Clinical Course, Laboratory Findings, and Prognosis of SARS-CoV-2 Infection in Infants up to 90 Days of Age: A Single-Center Experience and a Proposal for a Management Pathway
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
Demographic and Clinical Findings
4. Discussion
4.1. Clinical Presentation
4.2. Blood and Urine Test Findings
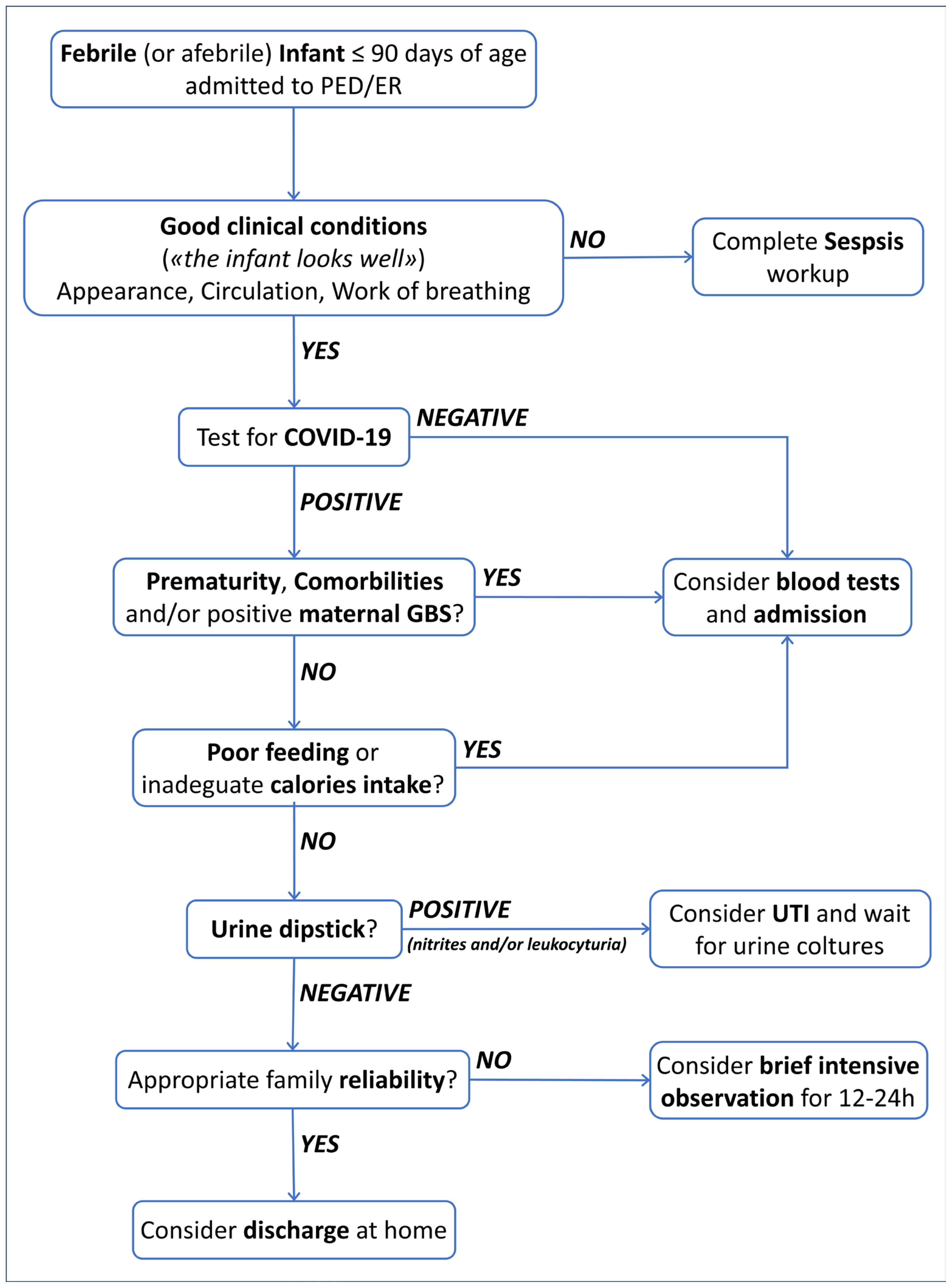
4.3. Management and Outcome
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venturini, E.; Montagnani, C.; Garazzino, S.; Donà, D.; Pierantoni, L.; Vecchio, A.L.; Nicolini, G.; Bianchini, S.; Krzysztofiak, A.; Galli, L.; et al. Treatment of children with COVID-19: Position paper of the Italian Society of Pediatric Infectious Disease. Ital. J. Pediatr. 2020, 46, 139. [Google Scholar] [CrossRef]
- Khoury, L.; Pillar, G.; Shehadeh, S. COVID-19 in neonates and infants younger than 6 months—A mild viral illness. Eur. J. Pediatr. 2023, 182, 3287–3291. [Google Scholar] [CrossRef]
- Dona’, D.; Montagnani, C.; Di Chiara, C.; Venturini, E.; Galli, L.; Vecchio, A.L.; Denina, M.; Olivini, N.; Bruzzese, E.; Campana, A.; et al. COVID-19 in Infants Less than 3 Months: Severe or Not Severe Disease? Viruses 2022, 14, 2256. [Google Scholar] [CrossRef]
- Gale, C.; A Quigley, M.; Placzek, A.; Knight, M.; Ladhani, S.; Draper, E.S.; Sharkey, D.; Doherty, C.; Mactier, H.; Kurinczuk, J.J. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: A prospective national cohort study using active surveillance. Lancet Child Adolesc. Health 2021, 5, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kanburoglu, M.K.; Tayman, C.; Oncel, M.Y.; Akin, I.M.; Can, E.; Demir, N.; Arayici, S.; Baser, D.O.; Caner, I.; Memisoglu, A.; et al. A Multicentered Study on Epidemiologic and Clinical Characteristics of 37 Neonates With Community-acquired COVID-19. Pediatr. Infect. Dis. J. 2020, 39, e297–e302. [Google Scholar] [CrossRef]
- Spoulou, V.; Noni, M.; Koukou, D.; Kossyvakis, A.; Michos, A. Clinical characteristics of COVID-19 in neonates and young infants. Eur. J. Pediatr. 2021, 180, 3041–3045. [Google Scholar] [CrossRef]
- Götzinger, F.; Santiago-Garcia, B.; Fumadó-Pérez, V.; Brinkmann, F.; Tebruegge, M.; ptbnet COVID-19 Study Group. The ability of the neonatal immune response to handle SARS-CoV-2 infection. Lancet Child Adolesc. Health 2021, 5, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Bellini, T.; Rotulo, G.A.; Caruggi, S.; Carta, S.; Bonato, I.; Piccotti, E. Characteristics of COVID-19 patients up to 6 months of age admitted to a paediatric emergency department. Acta Paediatr. 2022, 111, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Tang, M.; Yan, K.; Zhou, W. Clinical features of infants with SARS-CoV-2 infection: A systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 3394–3408. [Google Scholar] [CrossRef] [PubMed]
