Prevalence and Predictors of Depression in Women with Osteoarthritis: Cross-Sectional Analysis of Nationally Representative Survey Data
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Sample
2.3. Measures
2.4. Statistical Analysis
3. Results
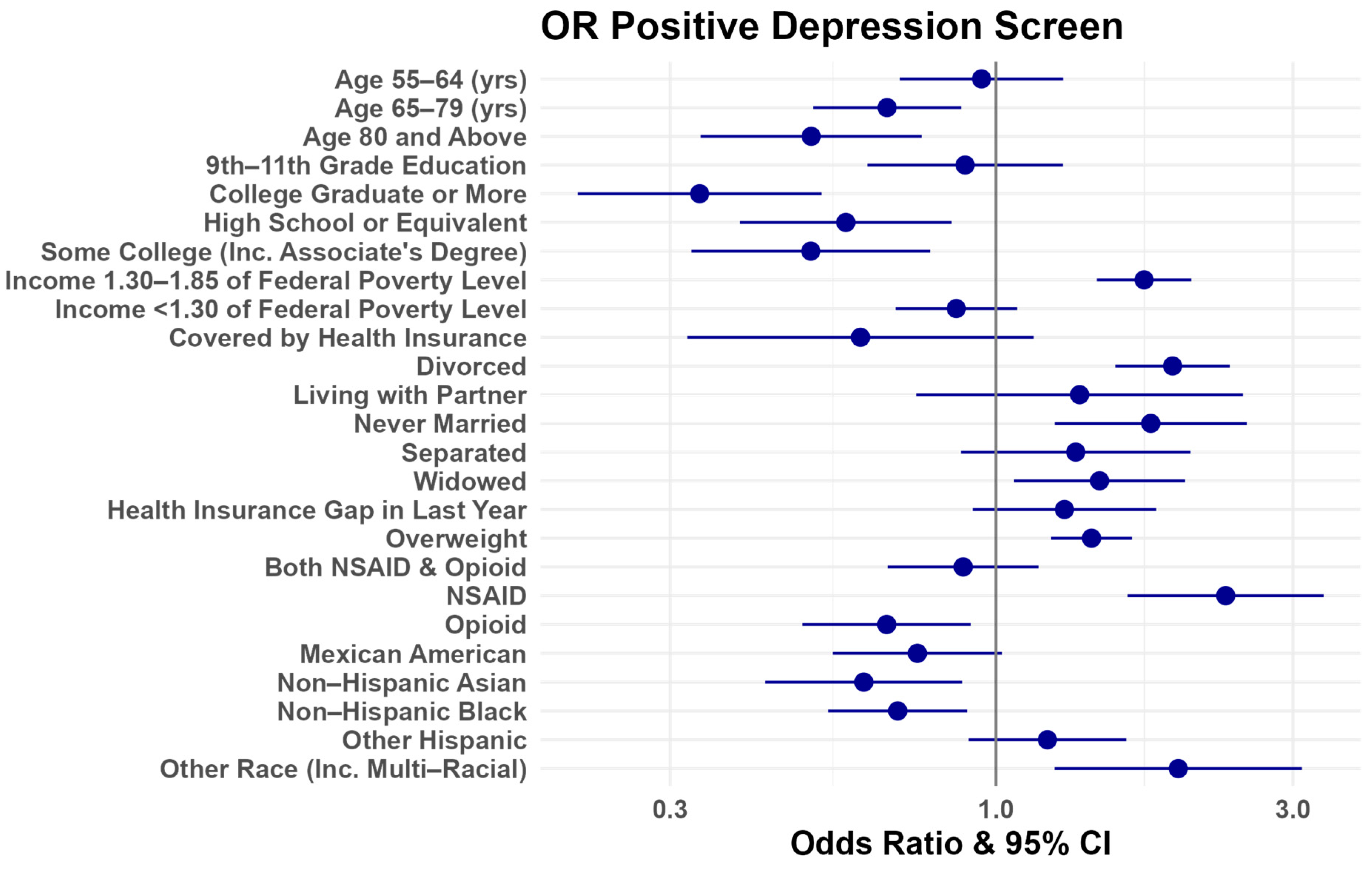
4. Discussion
5. Limitations and Recommendations for Future Research Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Center for Disease Control and Prevention (CDC). Osteoarthritis. Available online: https://www.cdc.gov/arthritis/types/osteoarthritis.htm#:~:text=OA%20affects%20over%2032.5%20million,of%20joint%20cartilage%20between%20bones.&text=Some%20people%20are%20concerned%20that,function%2C%20and%20quality%20of%20life (accessed on 22 January 2024).
