Usage Patterns of Traditional Chinese Medicine for Patients with Bipolar Disorder: A Population-Based Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
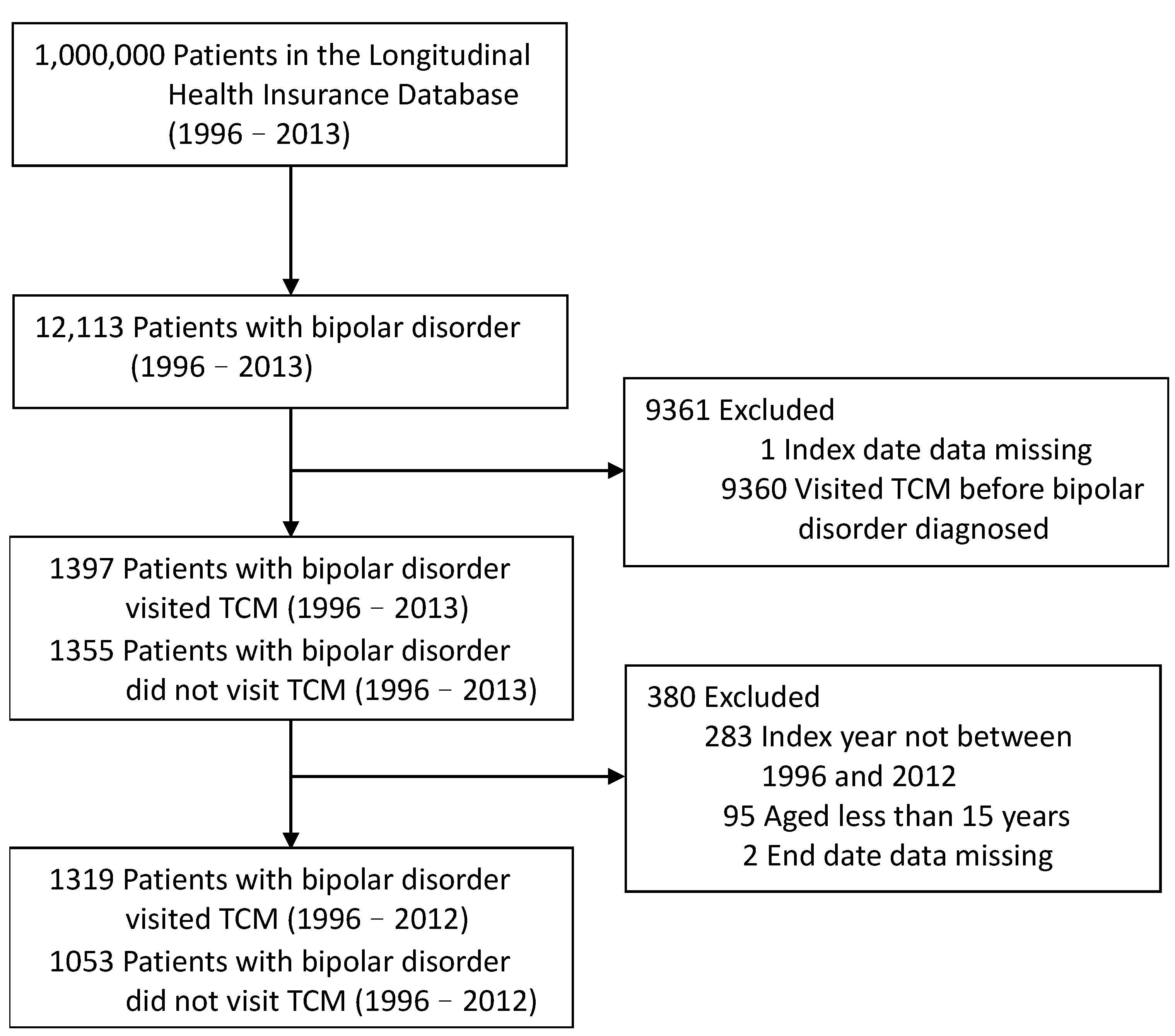
2.2. Study Population
2.3. Covariates of Comorbidities and Medications
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. TCM Treatment Interventions
3.3. Principal Diagnostic Codes Used by TCM Practitioners
3.4. Top Ten Most Commonly Prescribed Chinese Single Herbs and Herbal Formulae
4. Discussion
4.1. Demographic Characteristics
4.2. Acupuncture Treatment Intervention
4.3. Principal Diagnostic Codes Used by TCM Practitioners
4.4. Top Ten Most Commonly Prescribed Chinese Single Herbs and Herbal Formulae
4.5. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Ming, T.T.; Tohen, M.; Jones, P.B. Textbook in Psychiatric Epidemiology; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Shim, I.H.; Woo, Y.S.; Wang, H.R.; Bahk, W.M. Predictors of a Shorter Time to Hospitalization in Patients with Bipolar Disorder: Medication during the Acute and Maintenance Phases and Other Clinical Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, M.A.; Bowden, C.L.; Gitlin, M.J.; Keck, P.E.; Perlis, R.H.; Suppes, T.; Thase, M.E.; Wagner, K.D. Practice guideline for the treatment of patients with bipolar disorder (revision). Am. J. Psychiatry 2002, 159 (Suppl. S4), 1–50. [Google Scholar]
- National Collaborating Centre for Mental Health; National Institute for Health and Care Excellence. Clinical Guidelines. In Bipolar Disorder: The NICE Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care; The British Psychological Society and The Royal College of Psychiatrists: London, UK, 2014. [Google Scholar]
- Bai, Y.M.; Chang, C.J.; Tsai, S.Y.; Chen, Y.C.; Hsiao, M.C.; Li, C.T.; Tu, P.; Chang, S.W.; Shen, W.W.; Su, T.P. Taiwan consensus of pharmacological treatment for bipolar disorder. J. Chin. Med. Assoc. 2013, 76, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.A.; Keitner, G.I.; Miller, I.W.; Shea, M.T.; Keller, M.B. Course of illness and maintenance treatments for patients with bipolar disorder. J. Clin. Psychiatry 1995, 56, 5–13. [Google Scholar] [PubMed]
- Jarman, C.N.; Perron, B.E.; Kilbourne, A.M.; Teh, C.F. Perceived treatment effectiveness, medication compliance, and complementary and Alternative medicine use among veterans with bipolar disorder. J. Altern. Complement. Med. 2010, 16, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Perron, B.E.; Jarman, C.N.; Kilbourne, A.M. Access to conventional mental health and medical care among users of complementary and Alternative medicine with bipolar disorder. J. Nerv. Ment. Dis. 2009, 197, 287–290. [Google Scholar] [CrossRef]