- Bellini, T.; Papa, R.; Calevo, M.G.; Fueri, E.; Caruggi, S.; Bonato, I.; Piccotti, E. Repeated inflammatory markers may be useful for assessing febrile infants aged 29–90 days during early hospital surveillance. Acta Paediatr. 2023, 112, 1056–1057. [Google Scholar] [CrossRef]
- Rybak, A.; Aupiais, C.; Cotillon, M.; Basmaci, R.; de Pontual, L.; Bonacorsi, S.; Mariani, P.; Landraud, L.; Brichler, S.; Poilane, I.; et al. Reassessing the Performance of the “Step-By-Step” Approach to Febrile Infants 90 Days of Age and Younger in the Context of the COVID-19 Pandemic: A Multicentric Retrospective Study. Pediatr. Infect. Dis. J. 2022, 41, e365–e368. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, L.; Gomez, B.; Pintos, C.; Benito, J.; Mintegi, S. Prevalence of Bacterial Infection in Febrile Infant 61–90 Days Old Compared with Younger Infants. Pediatr. Infect. Dis. J. 2019, 38, 1163–1167. [Google Scholar] [CrossRef]
- Pantell, R.H.; Roberts, K.B.; Adams, W.G.; Dreyer, B.P.; Kuppermann, N.; O’leary, S.T.; Okechukwu, K.; Woods, C.R.; Infants, S.O.F. Evaluation and Management of Well-Appearing Febrile Infants 8 to 60 Days Old. Pediatrics 2021, 148, e2021052228. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.; Mintegi, S.; Bressan, S.; Da Dalt, L.; Gervaix, A.; Lacroix, L. Validation of the “Step-by-Step” Approach in the Management of Young Febrile Infants. Pediatrics 2016, 138, e20154381. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, I.; Alfvén, T.; Mossberg, M.; Tenland, M.; Fernandez, J.S.; Eklund, E.A.; Elfving, K. Age- and sex-specific prevalence of serious bacterial infections in febrile infants ≤60 days, in Sweden. Acta Paediatr. 2021, 110, 3069–3076. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, N.; Mahajan, P.; Dayan, P.S. Fever, Absolute Neutrophil Count, Procalcitonin, and the AAP Febrile Infant Guidelines. Pediatrics 2023, 151, e2022059862. [Google Scholar] [CrossRef] [PubMed]
- Piché-Renaud, P.-P.; Panetta, L.; Farrar, D.S.; Moore-Hepburn, C.; Drouin, O.; Papenburg, J.; Salvadori, M.I.; Laffin, M.; Kakkar, F.; Morris, S.K.; et al. Clinical manifestations and disease severity of SARS-CoV-2 infection among infants in Canada. PLoS ONE 2022, 17, e0272648. [Google Scholar] [CrossRef]