- Xu, Y.; Wu, Q. Trends and disparities in osteoarthritis prevalence among US adults, 2005–2018. Sci. Rep. 2021, 11, 21845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Cristina de Oliveira, N.; Alfieri, F.M.; Lima, A.R.S.; Portes, L.A. Lifestyle and Pain in Women With Knee Osteoarthritis. Am. J. Lifestyle Med. 2019, 13, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shah, D.; Gandhi, K.; Wei, W.; Dwibedi, N.; Webster, L.; Sambamoorthi, U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthr. Cartil. 2019, 27, 1618–1626. [Google Scholar] [CrossRef]
- White, A.G.; Birnbaum, H.G.; Janagap, C.; Buteau, S.; Schein, J. Direct and indirect costs of pain therapy for osteoarthritis in an insured population in the United States. J. Occup. Environ. Med. 2008, 50, 998–1005. [Google Scholar] [CrossRef]
- Gupta, S.; Hawker, G.A.; Laporte, A.; Croxford, R.; Coyte, P.C. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology 2005, 44, 1531–1537. [Google Scholar] [CrossRef]
- Stubbs, B.; Aluko, Y.; Myint, P.K.; Smith, T.O. Prevalence of depressive symptoms and anxiety in osteoarthritis: A systematic review and meta-analysis. Age Ageing 2016, 45, 228–235. [Google Scholar] [CrossRef]
- Marshall, D.A.; Liu, X.; Barnabe, C.; Yee, K.; Faris, P.D.; Barber, C.; Mosher, D.; Noseworthy, T.; Werle, J.; Lix, L. Existing comorbidities in people with osteoarthritis: A retrospective analysis of a population-based cohort in Alberta, Canada. BMJ Open 2019, 9, e033334. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; King, L.K. The Burden of Osteoarthritis in Older Adults. Clin. Geriatr. Med. 2022, 38, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, L.; Cicuttini, F.; Zhu, Z.; Han, W.; Antony, B.; Wluka, A.E.; Winzenberg, T.; Aitken, D.; Blizzard, L.; et al. Depression in patients with knee osteoarthritis: Risk factors and associations with joint symptoms. BMC Musculoskelet. Disord. 2021, 22, 40. [Google Scholar] [CrossRef]
- Nowinka, Z.; Alagha, M.A.; Mahmoud, K.; Jones, G.G. Predicting Depression in Patients with Knee Osteoarthritis Using Machine Learning: Model Development and Validation Study. JMIR Form. Res. 2022, 6, e36130. [Google Scholar] [CrossRef]
- Vajapey, S.P.; McKeon, J.F.; Krueger, C.A.; Spitzer, A.I. Outcomes of total joint arthroplasty in patients with depression: A systematic review. J. Clin. Orthop. Trauma 2021, 18, 187–198. [Google Scholar] [CrossRef]
- Wang, S.T.; Ni, G.X. Depression in Osteoarthritis: Current Understanding. Neuropsychiatr. Dis. Treat. 2022, 18, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.A. Depression in Older Adults: A Treatable Medical Condition. Prim. Care 2017, 44, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Karrouri, R.; Hammani, Z.; Benjelloun, R.; Otheman, Y. Major depressive disorder: Validated treatments and future challenges. World J. Clin. Cases 2021, 9, 9350–9367. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Pan, X.; Sambamoorthi, U. Depression treatment patterns among individuals with osteoarthritis: A cross sectional study. BMC Psychiatry 2013, 13, 121. [Google Scholar] [CrossRef]
- Hägg, S.; Jylhävä, J. Sex differences in biological aging with a focus on human studies. eLife 2021, 10, e63425. [Google Scholar] [CrossRef]
- Schepman, P.T.S.; Robinson, R.; Malhotra, D.; Emir, B.; Beck, C. Moderate to Severe Osteoarthritis Pain and Its Impact on Patients in the United States: A National Survey. J. Pain Res. 2021, 14, 2313–2326. [Google Scholar] [CrossRef]
- Van Dyne, A.; Moy, J.; Wash, K.; Thompson, L.; Skow, T.; Roesch, S.C.; Cronan, T. Health, Psychological and Demographic Predictors of Depression in People with Fibromyalgia and Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 3413. [Google Scholar] [CrossRef]
- Kokkeler, K.J.E.; Marijnissen, R.M.; Wardenaar, K.J.; Rhebergen, D.; van den Brink, R.H.S.; van der Mast, R.C.; Oude Voshaar, R.C. Subtyping late-life depression according to inflammatory and metabolic dysregulation: A prospective study. Psychol. Med. 2022, 52, 515–525. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data; Centers for Disease Control and Prevention (CDC): Hyattsville, MD, USA, 2022. [Google Scholar]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Health and Nutrition Examination Survey (NHANES) MEC Interviewers Procedures Manual; National Center for Health Statistics: Hyattsville, MD, USA, 2016; pp. 5-1–5-3.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Susman, H. RNHANES: Facilitates Analysis of CDC NHANES Data. R Package Version 1.1.0. 2016. Available online: http://github.com/silentspringinstitute/RNHANES (accessed on 9 July 2022).
