Improvements in Obstetric and Newborn Health Information Documentation following the Implementation of the Safer Births Bundle of Care at 30 Facilities in Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Management, Sites, and Population
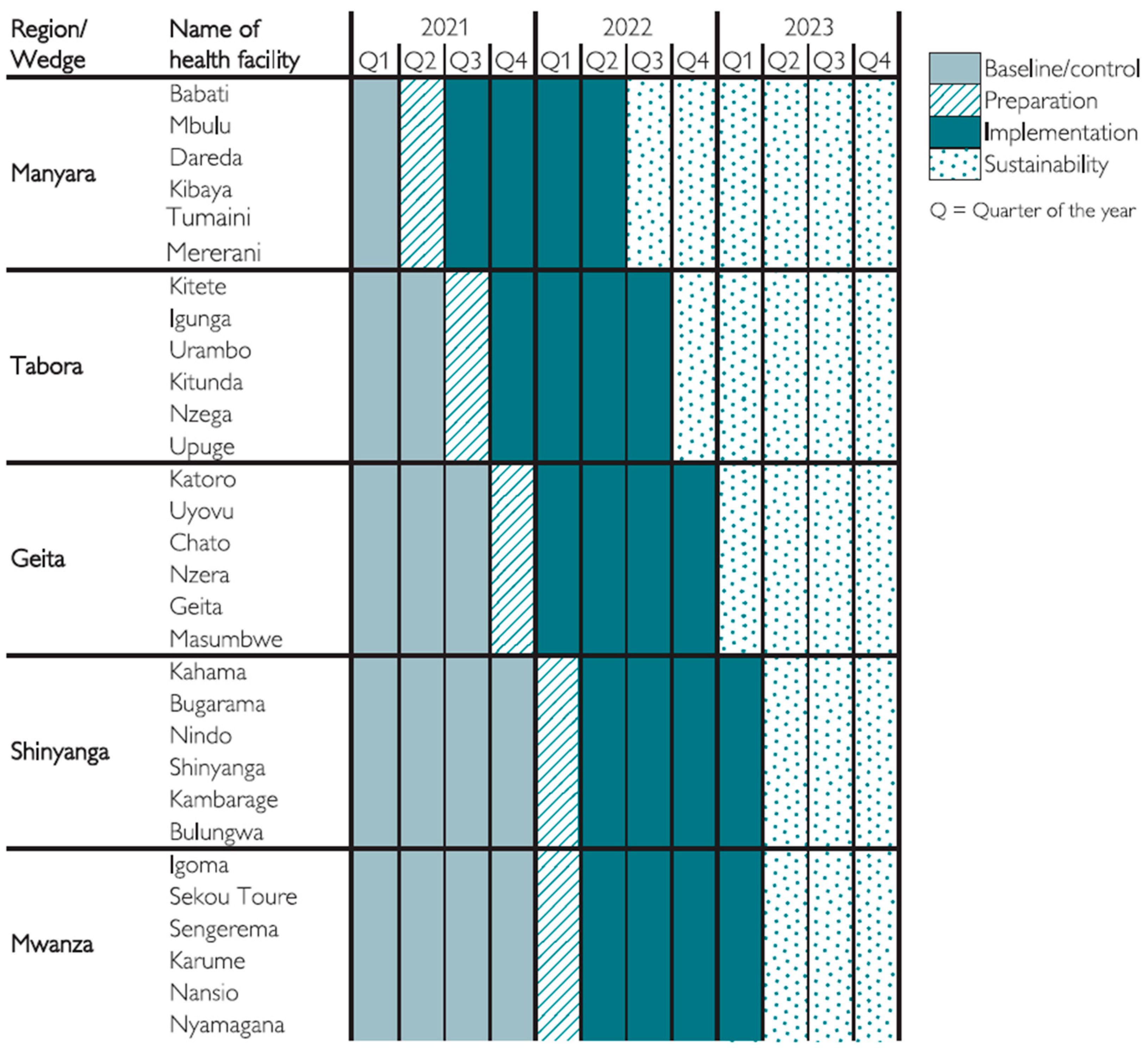
2.2. The SBBC Project and Implementation Strategy
2.3. Data Collection and Management
2.4. Variables Included in This Analysis
2.5. Statistical Methods
2.6. Ethical Considerations
3. Results
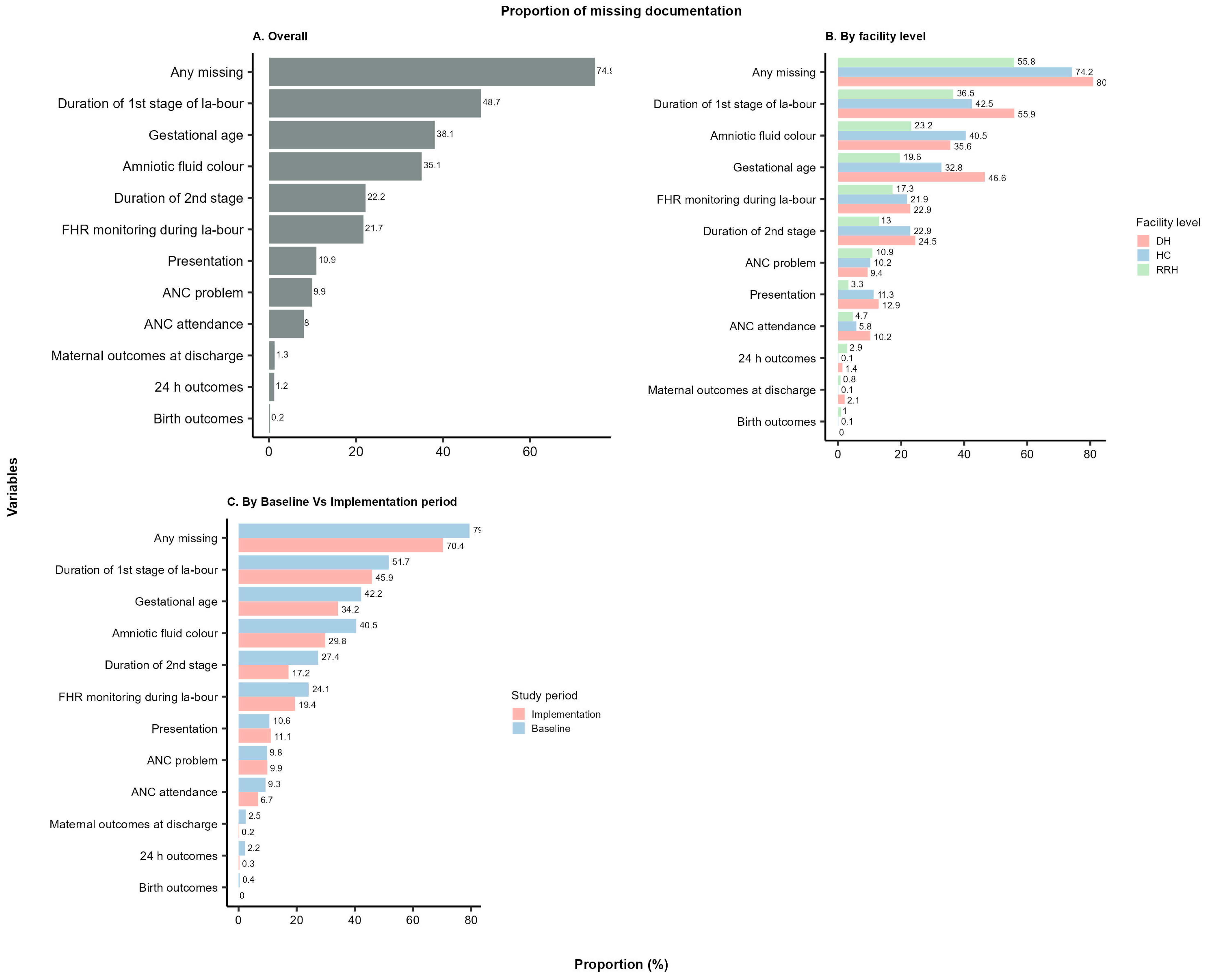
3.1. Overall Findings
3.2. Comparison of Non-Documentation across Health Facility Levels
3.3. Comparison of Non-Documentation across Regions
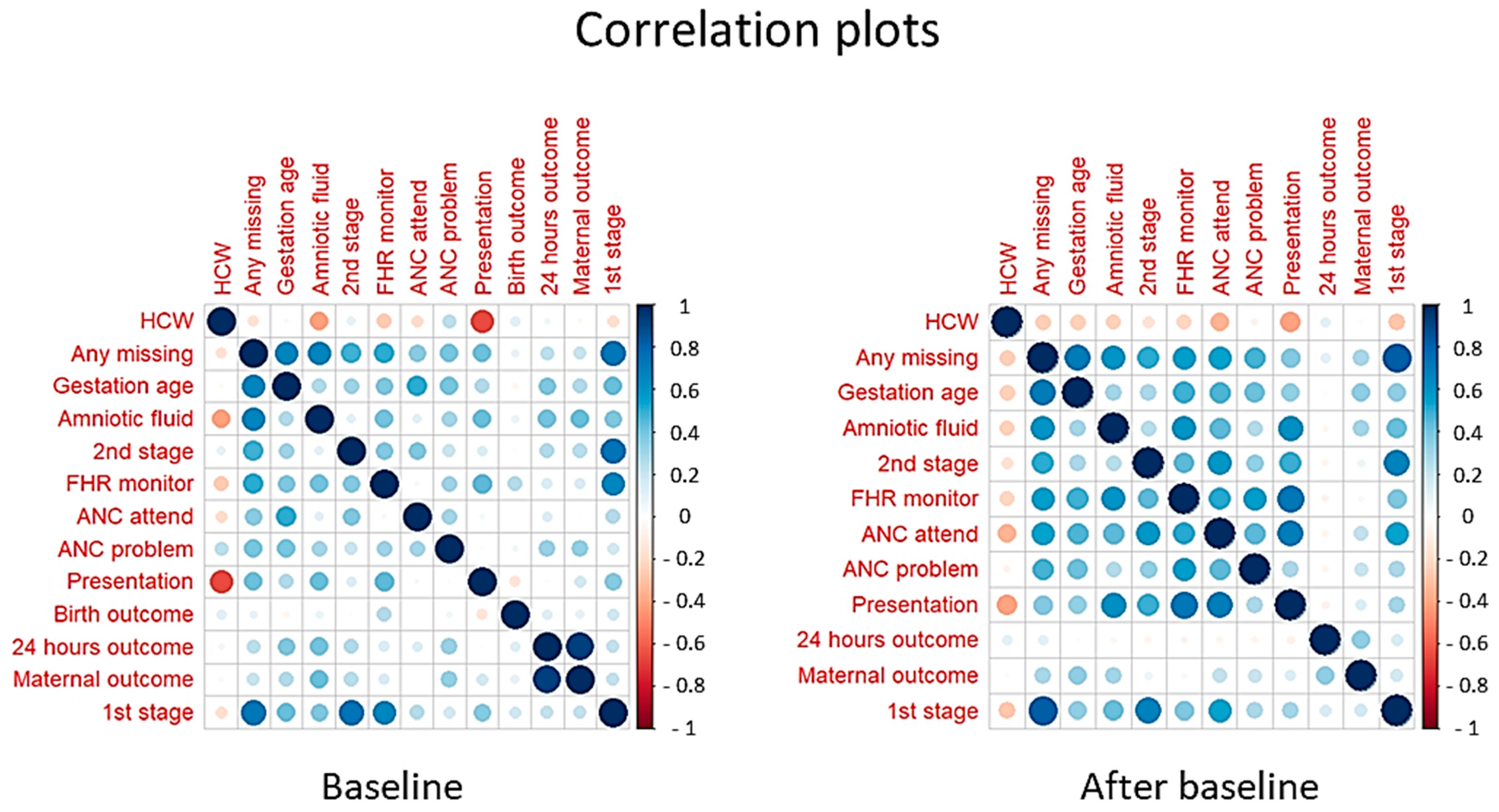
3.4. The Relative Number of HCWs and Case Note Documentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ending Preventable Newborn Deaths and Stillbirths by 2030: Moving