- Hassan, M.; Khalil, A.; Magboul, S.; Alomari, O.; Abdalla, T.; Alsliman, H.; Alhothi, A.; Al Maslamani, E.; AlAmri, M.; Soliman, A. Neonates and Young Infants with COVID-19 Presented with Sepsis-Like Syndrome: A Retrospective Case Controlled Study. Front. Pediatr. 2021, 9, 634844. [Google Scholar] [CrossRef]
- Servidio, A.G.; Visentin, G.; Conti, R.; Cozzi, G.; Travan, L.; Bua, J.; Barbi, E.; Amaddeo, A. Mild COVID-19 in hospitalised infants younger than 90 days. Acta Paediatr. 2023, 112, 483–485. [Google Scholar] [CrossRef]
- Brisca, G.; Mariani, M.; Rotulo, G.A.; Pirlo, D.; Romanengo, M.; Castagnola, E.; Piccotti, E.; Moscatelli, A. Clinical course of COVID-19 in children with pre-existing medical conditions. Acta Paediatr. 2021, 110, 1291–1292. [Google Scholar] [CrossRef]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Calò Carducci, F.I.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef]
- Aronson, P.L.; Louie, J.P.; Kerns, E.; Jennings, B.; Magee, S.; Wang, M.E.; Gupta, N.; Kovaleski, C.; McDaniel, L.M.; McDaniel, C.E.; et al. Prevalence of Urinary Tract Infection, Bacteremia, and Meningitis among Febrile Infants Aged 8 to 60 Days with SARS-CoV-2. JAMA Netw. Open 2023, 6, e2313354. [Google Scholar] [CrossRef]
- Orfanos, I.; Elfving, K.; Fernandez, J.S.; Wennlund, L.; Weiber, S.; Eklund, E.A.; Alfvén, T. Management and Outcome of Febrile Infants ≤60 days, with Emphasis on Infants ≤21 Days Old, in Swedish Pediatric Emergency Departments. Pediatr. Infect. Dis. J. 2022, 41, 537–543. [Google Scholar] [CrossRef]
- Schroeder, A.R.; Chang, P.W.; Shen, M.W.; Biondi, E.A.; Greenhow, T.L. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics 2015, 135, 965–971. [Google Scholar]
- Herreros, M.L.; Tagarro, A.; García-Pose, A.; Sánchez, A.; Cañete, A.; Gili, P. Performing a urine dipstick test with a clean-catch urine sample is an accurate screening method for urinary tract infections in young infants. Acta Paediatr. 2018, 107, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Sovtic, A.; Garelli, D.; Krivec, U.; Silvagni, D.; Corsini, I.; Colombo, M.; Giangreco, M.; Giannattasio, A.; Milani, G.P.; et al. SARS-CoV-2-related bronchiolitis: A multicentre international study. Arch. Dis. Child. 2023, 108, e15. [Google Scholar] [CrossRef] [PubMed]
- Merckx, J.; Morris, S.K.; Bitnun, A.; Gill, P.; El Tal, T.; Laxer, R.M.; Yeh, A.; Yea, C.; Ulloa-Gutierrez, R.; Brenes-Chacon, H.; et al. Infants hospitalized for acute COVID-19: Disease severity in a multicenter cohort study. Eur. J. Pediatr. 2022, 181, 2535–2539. [Google Scholar] [CrossRef]