- Keaton, D.; Lamkin, N.; Cassidy, K.A.; Meyer, W.J.; Ignacio, R.V.; Aulakh, L.; Blow, F.C.; Sajatovic, M. Utilization of herbal and nutritional compounds among older adults with bipolar disorder and with major depression. Int. J. Geriatr. Psychiatry 2009, 24, 1087–1093. [Google Scholar] [CrossRef][Green Version]
- Kilbourne, A.M.; Copeland, L.A.; Zeber, J.E.; Bauer, M.S.; Lasky, E.; Good, C.B. Determinants of complementary and Alternative medicine use by patients with bipolar disorder. Psychopharmacol. Bull. 2007, 40, 104–115. [Google Scholar]
- Andreescu, C.; Mulsant, B.H.; Emanuel, J.E. Complementary and Altern.ive medicine in the treatment of bipolar disorder—a review of the evidence. J. Affect. Disord. 2008, 110, 16–26. [Google Scholar] [CrossRef]
- Le, T.T.; McGrath, S.R.; Fasinu, P.S. Herb-drug Interactions in Neuropsychiatric Pharmacotherapy—A Review of Clinically Relevant Findings. Curr. Neuropharmacol. 2022, 20, 1736–1751. [Google Scholar] [CrossRef]
- Sharma, A.; Gerbarg, P.; Bottiglieri, T.; Massoumi, L.; Carpenter, L.L.; Lavretsky, H.; Muskin, P.R.; Brown, R.P.; Mischoulon, D. S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J. Clin. Psychiatry 2017, 78, e656–e667. [Google Scholar] [CrossRef] [PubMed]
- Abeysundera, H.; Gill, R. Possible SAMe-induced mania. BMJ Case Rep. 2018, 2018, bcr2018224338. [Google Scholar] [CrossRef]
- NCCIH. Complementary, Alternive, or Integrative Health: What’s In a Name? Available online: https://www.nccih.nih.gov/health/complementary-Altern.ive-or-integrative-health-whats-in-a-name (accessed on 4 February 2024).
- Unschuld, P.U.; Tessenow, H.; Jinsheng, Z.; Huang, D.; Nei, J.; Su, W. An Annotated Translation of Huang Di’s Inner Classic—Basic Questions: 2 Volumes, 1st ed.; University of California Press: Los Angeles, CA, USA, 2011. [Google Scholar]
- Unschuld, P.U.; Huang, D.; Nei, J.; Ling, S. The Ancient Classic on Needle Therapy, 1st ed.; University of California Press: Los Angeles, CA, USA, 2016. [Google Scholar]
- Zhang, Z.J.; Kang, W.H.; Li, Q.; Tan, Q.R. The beneficial effects of the herbal medicine Free and Easy Wanderer Plus (FEWP) for mood disorders: Double-blind, placebo-controlled studies. J. Psychiatr. Res. 2007, 41, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, J.K.; Yang, G.Y.; Lee, B.R.; Noh, S.H. Acupuncture for management of balance impairment in a patient with bipolar disorder. Jams J. Acupunct. Merid. Stud. 2013, 6, 56–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- NHIRD. Longitudinal Health Insurance Database (LHID). Available online: https://nhird.nhri.edu.tw/en/Data_Subsets.html (accessed on 4 February 2024).