Faster towards High-Quality Universal Health Coverage in 2020–2025. UNICEF and WHO. 2020. Available online: https://www.unicef.org/reports/ending-preventable-newborn-deaths-stillbirths-quality-health-coverage-2020-2025 (accessed on 28 November 2023).
- Levels & Trends in Child Mortality. Report 2015. Estimates Developed by the UN Inter-Agency Group for Child Mortality Estimation. United Nations Children’s Fund, World Health Organization, World Bank and United Nations (UN IGME). New York. 2018. Available online: https://childmortality.org/wp-content/uploads/2015/10/Levels-and-Trends-in-Child-Mortality-Report-2015.pdf (accessed on 28 November 2023).
- Levels and Trends in Child Mortality–UNICEF DATA. Available online: https://data.unicef.org/resources/levels-and-trends-in-child-mortality/ (accessed on 28 November 2023).
- Maternal and Newborn–Overview. Available online: https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/maternal-and-newborn-data (accessed on 28 November 2023).
- McClure, E.M.; Garces, A.L.; Hibberd, P.L.; Moore, J.L.; Goudar, S.S.; Saleem, S.; Esamai, F.; Patel, A.; Chomba, E.; Lokangaka, A.; et al. The Global Network Maternal Newborn Health Registry: A Multi-Country, Community-Based Registry of Pregnancy Outcomes. Reprod. Health 2020, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-quality Health Systems in the Sustainable Development Goals Era: Time for a Revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef] [PubMed]
- Mother and Newborn Information for Tracking Outcomes and Results (MoNITOR) World Health Organization. Available online: https://www.who.int/groups/mother-and-newborn-information-for-tracking-outcomes-and-results-(monitor) (accessed on 28 November 2023).