- Yoshida, K.; Alexander, B. Tableone: Create ‘Table 1’ to Describe Baseline Characteristics with or without Propensity Score Weights. R Package Version 0.13.0. 2021. Available online: https://CRAN.R-project.org/package=tableone (accessed on 9 July 2022).
- Lumley, T. Survey: Analysis of Complex Survey Samples, R Package Version 4.2; Computer software. 2023.
- Hothorn, A.Z.A.T. Diagnostic Checking in Regression Relationships. R News 2012, 2, 7–10. Available online: https://CRAN.R-project.org/doc/Rnews/ (accessed on 9 July 2022).
- Kaplan, J. fastDummies: Fast Creation of Dummy (Binary) Columns and Rows from Categorical Variables. 2020. R package version 1.6.3. Available online: https://CRAN.R-project.org/package=fastDummies (accessed on 9 July 2022).
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Forkmann, T.; Gauggel, S.; Spangenberg, L.; Brahler, E.; Glaesmer, H. Dimensional assessment of depressive severity in the elderly general population: Psychometric evaluation of the PHQ-9 using Rasch Analysis. J. Affect. Disord. 2013, 148, 323–330. [Google Scholar] [CrossRef]
- Kim, D.J. A Study on the Physical Activities, Mental Health, and Health-Related Quality of Life of Osteoarthritis Patients. Osong Public Health Res. Perspect. 2019, 10, 368–375. [Google Scholar] [CrossRef]
- Park, H.-M.; Kim, H.-S.; Lee, Y.-J. Knee osteoarthritis and its association with mental health and health-related quality of life: A nationwide cross-sectional study. Geriatr. Gerontol. Int. 2020, 20, 379–383. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Nam, S.-M. Effects of Activity Limitations on the Health-Related Quality of Life and Depression in Osteoarthritis Patients. KSPM 2020, 15, 109–116. [Google Scholar] [CrossRef]
- Holla, J.F.M.; Leeden, M.v.d.; Heymans, M.W.; Roorda, L.D.; Bierma-Zeinstra, S.M.A.; Boers, M.; Lems, W.F.; Steultjens, M.P.M.; Dekker, J. Three trajectories of activity limitations in early symptomatic knee osteoarthritis: A 5-year follow-up study. Ann. Rheum. Dis. 2014, 73, 1369. [Google Scholar] [CrossRef]
- Han, A.; Gellhorn, A.C. Trajectories of Quality of Life and Associated Risk Factors in Patients With Knee Osteoarthritis: Findings From the Osteoarthritis Initiative. Am. J. Phys. Med. Rehabil. 2018, 97, 620–627. [Google Scholar] [CrossRef]
- Bromberger, J.T.; Kravitz, H.M.; Chang, Y.F.; Cyranowski, J.M.; Brown, C.; Matthews, K.A. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol. Med. 2011, 41, 1879–1888. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef]
- Wluka, A.E.; Cicuttini, F.M.; Spector, T.D. Menopause, oestrogens and arthritis. Maturitas 2000, 35, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Vivian-Taylor, J.; Hickey, M. Menopause and depression: Is there a link? Maturitas 2014, 79, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, E.; Fereidooni, B.; Khazaei, S.; Hamzehei, R.; Tapak, L. The Relationship Between Depression and Menopause Symptoms: The PATH Model. Curr. Women’s Health Rev. 2021, 17, 92–98. [Google Scholar] [CrossRef]