- Chang, L.C.; Huang, N.; Chou, Y.J.; Lee, C.H.; Kao, F.Y.; Huang, Y.T. Utilization patterns of Chinese medicine and Western medicine under the National Health Insurance Program in Taiwan, a population-based study from 1997 to 2003. BMC Health Serv. Res. 2008, 8, 170. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Chen, F.P.; Chen, T.J.; Kung, Y.Y.; Chen, Y.C.; Chou, L.F.; Chen, F.J.; Hwang, S.J. Use frequency of traditional Chinese medicine in Taiwan. BMC Health Serv. Res. 2007, 7, 26. [Google Scholar] [CrossRef]
- Bolton, S.; Warner, J.; Harriss, E.; Geddes, J.; Saunders, K.E.A. Bipolar disorder: Trimodal age-at-onset distribution. Bipolar Disord. 2021, 23, 341–356. [Google Scholar] [CrossRef]
- Lavin, P.; Buck, G.; Almeida, O.P.; Su, C.L.; Eyler, L.T.; Dols, A.; Blumberg, H.P.; Forester, B.P.; Forlenza, O.V.; Gildengers, A.; et al. Clinical correlates of late-onset versus early-onset bipolar disorder in a global sample of older adults. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef]
- Goetz, M.; Novak, T.; Vesela, M.; Hlavka, Z.; Brunovsky, M.; Povazan, M.; Ptacek, R.; Sebela, A. Early stages of pediatric bipolar disorder: Retrospective analysis of a Czech inpatient sample. Neuropsychiatr. Dis. Treat. 2015, 11, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; Hawken, E.R.; Baldessarini, R.J.; Vázquez, G.H. Maintenance Pharmacological Treatment of Juvenile Bipolar Disorder: Review and Meta-Analyses. Int. J. NeuropsychoPharmacol. 2019, 22, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Szmulewicz, A.; Valerio, M.P.; Martino, D.J. Longitudinal analysis of cognitive performances in recent-onset and late-life Bipolar Disorder: A systematic review and meta-analysis. Bipolar Disord. 2020, 22, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Chien, I.C.; Lin, C.H.; Chou, Y.J.; Chou, P. Risk of hypertension in patients with bipolar disorder in Taiwan: A population-based study. Compr. Psychiatry 2013, 54, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.; Chien, I.C.; Lin, C.H. Increased risk of hyperlipidemia in patients with bipolar disorder: A population-based study. Gen. Hosp. Psychiatry 2015, 37, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Hu, L.Y.; Yeh, C.M.; Chiang, H.L.; Shen, C.C.; Chou, K.T.; Chen, T.J.; Lu, T.; Tzeng, C.H.; Liu, C.J. Chronic obstructive pulmonary disease associated with increased risk of bipolar disorder. Chron. Respir. Dis. 2017, 14, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.T.; West, A.E.; Eisner, L.; Baek, J.; Deckersbach, T. The Burden of Repeated Mood Episodes in Bipolar I Disorder: Results From the National Epidemiological Survey on Alcohol and Related Conditions. J. Nerv. Ment. Dis. 2016, 204, 87–94. [Google Scholar] [CrossRef]
- Cassidy, F.; Ahearn, E.; Carroll, B.J. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am. J. Psychiatry 1999, 156, 1417–1420. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Fagiolini, A.; Houck, P.; Kupfer, D.J. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009, 11, 657–662. [Google Scholar] [CrossRef]
- Kilbourne, A.; Cornelius, J.R.; Han, X.; Pincus, H.A.; Shad, M.; Salloum, I.; Conigliaro, J.; Haas, G.L. Burden of General Medical Conditions among Individuals with Bipolar Disorder. Bipolar Disord. 2004, 6, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.; Chien, I.C.; Lin, C.H. Increased risk of chronic obstructive pulmonary disease in patients with bipolar disorder: A population-based study. J. Affect. Disord. 2017, 220, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lasser, K.; Boyd, J.W.; Woolhandler, S.; Himmelstein, D.U.; McCormick, D.; Bor, D.H. Smoking and Mental IllnessA Population-Based Prevalence Study. JAMA 2000, 284, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Fagiolini, A.; Frank, E.; Houck, P.R.; Mallinger, A.G.; Swartz, H.A.; Buysse, D.J.; Ombao, H.; Kupfer, D.J. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J. Clin. Psychiatry 2002, 63, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.; Chien, I.C.; Lin, C.H. Increased risk of ischemic heart disease in patients with bipolar disorder: A population-based study. J. Affect. Disord. 2021, 281, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Ösby, U.; Brandt, L.; Correia, N.; Ekbom, A.; Sparén, P. Excess Mortality in Bipolar and Unipolar Disorder in Sweden. Arch. Gen. Psychiatry 2001, 58, 844–850. [Google Scholar] [CrossRef]