- Benova, L.; Moller, A.B.; Hill, K.; Vaz, L.M.E.; Morgan, A.; Hanson, C.; Semrau, K.; Al Arifees, S.; Moran, A.C. What is Meant by Validity in Maternal and Newborn Health Measurement? A Conceptual Framework for Understanding Indicator Validation. PLoS ONE 2020, 15, e0233969. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, K.; Hung, Y.W.; Irwin, B.R.; Grépin, K.A. Understanding the Challenges Associated with the Use of Data from Routine Health Information Systems in Low- and Middle-Income Countries: A systematic Review. Health Inf. Manag. J. 2020, 51, 135–148. [Google Scholar] [CrossRef]
- Moller, A.B.; Patten, J.H.; Hanson, C.; Morgan, A.; Say, L.; Diaz, T.; Moran, A.C. Monitoring Maternal and Newborn Health Outcomes Globally: A Brief History of Key Events and Initiatives. Trop. Med. Int. Health 2019, 24, 1342–1368. [Google Scholar] [CrossRef] [PubMed]
- Benova, L.; Moller, A.B.; Moran, A.C. “What Gets Measured Better Gets Done Better”: The Landscape of Validation of Global Maternal and Newborn Health Indicators through Key Informant Interviews. PLoS ONE 2019, 14, e0224746. [Google Scholar] [CrossRef] [PubMed]
- Everybody’s Business: Strengthening Health Systems to Improve Health Outcomes: WHO’s Framework for Action. 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/43918/9789241596077_eng.pdf?sequence=1 (accessed on 29 November 2023).
- Rumisha, S.F.; Lyimo, E.P.; Mremi, I.R.; Tungu, P.K.; Mwingira, V.S.; Mbata, D.; Malekia, S.E.; Joachim, C.; Mboera, L.E.G. Data Quality of the Routine Health Management Information System at the Primary Healthcare Facility and District Levels in Tanzania. BMC Med. Inform. Decis. Mak. 2020, 20, 340. [Google Scholar] [CrossRef]
- Målqvist, M.; Eriksson, L.; Nguyen, T.N.; Fagerland, L.I.; Hoa, D.P.; Wallin, L.; Ewald, U.; Persson, L.-A. Unreported Births and Deaths, a Severe Obstacle for Improved Neonatal Survival in Low-Income Countries. BMC Int. Health Hum. Rights 2008, 8, 4. [Google Scholar] [CrossRef]
- Dadzie, D.; Boadu, R.O.; Engmann, C.M.; Twum-Danso, N.A.Y. Evaluation of Neonatal Mortality Data Completeness and Accuracy in Ghana. PLoS ONE 2021, 16, e0239049. [Google Scholar] [CrossRef]
- Estifanos, A.S.; Gezahegn, R.; Keraga, D.W.; Kifle, A.; Procureur, F.; Hill, Z. ‘The False Reporter Will Get a Praise and the One Who Reported Truth Will Be Discouraged’: A Qualitative Study on Intentional Data Falsification by Frontline Maternal and Newborn Healthcare Workers in Two Regions in Ethiopia. BMJ Glob. Health 2022, 7, e008260. [Google Scholar] [CrossRef]
- Mboera, L.E.G.; Rumisha, S.F.; Mbata, D.; Mremi, I.R.; Lyimo, E.P.; Joachim, C. Data utilisation and factors influencing the performance of the health management information system in Tanzania. BMC Health Serv. Res. 2021, 21, 498. [Google Scholar] [CrossRef]
- German, C.; Kinyenje, E.; Yahya, T.; Hokororo, J.; Nungu, S.; Mohamed, M.; Degeh, M.; Nassoro, O.; Mwaisengela, S.; Bahegwa, R.; et al. The Use of Data for Planning and Services Improvement in Tanzanian Primary Healthcare Facilities: Experience from Star Rating Assessment. J. Serv. Sci. Manag. 2023, 16, 144–160. [Google Scholar] [CrossRef]
- District Health Information System 2 (DHIS2)|Open Health News. Available online: https://www.openhealthnews.com/resources/district-health-information-system-2-dhis2 (accessed on 29 November 2023).