- De Bernardo, G.; Giordano, M.; Zollo, G.; Chiatto, F.; Sordino, D.; De Santis, R.; Perrone, S. The clinical course of SARS-CoV-2 positive neonates. J. Perinatol. 2020, 40, 1462–1469. [Google Scholar] [CrossRef]
- Raschetti, R.; Vivanti, A.J.; Vauloup-Fellous, C.; Loi, B.; Benachi, A.; De Luca, D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020, 11, 5164. [Google Scholar] [CrossRef]
- Carter, B.; Roland, D.; Bray, L.; Harris, J.; Pandey, P.; Fox, J.; Carrol, E.D.; Neill, S. A systematic review of the organizational, environmental, professional and child and family factors influencing the timing of admission to hospital for children with serious infectious illness. PLoS ONE 2020, 15, e0236013. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.; McNally, J.D.; Stukel, T.A.; Lu, H.; Fisman, D.; Kwong, J.C.; Guttmann, A. Family and Child Risk Factors for Early-Life RSV Illness. Pediatrics 2021, 147, e2020029090. [Google Scholar] [CrossRef]
- Ungar, S.P.; Solomon, S.; Stachel, A.; Shust, G.F.; Clouser, K.N.; Bhavsar, S.M.; Lighter, J. Hospital and ICU Admission Risk Associated with Comorbidities among Children with COVID-19 Ancestral Strains. Clin. Pediatr. 2023, 62, 1048–1058. [Google Scholar] [CrossRef]
- Uka, A.; Buettcher, M.; Bernhard-Stirnemann, S.; Fougère, Y.; Moussaoui, D.; Kottanattu, L.; Wagner, N.; Zimmermann, P.; Ritz, N.; Albisetti, M.; et al. Factors associated with hospital and intensive care admission in paediatric SARS-CoV-2 infection: A prospective nationwide observational cohort study. Eur. J. Pediatr. 2022, 181, 1245–1255, Erratum in Eur. J. Pediatr. 2022, 181, 1257. https://doi.org/10.1007/s00431-021-04359-7. [Google Scholar] [CrossRef]
- Khamkar, A.M.; Mahindre, A.; Pote, P.D.; Suryawanshi, P.; Jose, G.E. Is COVID in Neonates Really Mild? Indian J. Pediatr. 2021, 88, 1270. [Google Scholar] [CrossRef]
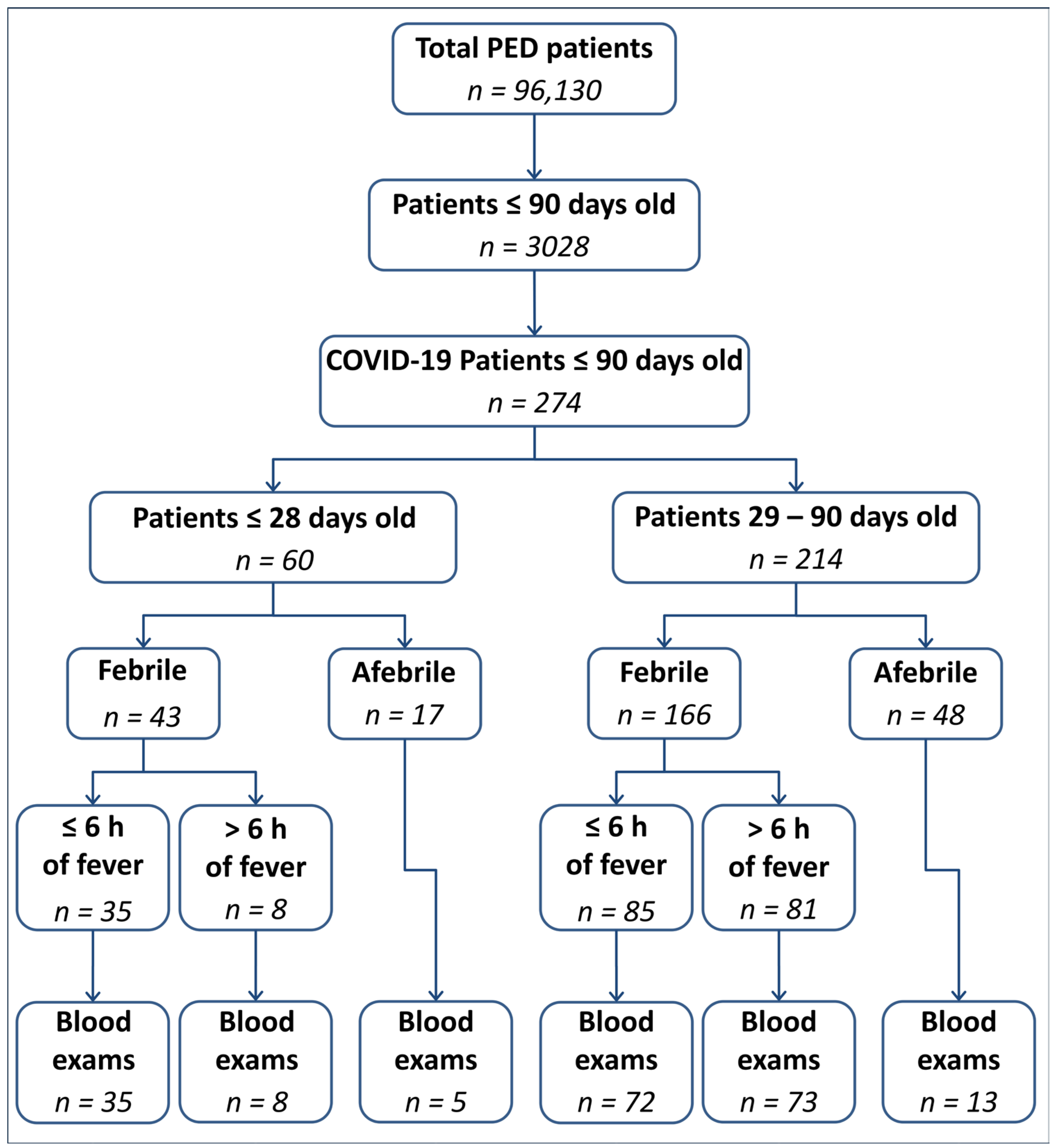
| Total n = 274 | Patients <29 Days Old n = 60 | Patients ≥29 Day Old n = 214 | p | |
|---|---|---|---|---|
| Age, median (IQR 25–75) | 47 (31–68) | 16 (10.75–22.25) | 54.5 (41–71) | <0.0001 |
| Sex, male (%) | 152 (55.0%) | 33 (55%) | 119 (55.5%) | 0.93 |
| Comorbidities, yes (%) | 23 (9%) | 5 (8.5%) | 18 (9%) | 0.81 |
| Older sibling, yes (%) | 76 (43.5%) | 22 (47%) | 54 (42%) | 0.58 |
| Maternal GBS, positive (%) | 17 (6.5%) | 6 (10.5%) | 11 (5.5%) | 0.19 |