- Chan, J.K.N.; Wong, C.S.M.; Yung, N.C.L.; Chen, E.Y.H.; Chang, W.C. Excess mortality and life-years lost in people with bipolar disorder: An 11-year population-based cohort study. Epidemiol. Psychiatr. Sci. 2021, 30, e39. [Google Scholar] [CrossRef]
- Yapici Eser, H.; Kacar, A.S.; Kilciksiz, C.M.; Yalçinay-Inan, M.; Ongur, D. Prevalence and Associated Features of Anxiety Disorder Comorbidity in Bipolar Disorder: A Meta-Analysis and Meta-Regression Study. Front. Psychiatry 2018, 9, 229. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Akiskal, H.S.; Angst, J.; Greenberg, P.E.; Hirschfeld, R.M.A.; Petukhova, M.; Kessler, R.C. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry 2007, 64, 543–552. [Google Scholar] [CrossRef]
- Schiweck, C.; Arteaga-Henriquez, G.; Aichholzer, M.; Edwin Thanarajah, S.; Vargas-Cáceres, S.; Matura, S.; Grimm, O.; Haavik, J.; Kittel-Schneider, S.; Ramos-Quiroga, J.A.; et al. Comorbidity of ADHD and adult bipolar disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 124, 100–123. [Google Scholar] [CrossRef]
- McElroy, S.L.; Frye, M.A.; Hellemann, G.; Altshuler, L.; Leverich, G.S.; Suppes, T.; Keck, P.E.; Nolen, W.A.; Kupka, R.; Post, R.M. Prevalence and correlates of eating disorders in 875 patients with bipolar disorder. J. Affect. Disord. 2011, 128, 191–198. [Google Scholar] [CrossRef]
- Regier, D.A.; Farmer, M.E.; Rae, D.S.; Locke, B.Z.; Keith, S.J.; Judd, L.L.; Goodwin, F.K. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990, 264, 2511–2518. [Google Scholar] [CrossRef]
- Pedersen, D.D. PsychNotes: Clinical Pocket Guide, 4th ed.; F.A. Davis: Philadelphia, PA, USA, 2014; p. 243. [Google Scholar]
- Suominen, K.; Mantere, O.; Valtonen, H.; Arvilommi, P.; Leppämäki, S.; Paunio, T.; Isometsä, E. Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment seeking. Bipolar Disord. 2007, 9, 698–705. [Google Scholar] [CrossRef]
- Jones, G.; Rong, C.; Vecera, C.M.; Gurguis, C.I.; Chudal, R.; Khairova, R.; Leung, E.; Ruiz, A.C.; Shahani, L.; Zanetti, M.V.; et al. The role of lithium treatment on comorbid anxiety symptoms in patients with bipolar depression. J. Affect. Disord. 2022, 308, 71–75. [Google Scholar] [CrossRef]
- Trevor Young, L.; Cooke, R.G.; Robb, J.C.; Levitt, A.J.; Joffe, R.T. Anxious and non-anxious bipolar disorder. J. Affect. Disord. 1993, 29, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Salloum, I.M. Bipolar disorder and comorbid alcoholism: Prevalence rate and treatment considerations. Bipolar Disord. 2006, 8, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.V.; Saraf, G.; Kozicky, J.; Beaulieu, S.; Sharma, V.; Parikh, S.V.; Cervantes, P.; Daigneault, A.; Walji, N.; Kauer-Sant’Anna, M.; et al. Remission and recurrence in bipolar disorder: The data from health outcomes and patient evaluations in bipolar disorder (HOPE-BD) study. J. Affect. Disord. 2020, 268, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xing, J.J.; Li, J.; Zeng, B.Y.; Liang, F.R. History of acupuncture research. Int. Rev. Neurobiol. 2013, 111, 1–23. [Google Scholar]
- Li, Y. Acupuncture journey to America: A turning point in 1971. J. Tradit. Chin. Med. Sci. 2014, 1, 81–83. [Google Scholar] [CrossRef]
- Upchurch, D.M.; Rainisch, B.W. A sociobehavioral wellness model of acupuncture use in the United States, 2007. J. Altern. Complement. Med. 2014, 20, 32–39. [Google Scholar] [CrossRef]
- Chen, F.P.; Kung, Y.Y.; Chen, T.J.; Hwang, S.J. Demographics and patterns of acupuncture use in the Chinese population: The Taiwan experience. J. Altern. Complement. Med. 2006, 12, 379–387. [Google Scholar] [CrossRef]
