Optimizing Invasive Neonatal Respiratory Care: A Systematic Review of Invasive Neurally Adjusted Ventilatory Assist
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Considering Studies for This Review
2.2. Search Methods for the Identification of Studies
2.3. Data Collection and Data Analysis
2.3.1. Selection of Studies
2.3.2. Data Extraction and Management
2.3.3. Data Synthesis
2.3.4. Quality of Evidence
2.3.5. Assessment of Heterogeneity
2.3.6. Assessment of Risk of Bias
3. Results
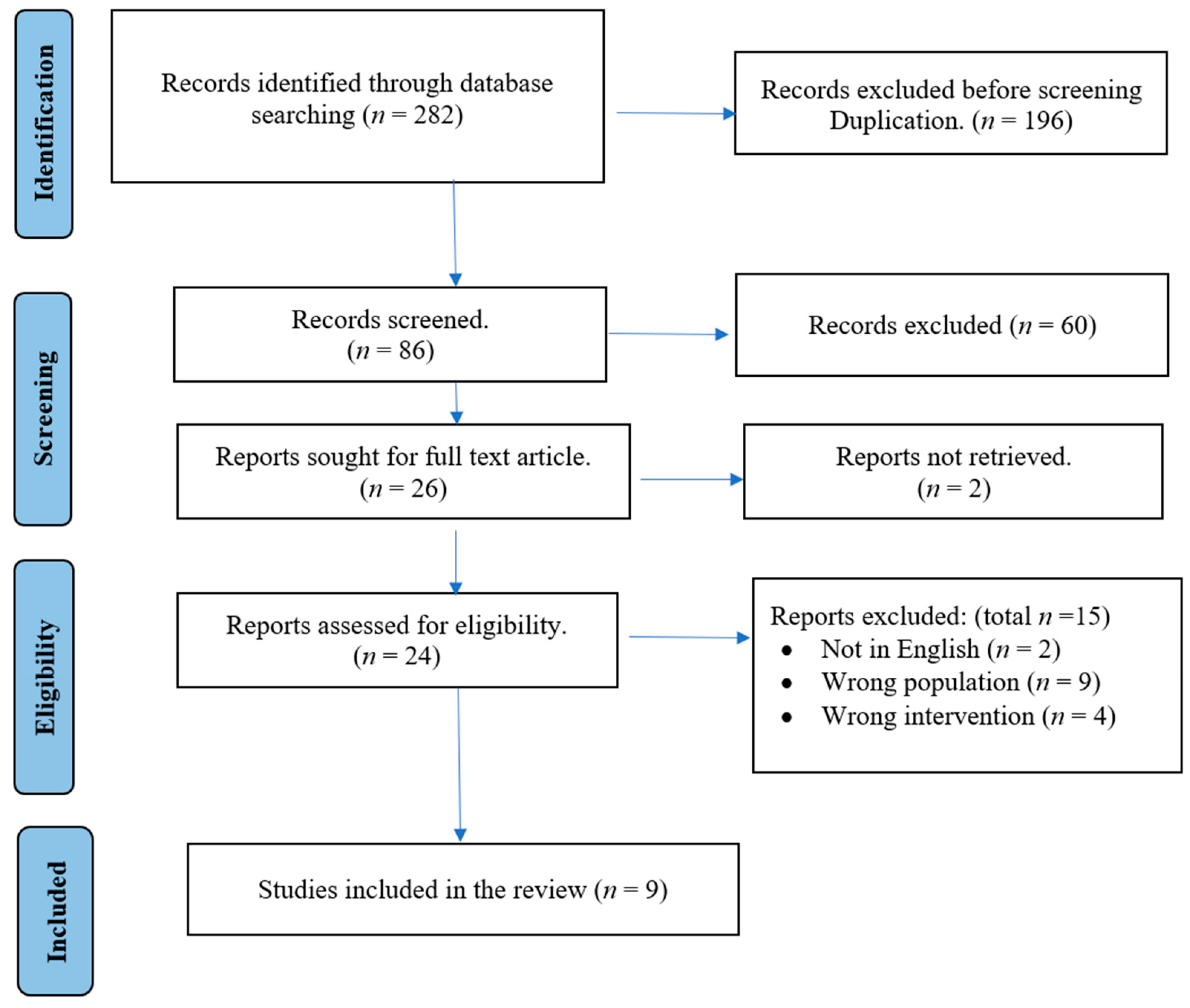
3.1. Study Selection
3.2. Study Characteristics
3.3. Level of Evidence and Grade of Recommendation
3.4. Methodological Quality and Risk of Bias
3.5. Impact on BPD Outcome
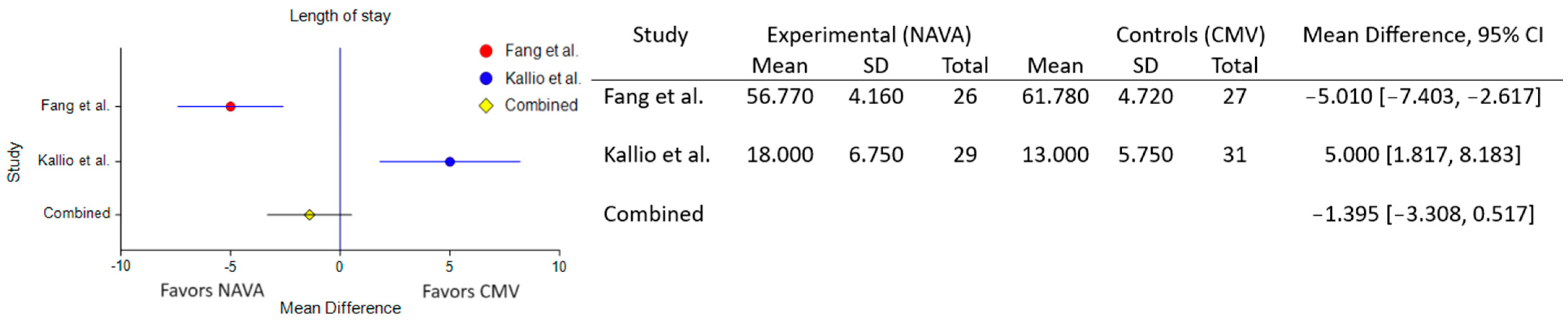
3.6. Impact on Length of Stay and Ventilator Days
3.7. Impact on Adverse Events
3.8. Impact on Ventilator Variables
4. Discussion
4.1. Practical Implications and Future Research Directions
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Blanch, L.; Villagra, A.; Sales, B.; Montanya, J.; Lucangelo, U.; Luján, M.; García-Esquirol, O.; Chacón, E.; Estruga, A.; Oliva, J.C.; et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015, 41, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Bosma, K.; Ferreyra, G.; Ambrogio, C.; Pasero, D.; Mirabella, L.; Braghiroli, A.; Appendini, L.; Mascia, L.; Ranieri, V.M. Patient-ventilator interaction and sleep in mechanically ventilated patients: Pressure support versus proportional assist ventilation. Crit. Care Med. 2007, 35, 1048–1054. [Google Scholar] [CrossRef]
- Colombo, D.; Cammarota, G.; Alemani, M.; Carenzo, L.; Barra, F.L.; Vaschetto, R.; Slutsky, A.S.; Della Corte, F.; Navalesi, P. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit. Care Med. 2011, 39, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; Miller, K.B.; Green, D.A.; Ostman, H.E.; Gennings, C.; Epstein, S.K. Ineffective triggering predicts increased duration of mechanical ventilation. Crit. Care Med. 2009, 37, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.K. How often does patient-ventilator asynchrony occur and what are the consequences? Respir. Care 2011, 56, 25–38. [Google Scholar] [CrossRef]
- Kyo, M.; Shimatani, T.; Hosokawa, K.; Taito, S.; Kataoka, Y.; Ohshimo, S.; Shime, N. Patient-ventilator asynchrony, impact on clinical outcomes and effectiveness of interventions: A systematic review and meta-analysis. J. Intensive Care 2021, 9, 50. [Google Scholar] [CrossRef]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Neumann, P.; Wrigge, H.; Zinserling, J.; Hinz, J.; Maripuu, E.; Andersson, L.G.; Putensen, C.; Hedenstierna, G. Spontaneous breathing affects the spatial ventilation and perfusion distribution during mechanical ventilatory support. Crit. Care Med. 2005, 33, 1090–1095. [Google Scholar] [CrossRef]