| Admission, yes (%) | 195 (71%) | 50 (83.5%) | 145 (67.5%) | 0.018 |
| LOS in h, median (IQR 25–75) | 48 (48–72) | 48 (48–78) | 48 (48–72) | 0.94 |
| Poor clinical condition, yes (%) | 8 (3%) | 3 (5%) | 5 (2.5%) | 0.27 |
| Asymptomatic, yes (%) | 19 (7%) | 6 (10%) | 13 (6%) | 0.29 |
| Fever, yes (%) | 209 (76%) | 43 (71.5%) | 166 (77.5%) | 0.34 |
| Fever < 6 h, yes (%) | 120 (57.5%) | 35 (81%) | 85 (51%) | 0.004 |
| Blood exams, yes (%) | 206 (75%) | 48 (80%) | 158 (74%) | 0.32 |
| Blood exams in fever, yes (%) | 188 (90%) | 43 (100%) | 145 (87%) | 0.013 |
| Blood exams in afebrile, yes (%) | 65 (24%) | 5 (29.5%) | 13 (27%) | 0.85 |
| Urine exams, yes (%) | 192 (70%) | 38 (63%) | 154 (78%) | 0.19 |
| Positive nitrites, yes (%) | 2 (1%) | 0 (%) | 2 (1.5%) | 0.47 |
| Leukocyturia, yes (%) | 9 (4.5%) | 0 (%) | 9 (6%) | 0.12 |
| Urine culture collected, yes (%) | 66 (34%) | 12 (31.5%) | 54 (35%) | 0.68 |
| Urine culture, positive (%) | 2 (3%) | 0 (0%) | 2 (3.5%) | 0.49 |
| Blood culture collected, yes (%) | 47 (23%) | 11 (23%) | 36 (22.5%) | 0.98 |
| Blood culture, positive (%) | 0 (0%) | 0 (%) | 0 (%) | / |
| RSV coinfection, yes (%) | 5 (2%) | 0 (0%) | 5 (2.5%) | 0.23 |
| GI symptoms, yes (%) | 18 (6.5%) | 3 (5%) | 15 (7%) | 0.57 |
| Poor feeding, yes (%) | 61 (22%) | 19 (31.5%) | 42 (19.5%) | 0.047 |
| LRT symptoms, yes (%) | 19 (7%) | 2 (3.5%) | 17 (8%) | 0.21 |
| URT symptoms, yes (%) | 93 (34%) | 21 (35%) | 72 (33.5%) | 0.84 |
| Dyspnea, yes (%) | 16 (6%) | 3 (3.5%) | 13 (6%) | 0.69 |
| Apnea, yes (%) | 6 (2%) | 1 (1.5%) | 5 (2.5%) | 0.75 |
| Cutaneous rash, yes (%) | 7 (2.5%) | 1 (1.5%) | 6 (3%) | 0.62 |
| Oxygen supplementation, yes (%) | 10 (3.5%) | 3 (3.5%) | 7 (3.5%) | 0.52 |
| HFNC, yes (%) | 1 (0.5%) | 0 (0%) | 1 (0.5%) | 0.59 |
| Total Patients n = 107 | Patients <29 Days Old n = 35 | Patients ≥29 Days Old n = 72 | p | |
|---|---|---|---|---|
| CRP in mg/dL, median (IQR 25–75) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.08) | 0.34 |
| CRP > 2 mg/dL, yes (%) | 11 (10%) | 0 (0%) | 11 (15%) | 0.014 |
| PCT in ng/mL, median (IQR 25–75) | 0.11 (0.05–0.14) | 0.11 (0.08–0.13) | 0.10 (0.05–0.14) | 0.85 |
| PCT > 0.5 ng/mL, yes (%) | 2 (2%) | 1 (3%) | 1 (1.5%) | 0.59 |
| WBC in cells/mm3, median (IQR 25–75) | 7100 (5300–8750) | 7900 (6000–8850) | 6650 (5150–8375) | 0.16 |
| WBC > 10,000 cells/mm3, yes (%) | 12 (11%) | 3 (8.5%) | 9 (12.5%) | 0.54 |
| ANC in cells/mm3, median (IQR 25–75) | 2300 (1750–3400) | 2800 (2100–3600) | 2150 (1500–3200) | 0.03 |