- Park, S. Mediating effect of a health-promoting lifestyle in the relationship between menopausal symptoms, resilience, and depression in middle-aged women. Health Care Women Int. 2020, 41, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Bhanu, C.; Frost, R.; Buszewicz, M.; Walters, K.R. A Systematic Review of Older Adults’ Attitudes Towards Depression and Its Treatment. Gerontologist 2020, 60, e93–e104. [Google Scholar] [CrossRef] [PubMed]
- Polacsek, M.; Boardman, G.H.; McCann, T.V. Help-seeking experiences of older adults with a diagnosis of moderate depression. Int. J. Ment. Health Nurs. 2019, 28, 278–287. [Google Scholar] [CrossRef]
- Atkins, J.; Naismith, S.L.; Luscombe, G.M.; Hickie, I.B. Elderly care recipients’ perceptions of treatment helpfulness for depression and the relationship with help-seeking. Clin. Interv. Aging 2015, 10, 287–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carlesso, L.C.; Jafarzadeh, S.R.; Stokes, A.; Felson, D.T.; Wang, N.; Frey-Law, L.; Lewis, C.E.; Nevitt, M.; Neogi, T.; Multicenter Osteoarthritis Study Group. Depressive symptoms and multi-joint pain partially mediate the relationship between obesity and opioid use in people with knee osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Nie, Y.; Zeng, Y.; Wu, Y.; Liu, Y.; Wu, L.; Shen, B. The trajectories of depression symptoms and comorbidity in knee osteoarthritis subjects. Clin. Rheumatol. 2022, 41, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Rodrigues, D.; Rodrigues, A.; Martins, T.; Pinto, J.; Amorim, D.; Almeida, A.; Pinto-Ribeiro, F. Correlation between pain severity and levels of anxiety and depression in osteoarthritis patients: A systematic review and meta-analysis. Rheumatology 2021, 61, 53–75. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Smith, G.D.; Lohoff, F.W. Prescription Opioid Use and Risk for Major Depressive Disorder and Anxiety and Stress-Related Disorders: A Multivariable Mendelian Randomization Analysis. JAMA Psychiatry 2021, 78, 151–160. [Google Scholar] [CrossRef]
- Abstracts From the 2022 Health Care Systems Research Network (HCSRN) Annual Conference. J. Patient Cent. Res. Rev. 2022, 9, 196–245. [CrossRef]
| Demographics | Depressed (%) | Not Depressed (%) | p |
|---|---|---|---|
| Age | 0.007 | ||
| 45–54 | 34.2 | 31.7 | |
| 55–64 | 36.6 | 32 | |
| 65–79 | 23.4 | 28.4 | |
| 80+ | 5.7 | 7.9 | |
| Race | <0.001 | ||
| Non-Hispanic White | 66.7 | 72.8 | |
| Non-Hispanic Black | 10.7 | 9.9 | |
| Non-Hispanic Asian | 2.1 | 4.4 | |
| Mexican American | 6.8 | 5.3 | |
| Other Hispanic | 7.8 | 4.8 | |
| Other/Multiracial | 5.8 | 2.7 | |
| Citizenship | 0.543 | ||
| US Citizen | 94.6 | 95 | |
| Not a US citizen | 5.4 | 5 | |
| Marital Status | <0.001 | ||
| Married | 46 | 65.2 | |
| Widowed | 22.2 | 16 | |
| Divorced | 18.6 | 10.7 | |
| Separated | 2.8 | 1.5 | |
| Never Married | 7.2 | 4.4 | |
| Living with Partner | 3.3 | 2.2 | |
| Lack Health Insurance Coverage | 11.8 | 9.1 | 0.006 |
| Gap in Insurance in Past Year | 7.2 | 3.6 | <0.001 |
| Pain Medication Use | <0.001 | ||
| None | 67.4 | 88 | |
| NSAIDS | 10.3 | 5.3 | |
| Opioids | 17 | 5.2 | |
| Both | 5.2 | 1.4 | |
| Family Income | <0.001 | ||
| >1.85 Poverty Level | 45.1 | 70.6 | |
| 1.35 < Poverty Level ≤ 1.85 | 15.3 | 10.9 | |
| Poverty Level ≤ 1.35 | 39.6 | 18.4 | |
| Education Level | <0.001 | ||
| Less than 9th grade | 9.2 | 4.5 | |
| 9th–11th grade | 15.5 | 8.2 | |