- Petrof, B.J.; Hussain, S.N. Ventilator-induced diaphragmatic dysfunction: What have we learned? Curr. Opin. Crit. Care 2016, 22, 67–72. [Google Scholar] [CrossRef]
- Putensen, C.; Zech, S.; Wrigge, H.; Zinserling, J.; Stüber, F.; Von Spiegel, T.; Mutz, N. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am. J. Respir. Crit. Care Med. 2001, 164, 43–49. [Google Scholar] [CrossRef]
- Radell, P.; Edström, L.; Stibler, H.; Eriksson, L.I.; Ansved, T. Changes in diaphragm structure following prolonged mechanical ventilation in piglets. Acta Anaesthesiol. Scand. 2004, 48, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Rodriguez, P.; Cabello, B.; Lellouche, F.; Brochard, L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006, 32, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Wrigge, H.; Zinserling, J.; Neumann, P.; Defosse, J.; Magnusson, A.; Putensen, C.; Hedenstierna, G. Spontaneous breathing improves lung aeration in oleic acid-induced lung injury. Anesthesiology 2003, 99, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Spahija, J.; de Marchie, M.; Albert, M.; Bellemare, P.; Delisle, S.; Beck, J.; Sinderby, C. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit. Care Med. 2010, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Delisle, S.; Ouellet, P.; Bellemare, P.; Tétrault, J.P.; Arsenault, P. Sleep quality in mechanically ventilated patients: Comparison between NAVA and PSV modes. Ann. Intensive Care 2011, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- De la Oliva, P.; Schüffelmann, C.; Gómez-Zamora, A.; Villar, J.; Kacmarek, R.M. Asynchrony, neural drive, ventilatory variability and COMFORT: NAVA versus pressure support in pediatric patients. A non-randomized cross-over trial. Intens. Care Med. 2012, 38, 838–846. [Google Scholar] [CrossRef]
- Beck, J.; Reilly, M.; Grasselli, G.; Mirabella, L.; Slutsky, A.S.; Dunn, M.S.; Sinderby, C. Patient-ventilator interaction during neurally adjusted ventilatory assist in low birth weight infants. Pediatr. Res. 2009, 65, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Mally, P.V.; Beck, J.; Sinderby, C.; Caprio, M.; Bailey, S.M. Neural Breathing Pattern and Patient-Ventilator Interaction During Neurally Adjusted Ventilatory Assist and Conventional Ventilation in Newborns. Pediatr. Crit. Care Med. 2018, 19, 48–55. [Google Scholar] [CrossRef]
- Holanda, M.A.; Vasconcelos, R.D.S.; Ferreira, J.C.; Pinheiro, B.V. Patient-ventilator asynchrony. J. Bras. Pneumol. 2018, 44, 321–333. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Govindaswami, B.; Nudelman, M.; Narasimhan, S.R.; Huang, A.; Misra, S.; Urquidez, G.; Kifle, A.; Stemmle, M.; Angell, C.; Patel, R.; et al. Eliminating Risk of Intubation in Very Preterm Infants with Noninvasive Cardiorespiratory Support in the Delivery Room and Neonatal Intensive Care Unit. BioMed Res. Int. 2019, 2019, 5984305. [Google Scholar] [CrossRef]