- Wu, M.Y.; Lee, Y.C.; Lin, C.L.; Huang, M.C.; Sun, M.F.; Yen, H.R. Trends in use of acupuncture among adults in Taiwan from 2002 to 2011: A nationwide population-based study. PLoS ONE 2018, 13, e0195490. [Google Scholar] [CrossRef]
- Lee, I.S.; Jo, H.J.; Lee, S.H.; Lee, H.; Lee, H.; Park, H.J.; Chae, Y. Fear of acupuncture enhances sympathetic activation to acupuncture stimulation. Acupunct. Med. 2013, 31, 276–281. [Google Scholar] [CrossRef]
- Qin, W.; Tian, J.; Bai, L.; Pan, X.; Yang, L.; Chen, P.; Dai, J.; Ai, L.; Zhao, B.; Gong, Q.; et al. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol. Pain 2008, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The emotional brain, fear, and the amygdala. Cell Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Strakowski, S.M.; Delbello, M.P.; Adler, C.M. The functional neuroanatomy of bipolar disorder: A review of neuroimaging findings. Mol. Psychiatry 2005, 10, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Eggermont, L.; Mitchell, A.J.; De Hert, M.; Correll, C.U.; Soundy, A.; Rosenbaum, S.; Vancampfort, D. The prevalence of pain in bipolar disorder: A systematic review and large-scale meta-analysis. Acta Psychiatr. Scand. 2015, 131, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Leo, R.J.; Singh, J. Migraine headache and bipolar disorder comorbidity: A systematic review of the literature and clinical implications. Scand. J. Pain 2016, 11, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Goesling, J.; Lin, L.A.; Clauw, D.L. Psychiatry and Pain Management: At the Intersection of Chronic Pain and Mental Health. Curr. Psychiatry Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Manji, H.K.; Quiroz, J.A.; Payne, J.L.; Singh, J.; Lopes, B.P.; Viegas, J.S.; Zarate, C.A. The underlying neurobiology of bipolar disorder. World Psychiatry 2003, 2, 136–146. [Google Scholar] [PubMed]
- Kurita, M. Noradrenaline plays a critical role in the switch to a manic episode and treatment of a depressive episode. Neuropsychiatr. Dis. Treat. 2016, 12, 2373–2380. [Google Scholar] [CrossRef]
- Lee, M.; Schwab, C.; McGeer, P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2011, 59, 152–165. [Google Scholar] [CrossRef]
- Benedetti, F.; Poletti, S.; Hoogenboezem, T.A.; Locatelli, C.; de Wit, H.; Wijkhuijs, A.J.M.; Colombo, C.; Drexhage, H.A. Higher Baseline Proinflammatory Cytokines Mark Poor Antidepressant Response in Bipolar Disorder. J. Clin. Psychiatry 2017, 78, e986–e993. [Google Scholar] [CrossRef] [PubMed]
- Macone, A.; Otis, J.A.D. Neuropathic Pain. Semin. Neurol. 2018, 38, 644–653. [Google Scholar] [PubMed]
- World Health Organization. Acupuncture Review and Analysis of Reports on Controlled Clinical Trials; World Health Organization: Geneva, Switzerland, 2003; pp. iii–iv. [Google Scholar]
- Arnold, M.D.; Thornbrough, L.M. Treatment of musculoskeletal pain with traditional Chinese herbal medicine. Phys. Med. Rehabil. Clin. N. Am. 1999, 10, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, J.E.; Roufogalis, B.D.; Chrubasik, S. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother. Res. 2007, 21, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Lewith, G.T. Traditional Chinese Medicine for treatment of fibromyalgia: A systematic review of randomized controlled trials. J. Altern. Complement. Med. 2010, 16, 397–409. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Hosseinzadeh, H. Medicinal herbs in the treatment of neuropathic pain: A review. Iran. J. Basic. Med. Sci. 2018, 21, 347–358. [Google Scholar]
- Gagnier, J.J.; Oltean, H.; van Tulder, M.W.; Berman, B.M.; Bombardier, C.; Robbins, C.B. Herbal Medicine for Low Back Pain: A Cochrane Review. Spine 2016, 41, 116–133. [Google Scholar] [CrossRef]