| HS Graduate/GED | 28 | 23.7 | |
| Some college/AA | 31.6 | 30.6 | |
| College graduate or greater | 15.6 | 32.9 | |
| Overweight | 55 | 41.8 | <0.001 |
| Time Since Last Healthcare Visit | 0.956 | ||
| Within 1 year | 11.7 | 11.5 | |
| More than 1 year | 88.3 | 88.6 | |
| Misc. Analgesic Use | 0 | 0 | - |
| Demographics | Received Treatment for Depression (%) | No Treatment for Depression (%) | p |
|---|---|---|---|
| Age | 0.966 | ||
| 45–54 | 31.8 | 32.3 | |
| 55–64 | 32.1 | 32 | |
| 65–79 | 27.6 | 27.4 | |
| 80+ | 8.5 | 8.3 | |
| Race | <0.001 | ||
| Non-Hispanic White | 80 | 68.6 | |
| Non-Hispanic Black | 7.6 | 11.1 | |
| Non-Hispanic Asian | 1.4 | 5.8 | |
| Mexican American | 3.3 | 6.2 | |
| Other Hispanic | 4.3 | 5.5 | |
| Other/Multiracial | 3.5 | 2.8 | |
| Citizenship | <0.001 | ||
| US Citizen | 97.8 | 93.5 | |
| Not a US Citizen | 2.2 | 6.5 | |
| Education | 0.013 | ||
| Less than 9th grade | 4.7 | 6 | |
| 9th–11th | 9.5 | 8.9 | |
| HS Graduate/GED | 23 | 24.1 | |
| Some college/AA | 33.5 | 29.3 | |
| College graduate+ | 29.4 | 31.7 | |
| Marital Status | <0.001 | ||
| Married | 56.6 | 64.9 | |
| Widowed | 20.6 | 15.6 | |
| Divorced | 13.3 | 10.9 | |
| Separated | 1.8 | 1.6 | |
| Never Married | 5 | 4.6 | |
| Living with Partner | 2.7 | 2.4 | |
| Pain Medication Use | <0.001 | ||
| None | 74.6 | 89.7 | |
| NSAID | 9.2 | 4.8 | |
| Opioid | 12.4 | 4.5 | |
| Both | 3.9 | 1 | |
| Overweight (Y) | 53.3 | 39 | <0.001 |
| Time since Last Healthcare Visit | 0.015 | ||
| Within 1 year | 23.8 | 11 | |
| More than 1 year | 76.2 | 89.0 | |
| Health Insurance (N) | 10.5 | 9.5 | <0.001 |
| Gap in Insurance in Past Year (Y) | 4.1 | 3.8 | 0.581 |
| Family Income | 0.001 | ||
| >1.85 Poverty Level | 63.8 | 68.7 | |
| 1.3 < poverty level < 1.85 | 12.4 | 11.1 | |
| Poverty Level ≤ 1.35 | 23.8 | 20.1 |
| (%) | |
|---|---|
| Mental Health Visit | 38.4 |
| Any Antidepressant Use | 82.6 |
| SSRI | 57.4 |
| SNRI | 16.4 |
| TCA | 7.9 |
| MAOI | 0.1 |
| Other | 12.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravi, A.; DeMarco, E.C.; Gebauer, S.; Poirier, M.P.; Hinyard, L.J. Prevalence and Predictors of Depression in Women with Osteoarthritis: Cross-Sectional Analysis of Nationally Representative Survey Data. Healthcare 2024, 12, 502. https://doi.org/10.3390/healthcare12050502
Ravi A, DeMarco EC, Gebauer S, Poirier MP, Hinyard LJ. Prevalence and Predictors of Depression in Women with Osteoarthritis: Cross-Sectional Analysis of Nationally Representative Survey Data. Healthcare. 2024; 12(5):502. https://doi.org/10.3390/healthcare12050502
Chicago/Turabian StyleRavi, Ananya, Elisabeth C. DeMarco, Sarah Gebauer, Michael P. Poirier, and Leslie J. Hinyard. 2024. "Prevalence and Predictors of Depression in Women with Osteoarthritis: Cross-Sectional Analysis of Nationally Representative Survey Data" Healthcare 12, no. 5: 502. https://doi.org/10.3390/healthcare12050502
APA StyleRavi, A., DeMarco, E. C., Gebauer, S., Poirier, M. P., & Hinyard, L. J. (2024). Prevalence and Predictors of Depression in Women with Osteoarthritis: Cross-Sectional Analysis of Nationally Representative Survey Data. Healthcare, 12(5), 502. https://doi.org/10.3390/healthcare12050502


_MD__MPH_PhD.png)