- Kamala, B.A.; Ersdal, H.L.; Mduma, E.; Moshiro, R.; Girnary, S.; Østrem, O.T.; Linde, J.; Dalen, I.; Søyland, E.; Bishanga, D.R.; et al. SaferBirths Bundle of Care Protocol: A Stepped-Wedge Cluster Implementation Project in 30 Public Health-Facilities in Five Regions, Tanzania. BMC Health Serv. Res. 2021, 21, 1117. [Google Scholar] [CrossRef] [PubMed]
- Siyam, A.; Ir, P.; York, D.; Antwi, J.; Amponsah, F.; Rambique, O.; Funzamo, C.; Azeez, A.; Mboera, L.; Kumalija, C.J.; et al. The Burden of Recording and Reporting Health Data in Primary Health Care Facilities in Five Low- and Lower-Middle Income Countries. BMC Health Serv. Res. 2021, 21, 691. [Google Scholar] [CrossRef] [PubMed]
- Avortri, G.S.; Nabukalu, J.B.; Nabyonga-Orem, J. Supportive Supervision to Improve Service Delivery in Low-Income Countries: Is There a Conceptual Problem or a Strategy Problem? BMJ Glob. Health 2019, 4 (Suppl. S9), e001151. [Google Scholar] [CrossRef] [PubMed]
- Unkels, R.; Alwy Al-Beity, F.; Julius, Z.; Mkumbo, E.; Pembe, A.B.; Hanson, C.; Molsted-Alvesson, H. Understanding Maternity Care Providers’ Use of data in Southern Tanzania. BMJ Glob. Health 2023, 8, e010937. [Google Scholar] [CrossRef] [PubMed]
- Shihundla, R.C.; Lebese, R.T.; Maputle, M.S. Effects of Increased Nurses’ Workload on Quality Documentation of Patient Information at selected Primary Health Care facilities in Vhembe District, Limpopo Province. Curationis 2016, 39, 1545. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, A.M.; Zegeye, D.T.; Assefa, S.; Yenit, M.K. Routine Health Information System Utilization and Factors Associated Thereof Among Health Workers at Government Health Institutions in East Gojjam Zone, Northwest Ethiopia. BMC Med. Inform. Decis. Mak. 2017, 17, 116. [Google Scholar] [CrossRef]
- Nutley, T.; Reynolds, H.W. Improving the Use of Health Data for Health sSystem Strengthening. Glob. Health Action 2013, 6, 20001. [Google Scholar] [CrossRef]
- Adane, A.; Adege, T.M.; Ahmed, M.M.; Anteneh, H.A.; Ayalew, E.S.; Berhanu, D.; Berhanu, N.; Getnet, M.; Bishaw, T.; Busza, J.; et al. Exploring Data Quality and Use of the Routine Health Information System in Ethiopia: A Mixed-Methods Study. BMJ Open. 2021, 11, e050356. [Google Scholar] [CrossRef]
- Markos, D.; Bogale, D. Documentation Status of the Modified World Health Organization Partograph in Public Health Institutions of Bale zone, Ethiopia. Reprod. Health 2015, 12, 81. [Google Scholar] [CrossRef][Green Version]
- Bhattacharya, A.A.; Umar, N.; Audu, A.; Allen, E.; Schellenberg, J.R.M.; Marchant, T. Quality of Routine Facility Data for Monitoring Priority Maternal and Newborn Indicators in DHIS2: A Case Study from Gombe State, Nigeria. PLoS ONE 2019, 14, e021126. [Google Scholar] [CrossRef]
- Health Sector Strategic Plan July 2021–June 2026 (HSSP V): Leaving No One Behind Ministry of Health, Community Development, Gender, Elderly and Children. 2021. Available online: https://mitu.or.tz/wp-content/uploads/2021/07/Tanzania-Health-Sector-Strategic-Plan-V-17-06-2021-Final-signed.pdf (accessed on 5 January 2024).
- Mutshatshi, T.E.; Mothiba, T.M.; Mamogobo, P.M.; Mbombi, M.O. Record-Keeping: Challenges Experienced by Nurses in Selected Public Hospitals. Curationis 2018, 41, e1–e6. [Google Scholar] [CrossRef]