| ANC < 1000 cells/mm3, yes (%) | 3 (3%) | 1 (3%) | 2 (3%) | 0.98 |
| ANC > 5000 cells/mm3, yes (%) | 6 (6%) | 0 (0%) | 6 (8.5%) | 0.07 |
| Total Patients n = 81 | Patients <29 Days Old n = 8 | Patients ≥29 Days Old n = 73 | p | |
|---|---|---|---|---|
| CRP in mg/dL, median (IQR 25–75) | 0.00 (0.00–0.00) | 0.00 (0.00–0.04) | 0.00 (0.00–0.00) | 0.51 |
| CRP > 2 mg/dL, yes (%) | 2 (2.5%) | 1 (12.5%) | 1 (1.5%) | 0.054 |
| PCT in ng/mL, median (IQR 25–75) | 0.11 (0.05–0.16) | 0.15 (0.10–0.19) | 0.10 (0.05–0.15) | 0.65 |
| PCT > 0.5 ng/mL, yes (%) | 1 (1%) | 0 (0%) | 1 (1.5%) | 0.73 |
| WBC in cells/mm3, median (IQR 25–75) | 7200 (5150–9800) | 10,200 (7400–11,000) | 6500 (4300–8900) | 0.005 |
| WBC > 10,000 cells/mm3, yes (%) | 19 (23.5%) | 5 (62.5%) | 14 (19%) | 0.006 |
| ANC in cells/mm3, median (IQR 25–75) | 1970 (1000–2525) | 2100 (1600–2550) | 1600 (1000–2500) | 0.25 |
| ANC < 1000 cells/mm3, yes (%) | 18 (22%) | 2 (25%) | 16 (22%) | 0.84 |
| ANC > 5000 cells/mm3, yes (%) | 5 (6%) | 1 (12.5%) | 4 (5.5%) | 0.43 |
| Total < 29 Days Old Patients n = 48 | Febrile Patients n = 43 | Afebrile Patients n = 5 | p | |
|---|---|---|---|---|
| Age, median (IQR 25–75) | 16 (10.75–22.25) | 16 (10.50–22.00) | 16 (11.00–23.00) | 0.67 |
| Sex, male (%) | 25 (52%) | 22 (51%) | 3 (60%) | 0.70 |
| CRP in mg/dL, median (IQR 25–75) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.60 |
| CRP > 2 mg/dL, yes (%) | 1 (2%) | 1 (2.5%) | 0 (0%) | 0.73 |
| PCT in ng/mL, median (IQR 25–75) | 0.11 (0.07–0.15) | 0.12 (0.08–0.15) | 0.08 (0.04–0.12) | 0.87 |
| PCT > 0.5 ng/mL, yes (%) | 1 (2%) | 1 (2.5%) | 0 (0%) | 0.73 |
| WBC in cells/mm3, median (IQR 25–75) | 7900 (6300–9200) | 7950 (6400–9975) | 8500 (6750–10,400) | 0.20 |
| WBC > 10,000 cells/mm3, yes (%) | 9 (19%) | 8 (18.5%) | 1 (20%) | 0.94 |
| ANC in cells/mm3, median (IQR 25–75) | 2500 (1950–3500) | 2450 (2000–3475) | 1500 (1450–2150) | 0.065 |
| ANC < 1000 cells/mm3, yes (%) | 1 (2%) | 3 (7%) | 0 (0%) | 0.54 |
| ANC > 5000 cells/mm3, yes (%) | 0 (0%) | 1 (2.5%) | 0 (0%) | 0.73 |
| Total 29–90 Days Old Patients n = 158 | Febrile Patients n = 145 | Afebrile Patients n = 13 | p | |
|---|---|---|---|---|
| Age, median (IQR 25–75) | 54.5 (41–71) | 55.5 (42–72) | 51.00 (37.75–67.75) | 0.26 |
| Sex, male (%) | 88 (55.5%) | 81 (56%) | 7 (54%) | 0.88 |
| CRP in mg/dL, median (IQR 25–75) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.27 |
| CRP > 2 mg/dL, yes (%) | 12 (7.5%) | 12 (8%) | 0 (0%) | 0.28 |