- Goldsmith, J.P.; Karotkin, E.; Suresh, G.; Keszler, M. Assisted Ventilation of the Neonate; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Finer, N.N.; Carlo, W.A.; Walsh, M.C.; Rich, W.; Gantz, M.G.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A.; Poole, W.K.; et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 2010, 362, 1970–1979. [Google Scholar] [CrossRef]
- Sinderby, C.; Navalesi, P.; Beck, J.; Skrobik, Y.; Comtois, N.; Friberg, S.; Gottfried, S.B.; Lindström, L. Neural control of mechanical ventilation in respiratory failure. Nat. Med. 1999, 5, 1433–1436. [Google Scholar] [CrossRef]
- Sinderby, C.; Beck, J.C. Neurally Adjusted Ventilatory Assist. In Principles and Practice of Mechanical Ventilation, 3rd ed.; Tobin, M.J., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2013; Chapter 13. [Google Scholar]
- European Respiratory Society; American Thoracic Society. ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- Longhini, F.; Ferrero, F.; De Luca, D.; Cosi, G.; Alemani, M.; Colombo, D.; Cammarota, G.; Berni, P.; Conti, G.; Bona, G.; et al. Neurally adjusted ventilatory assist in preterm neonates with acute respiratory failure. Neonatology 2015, 107, 60–67. [Google Scholar] [CrossRef]
- Matlock, D.N.; Bai, S.; Weisner, M.D.; Comtois, N.; Beck, J.; Sinderby, C.; Courtney, S.E. Work of Breathing in Premature Neonates: Noninvasive Neurally-Adjusted Ventilatory Assist versus Noninvasive Ventilation. Respir. Care 2020, 65, 946–953. [Google Scholar] [CrossRef]
- Breatnach, C.; Conlon, N.P.; Stack, M.; Healy, M.; O’Hare, B.P. A prospective crossover comparison of neurally adjusted ventilatory assist and pressure-support ventilation in a pediatric and neonatal intensive care unit population. Pediatr. Crit. Care Med. 2010, 11, 7–11. [Google Scholar] [CrossRef]
- Alander, M.; Peltoniemi, O.; Pokka, T.; Kontiokari, T. Comparison of pressure-, flow-, and NAVA-triggering in pediatric and neonatal ventilatory care. Pediatr. Pulmonol. 2012, 47, 76–83. [Google Scholar] [CrossRef]
- Sinderby, C.A.; Beck, J.C.; Lindström, L.H.; Grassino, A.E. Enhancement of signal quality in esophageal recordings of diaphragm EMG. J. Appl. Physiol. 1997, 82, 1370–1377. [Google Scholar] [CrossRef]
- Beck, J.; Sinderby, C.; Weinberg, J.; Grassino, A. Effects of muscle-to-electrode distance on the human diaphragm electromyogram. J. Appl. Physiol. 1995, 79, 975–985. [Google Scholar] [CrossRef]
- Stein, H.; Firestone, K.; Beck, J. Neurally Adjusted Ventilatory Assist (NAVA) Ventilation. In Manual of Neonatal Respiratory Care; Donn, S.M., Mammel, M.C., van Kaam, A.H.L.C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 443–454. [Google Scholar]