- Ersdal, H.; Mdoe, P.; Mduma, E.; Moshiro, R.; Guga, G.; Kvaløy, J.T.; Bundala, F.; Marwa, B.; Kamala, B. “Safer Births Bundle of Care” Implementation and Perinatal Impact at 30 Hospitals in Tanzania—Halfway Evaluation. Children 2023, 10, 255. [Google Scholar] [CrossRef]

| All | All Sites | Regional Referral Hospital | District Hospital Level | Health Centre Level | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline (N = 67,775) | Implementation (N = 70,667) | Baseline (N = 12,059) | Implementation (N = 10,151) | Baseline (N = 34,699) | Implementation (N = 39,699) | Baseline (N = 21,017) | Implementation (N = 20,817) | |
| Any missing p < 0.0001 | ||||||||
| Missing | 53,706 (79.2) | 49,463 (70.0) | 7278 (60.4) | 4857 (47.8) | 30,041 (86.6) | 30,024 (75.6) | 16,387 (78.0) | 14,582 (70.0) |
| Not missing | 14,069 (20.8) | 21,204 (30.0) | 4781 (39.6) | 5294 (52.2) | 4658 (13.4) | 9675 (24.4) | 4630 (22.0) | 6235 (30.0) |
| Gestational age p < 0.0001 | ||||||||
| Missing | 28,622 (42.2) | 24,168 (34.2) | 2419 (20.1) | 1935 (19.1) | 19,182 (55.3) | 15,533 (39.1) | 7021 (33.4) | 6700 (32.2) |
| Not missing | 39,153 (57.8) | 46,499 (65.8) | 9640 (79.9) | 8216 (80.9) | 15,517 (44.7) | 24,166 (60.9) | 13,996 (66.6) | 14,117 (67.8) |
| Amniotic fluid colour p < 0.0001 | ||||||||
| Missing | 27,517 (40.6) | 21,067 (29.8) | 3484 (28.9) | 1683 (16.6) | 13,558 (39.1) | 12,947 (32.6) | 10,475 (49.8) | 6437 (30.9) |
| Not missing | 40,258 (59.4) | 49,600 (70.2) | 8575 (71.1) | 8468 (83.4) | 21,141 (60.9) | 26,752 (67.4) | 10,542 (50.2) | 14,380 (69.1) |
| Duration of 2nd stage of labour p < 0.0001 | ||||||||
| Missing | 18,599 (27.4) | 12,160 (17.2) | 2353 (19.5) | 537 (5.3) | 11,770 (33.9) | 6520 (16.4) | 4476 (21.3) | 5103 (24.5) |
| Not missing | 49,176 (72.6) | 58,507 (82.8) | 9706 (80.5) | 9614 (94.7) | 22,929 (66.1) | 33,179 (83.6) | 16,541 (78.7) | 15,714 (75.5) |
| FHR monitoring during labour p < 0.0001 | ||||||||
| Missing | 16,403 (24.2) | 13,753 (19.5) | 2495 (20.7) | 1376 (13.6) | 9487 (27.3) | 7623 (19.2) | 4421 (21.0) | 4754 (22.8) |
| Not missing | 51,372 (75.8) | 56,914 (80.5) | 9564 (79.3) | 8775 (86.4) | 25,212 (72.7) | 32,076 (80.8) | 16,596 (79.0) | 16,063 (77.2) |
| ANC attendance p < 0.0001 | ||||||||
| Missing | 6323 (9.3) | 4707 (6.7) | 726 (6.0) | 300 (3.0) | 4517 (13.0) | 3084 (7.8) | 1080 (5.1) | 1323 (6.4) |
| Not missing | 61,452 (90.7) | 65,960 (93.3) | 11,333 (94.0) | 9851 (97.0) | 30,182 (87.0) | 36,615 (92.2) | 19,937 (94.9) | 19,494 (93.6) |
| ANC problem p = 0.4185 | ||||||||
| Missing | 6640 (9.8) | 7016 (9.9) | 1316 (10.9) | 1086 (10.7) | 3636 (10.5) | 3351 (8.4) | 1688 (8.0) | 2579 (12.4) |
| Not missing | 61,135 (90.2) | 63,651 (90.1) | 10,743 (89.1) | 9065 (89.3) | 31,063 (89.5) | 36,348 (91.6) | 19,329 (92.0) | 18,238 (87.6) |
| Presentation p = 0.0183 | ||||||||
| Missing | 7255 (10.7) | 7845 (11.1) | 495 (4.1) | 243 (2.4) | 4823 (13.9) | 4828 (12.2) | 1937 (9.2) | 2774 (13.3) |
| Not missing | 60,520 (89.3) | 62,822 (88.9) | 11,564 (95.9) | 9908 (97.6) | 29,876 (86.1) | 34,871 (87.8) | 19,080 (90.8) | 18,043 (86.7) |
| Birth outcome (at 30 min p < 0.0001) | ||||||||