- Chen, L.; Michalsen, A. Management of chronic pain using complementary and integrative medicine. Bmj 2017, 357, j1284. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.Z.; Sawadogo, R.; Tan, T.; Yuan, C.S. Effects of Herbal Medicines on Pain Management. Am. J. Chin. Med. 2020, 48, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.I.; Kemp, D.E.; Soczynska, J.K.; McIntyre, R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: A systematic review of the literature. J. Clin. Psychiatry 2009, 70, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Aggio, V.; Pratesi, M.L.; Greco, G.; Furlan, R. Neuroinflammation in Bipolar Depression. Front. Psychiatry 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Jung, H.G.; Myint, A.M.; Kim, H.; Park, S.H. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007, 104, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Ceresér, K.M.; Goi, P.D.; Fries, G.R.; Teixeira, A.L.; Fernandes, B.S.; Belmonte-de-Abreu, P.S.; Kauer-Sant’Anna, M.; Kapczinski, F.; Gama, C.S. Serum levels of IL-6, IL-10 and TNF-α in patients with bipolar disorder and schizophrenia: Differences in pro- and anti-inflammatory balance. Braz. J. Psychiatry 2011, 33, 268–274. [Google Scholar]
- Tian, B.; Tian, M.; Huang, S.M. Advances in phytochemical and modern pharmacological research of Rhizoma Corydalis. Pharm. Biol. 2020, 58, 265–275. [Google Scholar] [CrossRef]
- Wu, H.; Wang, P.; Liu, M.; Tang, L.; Fang, J.; Zhao, Y.; Zhang, Y.; Li, D.; Xu, H.; Yang, H. A 1H-NMR-Based Metabonomic Study on the Anti-Depressive Effect of the Total Alkaloid of Corydalis Rhizoma. Molecules 2015, 20, 10047–10064. [Google Scholar] [CrossRef]
- Henkes, H.; Franz, M.; Kendall, O.; Monroe, J.; Legaspi, A.; LeDoux, J.; Haese, C.; Williams, D.; McCall, S.; Johnson, A.D.; et al. Evaluation of the anxiolytic properties of tetrahydropalmatine, a Corydalis yanhusuo compound, in the male Sprague-Dawley rat. Aana J. 2011, 79 (Suppl. S4), S75–S80. [Google Scholar]
- Yuan, C.S.; Mehendale, S.R.; Wang, C.Z.; Aung, H.H.; Jiang, T.; Guan, X.; Shoyama, Y. Effects of Corydalis yanhusuo and Angelicae dahuricae on cold pressor-induced pain in humans: A controlled trial. J. Clin. Pharmacol. 2004, 44, 1323–1327. [Google Scholar] [CrossRef]
- Hermesdorf, M.; Berger, K.; Baune, B.T.; Wellmann, J.; Ruscheweyh, R.; Wersching, H. Pain Sensitivity in Patients with Major Depression: Differential Effect of Pain Sensitivity Measures. Somatic Cofactors, and Disease Characteristics. J. Pain 2016, 17, 606–616. [Google Scholar] [CrossRef]
- Michaelides, A.; Zis, A. Depression, anxiety and acute pain: Links and management challenges. Postgrad. Med. 2019, 131, 438–444. [Google Scholar] [CrossRef]
- Shu, H.; Arita, H.; Hayashida, M.; Zhang, L.; An, K.; Huang, W.; Hanaoka, K. Anti-hypersensitivity effects of Shu-jing-huo-xue-tang, a Chinese herbal medicine, in CCI-neuropathic rats. J. Ethnopharmacol. 2010, 131, 464–470. [Google Scholar] [CrossRef]
- Sonohata, M.; Furue, H.; Katafuchi, T.; Yasaka, T.; Doi, A.; Kumamoto, E.; Yoshimura, M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J. Physiol. 2004, 555 Pt 2, 515–526. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. The pharmacological effects of Salvia species on the central nervous system. Phytother. Res. 2006, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.M.; Chen, Y.Y.; Xu, D.Q.; Fu, R.J.; Yue, S.J.; Zhao, Q.; Huang, Y.X.; Bai, X.; Wang, M.; Xing, L.M.; et al. An integrated strategy for discovering effective components of Shaoyao Gancao decoction for treating neuropathic pain by the combination of partial least-squares regression and multi-index comprehensive method. J. Ethnopharmacol. 2020, 260, 113050. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, G.; Gao, J.; Liu, Y.; Zhang, C.; Liu, C.; Li, H.; He, Q.; Li, J.; Wang, J.; et al. Xue-Fu-Zhu-Yu capsule in the treatment of qi stagnation and blood stasis syndrome: A study protocol for a randomised controlled pilot and feasibility trial. Trials 2018, 19, 515. [Google Scholar] [CrossRef] [PubMed]