- Shi, Y.; Muniraman, H.; Biniwale, M.; Ramanathan, R. A Review on Non-invasive Respiratory Support for Management of Respiratory Distress in Extremely Preterm Infants. Front. Pediatr. 2020, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Emeriaud, G.; Liu, Y.; Sinderby, C. Neurally-adjusted ventilatory assist (NAVA) in children: A systematic review. Minerva Anestesiol. 2016, 82, 874–883. [Google Scholar] [PubMed]
- Jensen, E.A.; Edwards, E.M.; Greenberg, L.T.; Soll, R.F.; Ehret, D.E.Y.; Horbar, J.D. Severity of Bronchopulmonary Dysplasia Among Very Preterm Infants in the United States. Pediatrics 2021, 148, e2020030007. [Google Scholar] [CrossRef] [PubMed]
- Baez Hernandez, N.; Milad, A.; Li, Y.; Van Bergen, A.H. Utilization of Neurally Adjusted Ventilatory Assist (NAVA) Mode in Infants and Children Undergoing Congenital Heart Surgery: A Retrospective Review. Pediatr. Cardiol. 2019, 40, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Baudin, F.; Emeriaud, G.; Essouri, S.; Beck, J.; Javouhey, E.; Guerin, C. Neurally adjusted ventilatory assist decreases work of breathing during non-invasive ventilation in infants with severe bronchiolitis. Crit. Care 2019, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Crulli, B.; Khebir, M.; Toledano, B.; Vobecky, S.; Poirier, N.; Emeriaud, G. Neurally Adjusted Ventilatory Assist After Pediatric Cardiac Surgery: Clinical Experience and Impact on Ventilation Pressures. Respir. Care 2018, 63, 208–214. [Google Scholar] [CrossRef]
- Gibu, C.K.; Cheng, P.Y.; Ward, R.J.; Castro, B.; Heldt, G.P. Feasibility and physiological effects of noninvasive neurally adjusted ventilatory assist in preterm infants. Pediatr. Res. 2017, 82, 650–657. [Google Scholar] [CrossRef]
- Hunt, K.A.; Dassios, T.; Greenough, A. Proportional assist ventilation (PAV) versus neurally adjusted ventilator assist (NAVA): Effect on oxygenation in infants with evolving or established bronchopulmonary dysplasia. Eur. J. Pediatr. 2020, 179, 901–908. [Google Scholar] [CrossRef]
- Kallio, M.; Mahlman, M.; Koskela, U.; Aikio, O.; Suo-Palosaari, M.; Pokka, T.; Saarela, T.; Hallman, M. NIV NAVA versus Nasal CPAP in Premature Infants: A Randomized Clinical Trial. Neonatology 2019, 116, 380–384. [Google Scholar] [CrossRef]
- McKinney, R.L.; Keszler, M.; Truog, W.E.; Norberg, M.; Sindelar, R.; Wallström, L.; Schulman, B.; Gien, J.; Abman, S.H. Multicenter Experience with Neurally Adjusted Ventilatory Assist in Infants with Severe Bronchopulmonary Dysplasia. Am. J. Perinatol. 2021, 38, e162–e166. [Google Scholar] [CrossRef]
- Meinen, R.D.; Alali, Y.I.; Al-Subu, A.; Wilhelm, M.; Wraight, C.L.; McAdams, R.M.; Limjoco, J.J.; McCulley, D.J. Neurally-Adjusted Ventilatory Assist Can Facilitate Extubation in Neonates With Congenital Diaphragmatic Hernia. Respir. Care 2021, 66, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.K.; Lee, J.; Jun, Y.H. Neural feedback is insufficient in preterm infants during neurally adjusted ventilatory assist. Pediatr. Pulmonol. 