| Missing | 281 (0.4) | 0 (0.0) | 229 (1.9) | 0 (0.0) | 31 (0.1) | 0 (0.0) | 21 (0.1) | 0 (0.0) |
| Not missing | 67,494 (99.6) | 70,667 (100.0) | 11,830 (98.1) | 10,151 (100.0) | 34,668 (99.9) | 39,699 (100.0) | 20,996 (99.9) | 20,817 (100.0) |
| 24 h outcomes p < 0.0001 | ||||||||
| Missing | 1452 (2.1) | 193 (0.3) | 544 (4.5) | 72 (0.7) | 876 (2.5) | 113 (0.3) | 32 (0.2) | 8 (0.0) |
| Not missing | 66,323 (97.9) | 70,474 (99.7) | 11,515 (95.5) | 10,079 (99.3) | 33,823 (97.5) | 39,586 (99.7) | 20,985 (99.8) | 20,809 (100.0) |
| Maternal outcome at discharge p < 0.0001 | ||||||||
| Missing | 1653 (2.4) | 141 (0.2) | 160 (1.3) | 16 (0.2) | 1450 (4.2) | 116 (0.3) | 43 (0.2) | 9 (0.0) |
| Not missing | 66,122 (97.6) | 70,526 (99.8) | 11,899 (98.7) | 10,135 (99.8) | 33,249 (95.8) | 39,583 (99.7) | 20,974 (99.8) | 20,808 (100.0) |
| Duration of 1st stage of labour p < 0.0001 | ||||||||
| Missing | 35,075 (51.8) | 32,435 (45.9) | 5093 (42.2) | 3020 (29.8) | 21,472 (61.9) | 20,145 (50.7) | 8510 (40.5) | 9270 (44.5) |
| Not missing | 32,700 (48.2) | 38,232 (54.1) | 6966 (57.8) | 7131 (70.2) | 13,227 (38.1) | 19,554 (49.3) | 12,507 (59.5) | 11,547 (55.5) |
| All | Manyara | Tabora | Geita | Shinyanga | Mwanza | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (N = 4943) | Implementation (N = 15,658) | Baseline (N = 7382) | Implementation (N = 14,365) | Baseline (N = 19,307) | Implementation (N = 19,073) | Baseline (N = 15,545) | Implementation (N = 8453) | Baseline (N = 20,598) | Implementation (N = 13,118) | |
| Any missing p < 0.0001 | ||||||||||
| Missing | 4312 (87.2) | 11,378 (72.7) | 6237 (84.5) | 10,334 (71.9) | 14,936 (77.4) | 13,206 (69.2) | 11,042 (71.0) | 5733 (67.8) | 17,179 (83.4) | 8812 (67.2) |
| Not missing | 631 (12.8) | 4280 (27.3) | 1145 (15.5) | 4031 (28.1) | 4371 (22.6) | 5867 (30.8) | 4503 (29.0) | 2720 (32.2) | 3419 (16.6) | 4306 (32.8) |
| Gestational age p < 0.0001 | ||||||||||
| Missing | 3031 (61.3) | 5056 (32.3) | 4888 (66.2) | 7221 (50.3) | 7939 (41.1) | 6814 (35.7) | 3689 (23.7) | 1984 (23.5) | 9075 (44.1) | 3093 (23.6) |
| Not missing | 1912 (38.7) | 10,602 (67.7) | 2494 (33.8) | 7144 (49.7) | 11,368 (58.9) | 12,259 (64.3) | 11,856 (76.3) | 6469 (76.5) | 11,523 (55.9) | 10,025 (76.4) |
| Amniotic fluid colour p < 0.0001 | ||||||||||
| Missing | 2529 (51.2) | 6661 (42.5) | 2413 (32.7) | 2448 (17.0) | 9114 (47.2) | 6668 (35.0) | 6789 (43.7) | 3520 (41.6) | 6672 (32.4) | 1770 (13.5) |
| Not missing | 2414 (48.8) | 8997 (57.5) | 4969 (67.3) | 11,917 (83.0) | 10,193 (52.8) | 12,405 (65.0) | 8756 (56.3) | 4933 (58.4) | 13,926 (67.6) | 11,348 (86.5) |
| Duration of 2nd stage of labour p < 0.0001 | ||||||||||
| Missing | 1928 (39.0) | 1923 (12.3) | 1861 (25.2) | 836 (5.8) | 4317 (22.4) | 5009 (26.3) | 3849 (24.8) | 1832 (21.7) | 6644 (32.3) | 2560 (19.5) |
| Not missing | 3015 (61.0) | 13,735 (87.7) | 5521 (74.8) | 13,529 (94.2) | 14,990 (77.6) | 14,064 (73.7) | 11,696 (75.2) | 6621 (78.3) | 13,954 (67.7) | 10,558 (80.5) |
| FHR monitoring during labour p < 0.0001 | ||||||||||
| Missing | 1733 (35.1) | 3615 (23.1) | 2012 (27.3) | 2283 (15.9) | 5019 (26.0) | 5275 (27.7) | 2155 (13.9) | 963 (11.4) | 5484 (26.6) | 1617 (12.3) |
| Not missing | 3210 (64.9) | 12,043 (76.9) | 5370 (72.7) | 12,082 (84.1) | 14,288 (74.0) | 13,798 (72.3) | 13,390 (86.1) | 7490 (88.6) | 15,114 (73.4) | 11,501 (87.7) |