- Shergis, J.L.; Ni, X.; Sarris, J.; Zhang, A.L.; Guo, X.; Xue, C.C.; Lu, C.; Hugel, H. Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine 2017, 34, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.H.; Hsieh, M.T.; Lee, Y.S.; Lin, Y.C.; Liao, J. Anxiolytic effect of seed of Ziziphus jujuba in mouse models of anxiety. J. EthnoPharmacol. 2000, 72, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Chiao, Y.W.; Livneh, H.; Guo, H.R.; Chen, W.J.; Lu, M.C.; Lin, M.C.; Yeh, C.C.; Tsai, T.Y. Use of Chinese Herbal Medicines Is Related to a Reduction in Depression Risk Among Patients with Insomnia: A Matched Cohort Study. Front. Neurol. 2020, 11, 583485. [Google Scholar] [CrossRef]
- Yeh, C.H.; Arnold, C.K.; Chen, Y.H.; Lai, J.N. Suan zao ren tang as an original treatment for sleep difficulty in climacteric women: A prospective clinical observation. Evid. Based Complement. Altern. Med. 2011, 2011, 673813. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Zhou, X.L.; Xu, M.B.; Jin, T.Y.; Rong, P.Q.; Zheng, G.Q.; Lin, Y. Suanzaoren Formulae for Insomnia: Updated Clinical Evidence and Possible Mechanisms. Front. Pharmacol. 2018, 9, 76. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Q.; Huang, Y.; Tang, K.; Yang, X.; Cao, Z. In Silico Study of Anti-Insomnia Mechanism for Suanzaoren Prescription. Front. Pharmacol. 2019, 10, 925. [Google Scholar] [CrossRef]
- Chen, F.P.; Jong, M.S.; Chen, Y.C.; Kung, Y.Y.; Chen, T.J.; Chen, F.J.; Hwang, S.J. Prescriptions of Chinese Herbal Medicines for Insomnia in Taiwan during 2002. Evid Based Complement Altern. Med. 2011, 2011, 236341. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Liu, L.; Ming, S.P.; Fang, J.; Wu, D.N. Tian Wang Bu Xin Dan for Insomnia: A Systematic Review of Efficacy and Safety. Evid. Based Complement. Altern. Med. 2019, 2019, 4260801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Kang, W.H.; Tan, Q.R.; Li, Q.; Gao, C.G.; Zhang, F.G.; Wang, H.H.; Ma, X.C.; Chen, C.; Wang, W.; et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: A double-blind, randomized, placebo-controlled study. J. Psychiatr. Res. 2007, 41, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Lu, M.K.; Yang, Y.K.; Huang, M.C.; Yeh, T.L.; Chen, W.J.; Lu, R.B.; Kuo, P.H. Empirically derived subgroups of bipolar I patients with different comorbidity patterns of anxiety and substance use disorders in Han Chinese population. J. Affect. Disord. 2012, 136, 81–89. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total | Non-TCM | TCM | p-Value |
|---|---|---|---|---|
| N = 2372 | n = 1053 (44.4) | n = 1319 (55.6) | ||
| Mean ± SD a | Mean ± SD a | |||
| Age (year) * | 48.2 ± 19.0 | 44.3 ± 16.8 | <0.0001 | |
| N | n (%) | n (%) | ||
| 15–39 | 996 | 410 (38.9) | 586 (44.4) | |
| 40–64 | 942 | 399 (37.9) | 543 (41.2) | |
| ≥65 | 434 | 244 (23.2) | 190 (14.4) | |
| Gender * | <0.0001 | |||
| Female | 1091 | 386 (36.7) | 705 (53.4) | |
| Male | 1281 | 667 (63.3) | 614 (46.6) | |
| Follow-up duration (year) a | 8.6 ± 4.8 | 8.3 ± 4.3 | 0.1644 | |
| Comorbidities * | ||||
| Diabetes mellitus | 328 | 149 (14.2) | 179 (13.6) | 0.6847 |
| Hypertension | 576 | 261 (24.8) | 315 (23.9) | 0.6097 |
| Hyperlipidemia | 376 | 165 (15.7) | 211 (16.0) | 0.8282 |
| Ischemic heart disease | 74 | 32 (3.0) | 42 (3.2) | 0.8397 |
| COPD | 492 | 205 (19.5) | 287 (21.8) | 0.1716 |
| Anxiety | 639 | 218 (20.7) | 421 (31.9) | <0.0001 |
| Attention-deficit disorder | 12 | 5 (0.5) | 7 (0.5) | 0.8489 |
| Eating disorder, unspecified | 3 | 1(0.1) | 2 (0.2) | 0.6997 |
| Bulimia nervosa | 7 | 1(0.1) | 6 (0.5) | 0.1084 |
| Alcoholism | 107 | 35 (3.3) | 72 (5.5) | 0.0128 |
| Tobacco use | 26 | 7(0.7) | 19 (1.4) | 0.0714 |
| Obesity | 18 | 6(0.6) | 12 (0.9) | 0.3431 |
| Medications * | ||||
| Lithium carbonate | 486 | 128 (12.2) | 358 (27.1) | <0.0001 |
| Carbamazepine | 413 | 141 (13.4) | 272 (20.6) | <0.0001 |
| Valproic acid | 523 | 152 (14.4) | 371 (28.1) | <0.0001 |
| Lamotrigine | 56 | 21 (2.0) | 35 (2.7) | 0.2934 |
| Topiramate | 26 | 7 (0.7) | 19 (1.4) | 0.0714 |
| Aripiprazole | 23 | 0 (0.0) | 23 (1.7) | <0.0001 |
| Loxapine | 39 | 21 (2.0) | 18 (1.4) | 0.2309 |
| Chinese herbal medicine | ||||
| Herb | 1043 | - | 1043 (79.1) | - |
| Formula | 1155 | - | 1155 (87.6) | - |
| Acupuncture | 120 | - | 120 (9.1) | - |