2019, 54, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Kamei, Y.; Hiroma, T.; Nakamura, T. Neurally adjusted ventilatory assist in extremely low-birthweight infants. Pediatr. Int. 2018, 60, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Protain, A.P.; Firestone, K.S.; McNinch, N.L.; Stein, H.M. Evaluating peak inspiratory pressures and tidal volume in premature neonates on NAVA ventilation. Eur. J. Pediatr. 2021, 180, 167–175. [Google Scholar] [CrossRef]
- Rosterman, J.L.; Pallotto, E.K.; Truog, W.E.; Escobar, H.; Meinert, K.A.; Holmes, A.; Dai, H.; Manimtim, W.M. The impact of neurally adjusted ventilatory assist mode on respiratory severity score and energy expenditure in infants: A randomized crossover trial. J. Perinatol. 2018, 38, 59–63. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jung, Y.H.; Jang, J.; Kim, H.S.; Shin, S.H.; Choi, C.W.; Kim, E.K.; Kim, B.I. Respiratory severity score as a predictive factor for severe bronchopulmonary dysplasia or death in extremely preterm infants. BMC Pediatr. 2019, 19, 121. [Google Scholar] [CrossRef]
- Chakkarapani, A.A.; Adappa, R.; Mohammad Ali, S.K.; Gupta, S.; Soni, N.B.; Chicoine, L.; Hummler, H.D. “Current concepts of mechanical ventilation in neonates”—Part 1: Basics. Int. J. Pediatr. Adolesc. Med. 2020, 7, 13–18. [Google Scholar] [CrossRef]
- Forsyth, L.; Graham, K.; Harbour, R. SIGN 50: A Guideline Developers’ Handbook; Scottish Intercollegiate Guidlines Network: Edinburgh, UK, 2004. [Google Scholar]
- West, S.L.; Gartlehner, G.; Mansfield, A.J.; Poole, C.; Tant, E.; Lenfestey, N.; Lux, L.J.; Amoozegar, J.; Morton, S.C.; Carey, T.C.; et al. AHRQ Methods for Effective Health Care. In Comparative Effectiveness Review Methods: Clinical Heterogeneity; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2010. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Fang, S.J.; Su, C.H.; Liao, D.L.; Chen, C.C.; Chung, M.Y.; Chen, F.S.; Huang, H.C.; Ou-Yang, M.C. Neurally adjusted ventilatory assist for rapid weaning in preterm infants. Pediatr. Int. 2023, 65, e15360. [Google Scholar] [CrossRef]
- Kallio, M.; Koskela, U.; Peltoniemi, O.; Kontiokari, T.; Pokka, T.; Suo-Palosaari, M.; Saarela, T. Neurally adjusted ventilatory assist (NAVA) in preterm newborn infants with respiratory distress syndrome-a randomized controlled trial. Eur. J. Pediatr. 2016, 175, 1175–1183. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.S.; Sohn, J.A.; Lee, J.A.; Choi, C.W.; Kim, E.K.; Kim, B.I.; Choi, J.H. Randomized crossover study of neurally adjusted ventilatory assist in preterm infants. J. Pediatr. 2012, 161, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Parikka, V.; Lehtonen, L.; Azimi, S.; Porres, I.; Soukka, H. Neurally adjusted ventilatory assist in ventilated very preterm infants: A crossover study. Pediatr. Pulmonol. 2021, 56, 3857–3862. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Alosh, H.; Ethington, P.; White, D.B. Prospective crossover comparison between NAVA and pressure control ventilation in premature neonates less than 1500 grams. J. Perinatol. 2013, 33, 452–456. [Google Scholar] [CrossRef]
