthcare, 12(6), 679. https://doi.org/10.3390/healthcare12060679