| PCT in ng/mL, median (IQR 25–75) | 0.09 (0.03–0.14) | 0.10 (0.05–0.15) | 0.00 (0.00–0.05) | 0.32 |
| PCT > 0.5 ng/mL, yes (%) | 2 (1.5%) | 2 (1.5%) | 0 (0%) | 0.66 |
| WBC in cells/mm3, median (IQR 25–75) | 6600 (4800–8900) | 6600 (4800–8900) | 8900 (5900–9550) | 0.19 |
| WBC > 10,000 cells/mm3, yes (%) | 25(16%) | 23 (16%) | 2(15%) | 0.96 |
| ANC in cells/mm3, median (IQR 25–75) | 2000 (1285–2800) | 2000 (1200–2700) | 1700 (1400–2500) | 0.59 |
| ANC < 1000 cells/mm3, yes (%) | 20 (12.5%) | 18 (12.5%) | 2 (15%) | 0.75 |
| ANC > 5000 cells/mm3, yes (%) | 10 (6%) | 10 (7%) | 0 (0%) | 0.32 |
| Hospitalization | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| No | Yes | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | |
| Sex | 0.893 | 1.06 (0.628–1.79) | ||||
| Female | 36 (29.5%) | 86 (70.5%) | ||||
| Male | 43 (28.3%) | 109 (71.7%) | ||||
| Maternal positive GBS | 3 (17.6%) | 14 (82.4%) | 0.572 | 1.79 (0.499–6.45) | ||
| Comorbidities | 4 (17.4%) | 19 (82.6%) | 0.457 | 1.68 (0.548–5.13) | ||
| Older sibling | 11 (14.5%) | 65 (85.5%) | 0.546 | 1.31 (0.579–2.98) | ||
| Poor clinical condition | 0 (0) | 8 (100%) | 0.11 | 7.12 (0.406–125) * | ||
| Fever | 54 (25.8%) | 155 (74.2%) | 0.06 | 1.79 (0.997–3.23) | 0.002 | 2.79 (1.44–5.39) |
| Dyspnea | 0 (0) | 16 (100%) | 0.008 | 14.6 (0.866–247) * | 0.986 | / |
| URT symptoms | 24 (25.8%) | 69 (74.2%) | 0.482 | 1.25 (0.715–2.2) | ||
| LRT symptoms | 1 (5.3%) | 18 (94.7%) | 0.017 | 7.93 (1.04–60.5) | 0.128 | 5.43 (0.61–47.98) |
| Vomiting | 0 (0) | 5 (100%) | 0.326 | 4.59 (0.251–84.0) * | ||
| Diarrhea | 7 (50%) | 7 (50%) | 0.125 | 0.383 (0.130–1.13) | ||
| Poor feeding | 9 (15%) | 51 (85%) | 0.009 | 2.75 (1.28–5.91) | 0.015 | 2.78 (1.21–6.35) |
| Cutaneous rash | 1 (14.3%) | 6 (85.7%) | 0.677 | 2.48 (0.293–20.9) | ||
| WBC > 10,000/mmc | 0 (0) | 15 (100%) | 0.213 | 5.69 (0.323–100) * | ||
| ANC > 5000/mmc | 1 (16.7%) | 5 (83.3%) | 0.58 | 0.745 (0.08–6.85) | ||
| CRP > 2 mg/dL | 0 (0) | 1 (100%) | 1 | 1.10 (0.05–22.4) * | ||
| PCT > 0.5 ng/mL | 0 (0) | 2 (100%) | 1 | 1.04 (0.04–22.9) * | ||
| Age (days, median) | 57 | 42 | <0.001 | 0.98 (0.968–0.992) | <0.001 | 0.976 (0.964–0.989) |
| LOS (h, Median) | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| p | Estimate (95% CI) | p | Estimate (95% CI) | ||
| Sex (male) | 48 | 0.183 | 4.48 (−14.8–2.85) | ||
| Maternal positive GBS | 84 | 0.020 | 20.3 (3.29–37.3) | 0.154 | 19.10 (−7.35–45.6) |
| Comorbidities | 72 | 0.051 | 14.7 (−0.06–29.4) | ||
| Older sibling | 48 | 0.246 | 6 (−4.18–16.2) | ||