- Shetty, S.; Hunt, K.; Peacock, J.; Ali, K.; Greenough, A. Crossover study of assist control ventilation and neurally adjusted ventilatory assist. Eur. J. Pediatr. 2017, 176, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Kim, H.S.; Lee, J.; Shin, S.H.; Kim, E.K.; Choi, J.H. Neurally Adjusted Ventilatory Assist in Preterm Infants with Established or Evolving Bronchopulmonary Dysplasia on High-Intensity Mechanical Ventilatory Support: A Single-Center Experience. Pediatr. Crit. Care Med. 2016, 17, 1142–1146. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef]
- Nuthakki, S.; Ahmad, K.; Johnson, G.; Cuevas Guaman, M. Bronchopulmonary Dysplasia: Ongoing Challenges from Definitions to Clinical Care. J. Clin. Med. 2023, 12, 3864. [Google Scholar] [CrossRef]
- Jensen, E.A.; Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. Part A Clin. Mol. Teratol. 2014, 100, 145–157. [Google Scholar] [CrossRef]
- Onland, W.; Debray, T.P.; Laughon, M.M.; Miedema, M.; Cools, F.; Askie, L.M.; Asselin, J.M.; Calvert, S.A.; Courtney, S.E.; Dani, C.; et al. Clinical prediction models for bronchopulmonary dysplasia: A systematic review and external validation study. BMC Pediatr. 2013, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Romijn, M.; Dhiman, P.; Finken, M.J.J.; van Kaam, A.H.; Katz, T.A.; Rotteveel, J.; Schuit, E.; Collins, G.S.; Onland, W.; Torchin, H. Prediction Models for Bronchopulmonary Dysplasia in Preterm Infants: A Systematic Review and Meta-Analysis. J. Pediatr. 2023, 258, 113370. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.C.; Batey, N.; Luu, K.L.; Prayle, A.; Sharkey, D. Bronchopulmonary dysplasia prediction models: A systematic review and meta-analysis with validation. Pediatr. Res. 2023, 94, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Rossor, T.E.; Hunt, K.A.; Shetty, S.; Greenough, A. Neurally adjusted ventilatory assist compared to other forms of triggered ventilation for neonatal respiratory support. Cochrane Database Syst. Rev. 2017, 10, CD012251. [Google Scholar] [CrossRef]
- Greenough, A.; Rossor, T.E.; Sundaresan, A.; Murthy, V.; Milner, A.D. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst. Rev. 2016, 9, CD000456. [Google Scholar] [CrossRef]
| Study, Year | Study Type | Inclusion, Exclusion, and Comparison Criteria | Sample Size | BW (g) Mean ± SD or Median (Range) | GA (Weeks) Median | Enrollment Age Median (Range) |
|---|---|---|---|---|---|---|
| Fang, 2022 [56] | RCT | GA < 32 weeks, intubated for delivery room resuscitation. Exclusion: lethal anomalies, BW < 500 g. Comparison: SIMV or SIMV PS. | 53 | 1207.9 ± 47.2 | 29.0 ± 0.3 | <24 h |