| No. | Internal Medicine of TCM | Acupuncture | ||||
|---|---|---|---|---|---|---|
| ICD-9-CM | Indication | Frequency | ICD-9-CM | Indication | Frequency | |
| 1 | 780.5 | Sleep disturbances | 470 | 719.4 | Pain in joint | 20 |
| 2 | 719.4 | Pain in joint | 315 | 729.1 | Myalgia and myositis, unspecified | 18 |
| 3 | 460 | Acute nasopharyngitis | 240 | 724.2 | Lumbago | 15 |
| 4 | 729.1 | Myalgia and myositis, unspecified | 236 | 924.1 | Contusion of knee and lower leg | 11 |
| 5 | 724.2 | Lumbago | 212 | 845.0 | Ankle sprain | 8 |
| 6 | 786.2 | Cough | 182 | 840.9 | Sprains and strains of unspecified site of the shoulder and upper arm | 7 |
| 7 | 784.0 | Headache | 180 | 923.1 | Contusion of elbow and forearm | 7 |
| 8 | 924.2 | Contusion of ankle and foot excluding toe(s) | 131 | 923.2 | Contusion of wrist and hand(s) except finger(s) alone | 7 |
| 9 | 847.2 | Sprain of lumbar | 127 | 844.9 | Sprains and strains of unspecified site of the knee and leg | 6 |
| 10 | 564.0 | Constipation | 118 | 724.5 | Backache, unspecified | 5 |
| No. | Single Herb | Herbal Formula | ||
|---|---|---|---|---|
| Pin-Yin Name (Latin Name) | Frequency | Pin-Yin Name | Frequency | |
| 1 | Yanhusuo (Corydalis yanhusuo W. T. Wang) | 347 | Shu-Jing-Huo-Xue-Tang | 357 |
| 2 | Danshen (Salvia miltiorrhiza Bunge) | 297 | Jia-Wei-Xiao-Yao-San | 347 |
| 3 | Huangqin (Scutellaria baicalensis Georgi) | 241 | Shao-Yao-Gan-Cao-Tang | 312 |
| 4 | Gegen (Pueraria lobate) | 215 | Suan-Zao-Ren-Tang | 261 |
| 5 | Jiegeng (Platycodon grandiflorum) | 209 | Ge-Gen-Tang | 255 |
| 6 | Gancao (Glycyrrhiza uralensis Fisch.) | 197 | Xue-Fu-Zhu-Yu-Tang | 231 |
| 7 | Suanzaoren (Ziziphus jujube Mill. var. spinosa) | 197 | Tian-Wang-Bu-Xin-Dan | 216 |
| 8 | Duzhong (Eucommis ulmoidea Oliv.) | 191 | Chai-Hu-Jia-Long-Gu-Mu-Li-Tang | 213 |
| 9 | Dahuang (Rheum palmatum L.) | 189 | Gan-Mai-Da-Zao-Tang | 210 |
| 10 | Baizhi (Angelica dahurica) | 187 | Chuan-Xiong-Cha-Tiao-San | 207 |
| No. | Single Herb | Herbal Formula | ||
|---|---|---|---|---|
| Pin-Yin Name (Latin Name) | Frequency | Pin-Yin Name | Frequency | |
| 1 | Yejiaoteng (Polygonum multiflorumThunb) | 376 | Suan-Zao-Ren-Tang | 495 |
| 2 | Yuanzhi (Polygala tenuifolia Willd) | 269 | Chai-Hu-Jia-Long-Gu-Mu-Li-Tang | 404 |
| 3 | Suanzaoren (Ziziphus jujuba Mill.) | 267 | Tian-Wang-Bu-Xin-Dan | 387 |
| 4 | Fushen (PoriaCocos Sclerotium) | 258 | Gan-Mai-Da-Zao-Tang | 351 |
| 5 | Hehuanpi (Albizia julibrissin sensu Baker.) | 216 | Jia-Wei-Xiao-Yao-San | 319 |
| 6 | Danshen (Salvia miltiorrhiza Bge.) | 195 | Wen-Dan-Tang | 270 |
| 7 | Botsujen (Platycladus orientalis (L.) Franco) | 157 | Gui-Pi-Tang | 145 |
| 8 | Gegen (Pueraria lobata (Willd.) Ohwi) | 133 | Jia-Wei-Xiao-Yao-San | 137 |
| 9 | Huanglian (Coptis chinensis Franch.) | 129 | Long-Dan-Xie-Gan-Tang | 100 |
| 10 | Shichangpu (Acorus gramineus Soland.) | 125 | Liu-Wei-Di-Huang-Wan | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-P.; Yang, S.-T.; Hu, K.-C.; Satyanarayanan, S.K.; Su, K.-P. Usage Patterns of Traditional Chinese Medicine for Patients with Bipolar Disorder: A Population-Based Study in Taiwan. Healthcare 2024, 12, 490. https://doi.org/10.3390/healthcare12040490
Chen S-P, Yang S-T, Hu K-C, Satyanarayanan SK, Su K-P. Usage Patterns of Traditional Chinese Medicine for Patients with Bipolar Disorder: A Population-Based Study in Taiwan. Healthcare. 2024; 12(4):490. https://doi.org/10.3390/healthcare12040490
Chicago/Turabian StyleChen, Shu-Ping, Su-Tso Yang, Kai-Chieh Hu, Senthil Kumaran Satyanarayanan, and Kuan-Pin Su. 2024. "Usage Patterns of Traditional Chinese Medicine for Patients with Bipolar Disorder: A Population-Based Study in Taiwan" Healthcare 12, no. 4: 490. https://doi.org/10.3390/healthcare12040490
APA StyleChen, S.-P., Yang, S.-T., Hu, K.-C., Satyanarayanan, S. K., & Su, K.-P. (2024). Usage Patterns of Traditional Chinese Medicine for Patients with Bipolar Disorder: A Population-Based Study in Taiwan. Healthcare, 12(4), 490. https://doi.org/10.3390/healthcare12040490