| Poor clinical condition | 108 | <0.001 | 46.2 (25.2–67.3) | 0.190 | 25.14 (−12.77–63.1) |
| Fever | 48 | 0.629 | −2.69 (−13.6–8.26) | ||
| Dyspnea | 84 | 0.003 | 23.8 (8.21–39.4) | 0.979 | −0.443 (−34.22–33.3) |
| URT symptoms | 72 | 0.185 | 6.18 (−2.97–15.3) | ||
| LRT symptoms | 96 | <0.001 | 26.1 (11.4–40.7) | 0.139 | 28.47 (−9.5–66.4) |
| Vomiting | 72 | 0.338 | 13.4 (−14.2–41) | ||
| Diarrhea | 48 | 0.441 | −9.19 (−32.7–14.3) | ||
| Poor feeding | 48 | 0.006 | 13.8 (3.99–23.7) | ||
| Cutaneous rash | 60 | 0.854 | 2.37 (−23–27.7) | ||
| WBC > 10,000/mmc | 72 | 0.210 | 11.3 (−6.46–29) | ||
| ANC > 5000/mmc | 48 | 0.249 | −17 (−46–12.1) | ||
| CRP > 2 mg/dL | 96 | 0.183 | 24.7 (−11.9–61.4) | ||
| PCT > 0.5 ng/mL | 120 | 0.012 | 58.2 (13.3–103.1) | 0.020 | 54.64 (9.07–100.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, T.; Brisca, G.; Orfanos, I.; Mariani, M.; Pezzotta, F.; Giordano, B.; Pastorino, A.; Misley, S.; Formigoni, C.; Fueri, E.; et al. Clinical Course, Laboratory Findings, and Prognosis of SARS-CoV-2 Infection in Infants up to 90 Days of Age: A Single-Center Experience and a Proposal for a Management Pathway. Healthcare 2024, 12, 528. https://doi.org/10.3390/healthcare12050528
Bellini T, Brisca G, Orfanos I, Mariani M, Pezzotta F, Giordano B, Pastorino A, Misley S, Formigoni C, Fueri E, et al. Clinical Course, Laboratory Findings, and Prognosis of SARS-CoV-2 Infection in Infants up to 90 Days of Age: A Single-Center Experience and a Proposal for a Management Pathway. Healthcare. 2024; 12(5):528. https://doi.org/10.3390/healthcare12050528
Chicago/Turabian StyleBellini, Tommaso, Giacomo Brisca, Ioannis Orfanos, Marcello Mariani, Federico Pezzotta, Benedetta Giordano, Andrea Pastorino, Silvia Misley, Clelia Formigoni, Elena Fueri, and et al. 2024. "Clinical Course, Laboratory Findings, and Prognosis of SARS-CoV-2 Infection in Infants up to 90 Days of Age: A Single-Center Experience and a Proposal for a Management Pathway" Healthcare 12, no. 5: 528. https://doi.org/10.3390/healthcare12050528
APA StyleBellini, T., Brisca, G., Orfanos, I., Mariani, M., Pezzotta, F., Giordano, B., Pastorino, A., Misley, S., Formigoni, C., Fueri, E., Ferretti, M., Marin, M., Finetti, M., Piccotti, E., Castagnola, E., & Moscatelli, A. (2024). Clinical Course, Laboratory Findings, and Prognosis of SARS-CoV-2 Infection in Infants up to 90 Days of Age: A Single-Center Experience and a Proposal for a Management Pathway. Healthcare, 12(5), 528. https://doi.org/10.3390/healthcare12050528