| Kallio, 2016 [57] | RCT | GA 28–36 6/7 weeks, on invasive ventilation for RDS for at least 4 h. Exclusion: diaphragm defects, inability to insert gastric tube, severe asphyxia, chromosomal abnormalities. Comparison: patient-triggered PC ventilation. | 60 | 1735.9 ± 812 | 31.6 ± 2.6 | 9.3 (2.3–49) days |
| Lee, 2012 [58] | Randomized Crossover | GA < 37 weeks, on invasive ventilation with spontaneous breathing. Exclusion: major anomalies, IVH (grade III+), phrenic nerve palsy. Comparison: SIMV with PS (4 h crossover). | 19 | 1210 (670–2580) | 29.1 (25–36.4) | 7 (2–70) days |
| Oda, 2021 [59] | Observational Crossover | GA < 30 weeks, on invasive ventilation with desaturation events. Exclusion: major anomalies. Comparison: SIMV + PS (3 h crossover). | 20 | 610 (400–1160) | 26 4/7 (23–29 3/7) | 20 (1–82) days |
| Stein, 2013 [60] | Prospective Crossover | Low-birth-weight infants on invasive ventilation. Comparison: PCV (4 h crossover). | 5 | 697 (370–1140) | 26.2 (25–29) | 24 (6–34) days |
| Rosterman, 2018 [48] | Randomized Crossover | GA > 22 weeks, stable on MV. Exclusion: phrenic nerve palsy, respiratory suppression due to sedation or neurologic compromise. Comparison: SIMV (PC)+PS (12 h crossover). | 22 | 734 (432 to 3165) | 26 4/7 (23 to 39) | 40 (3 to 135) days |
| Hunt, 2020 [41] | Crossover | Born < 32 weeks, ventilated beyond 1 week. Comparison: A/C or SIMV (2 h crossover). | 18 | 750 (454–950) | 25.3 (23.6–30.3) | 20.5 (8–58) days |
| Shetty, 2017 [61] | Crossover | GA < 32 weeks, on invasive ventilation for > 2 weeks. Comparison: A/C or SIMV. | 9 | 750 (545–830) | 25 (22–27) | 20 (8–84) days |
| Jung, 2020 [62] | Retrospective | GA < 32 weeks on mechanical ventilation with RSS > 4. Comparison: SIMV-PC (PS)—(pre- and post-NAVA conversion). | 29 | 680 (370–1230) | 25.4 (23.4–30.3) | 32.1 (26.4–43.3) days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasundaram, P.; Sakr, M. Optimizing Invasive Neonatal Respiratory Care: A Systematic Review of Invasive Neurally Adjusted Ventilatory Assist. Healthcare 2024, 12, 632. https://doi.org/10.3390/healthcare12060632
Balasundaram P, Sakr M. Optimizing Invasive Neonatal Respiratory Care: A Systematic Review of Invasive Neurally Adjusted Ventilatory Assist. Healthcare. 2024; 12(6):632. https://doi.org/10.3390/healthcare12060632
Chicago/Turabian StyleBalasundaram, Palanikumar, and Mohamed Sakr. 2024. "Optimizing Invasive Neonatal Respiratory Care: A Systematic Review of Invasive Neurally Adjusted Ventilatory Assist" Healthcare 12, no. 6: 632. https://doi.org/10.3390/healthcare12060632
APA StyleBalasundaram, P., & Sakr, M. (2024). Optimizing Invasive Neonatal Respiratory Care: A Systematic Review of Invasive Neurally Adjusted Ventilatory Assist. Healthcare, 12(6), 632. https://doi.org/10.3390/healthcare12060632