| ANC attendance p < 0.0001 | ||||||||||
| Missing | 708 (14.3) | 970 (6.2) | 1442 (19.5) | 579 (4.0) | 1256 (6.5) | 1529 (8.0) | 692 (4.5) | 893 (10.6) | 2225 (10.8) | 736 (5.6) |
| Not missing | 4235 (85.7) | 14,688 (93.8) | 5940 (80.5) | 13,786 (96.0) | 18,051 (93.5) | 17,544 (92.0) | 14,853 (95.5) | 7560 (89.4) | 18,373 (89.2) | 12,382 (94.4) |
| ANC problem p = 0.4185 | ||||||||||
| Missing | 936 (18.9) | 1472 (9.4) | 1273 (17.2) | 1348 (9.4) | 1461 (7.6) | 2681 (14.1) | 533 (3.4) | 726 (8.6) | 2437 (11.8) | 789 (6.0) |
| Not missing | 4007 (81.1) | 14,186 (90.6) | 6109 (82.8) | 13,017 (90.6) | 17,846 (92.4) | 16,392 (85.9) | 15,012 (96.6) | 7727 (91.4) | 18,161 (88.2) | 12,329 (94.0) |
| Presentation p = 0.0183 | ||||||||||
| Missing | 259 (5.2) | 1157 (7.4) | 424 (5.7) | 913 (6.4) | 2,59 (13.3) | 3333 (17.5) | 1271 (8.2) | 1487 (17.6) | 2742 (13.3) | 955 (7.3) |
| Not missing | 4684 (94.8) | 14,501 (92.6) | 6958 (94.3) | 13,452 (93.6) | 16,748 (86.7) | 15,740 (82.5) | 14,274 (91.8) | 6966 (82.4) | 17,856 (86.7) | 12,163 (92.7) |
| Birth outcomes (at 30 min p < 0.0001) | ||||||||||
| Missing | 6 (0.1) | 0 (0.0) | 11 (0.1) | 0 (0.0) | 30 (0.2) | 0 (0.0) | 215 (1.4) | 0 (0.0) | 19 (0.1) | 0 (0.0) |
| Not missing | 4937 (99.9) | 15,658 (100.0) | 7371 (99.9) | 14,365 (100.0) | 19,277 (99.8) | 19,073 (100.0) | 15,330 (98.6) | 8453 (100.0) | 20,579 (99.9) | 13,118 (100.0) |
| 24 h outcomes p < 0.0001 | ||||||||||
| Missing | 630 (12.7) | 61 (0.4) | 212 (2.9) | 25 (0.2) | 48 (0.2) | 4 (0.0) | 253 (1.6) | 86 (1.0) | 309 (1.5) | 17 (0.1) |
| Not missing | 4313 (87.3) | 15,597 (99.6) | 7170 (97.1) | 14,340 (99.8) | 19,259 (99.8) | 19,069 (100.0) | 15,292 (98.4) | 8367 (99.0) | 20,289 (98.5) | 13,101 (99.9) |
| Maternal outcomes at discharge p < 0.0001 | ||||||||||
| Missing | 457 (9.2) | 61 (0.4) | 57 (0.8) | 18 (0.1) | 53 (0.3) | 12 (0.1) | 998 (6.4) | 41 (0.5) | 88 (0.4) | 9 (0.1) |
| Not missing | 4486 (90.8) | 15,597 (99.6) | 7325 (99.2) | 14,347 (99.9) | 19,254 (99.7) | 19,061 (99.9) | 14,547 (93.6) | 8412 (99.5) | 20,510 (99.6) | 13,109 (99.9) |
| Duration of 1st stage of labour p < 0.0001 | ||||||||||
| Missing | 3080 (62.3) | 7905 (50.5) | 3806 (51.6) | 5073 (35.3) | 7915 (41.0) | 8486 (44.5) | 7025 (45.2) | 4262 (50.4) | 13,249 (64.3) | 6709 (51.1) |
| Not missing | 1863 (37.7) | 7753 (49.5) | 3576 (48.4) | 9292 (64.7) | 11,392 (59.0) | 10,587 (55.5) | 8520 (54.8) | 4191 (49.6) | 7349 (35.7) | 6409 (48.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamala, B.A.; Ersdal, H.; Moshiro, R.; Mduma, E.; Baker, U.; Guga, G.; Kvaløy, J.T.; Bishanga, D.R.; Bundala, F.; Marwa, B.; et al. Improvements in Obstetric and Newborn Health Information Documentation following the Implementation of the Safer Births Bundle of Care at 30 Facilities in Tanzania. Healthcare 2024, 12, 319. https://doi.org/10.3390/healthcare12030319
Kamala BA, Ersdal H, Moshiro R, Mduma E, Baker U, Guga G, Kvaløy JT, Bishanga DR, Bundala F, Marwa B, et al. Improvements in Obstetric and Newborn Health Information Documentation following the Implementation of the Safer Births Bundle of Care at 30 Facilities in Tanzania. Healthcare. 2024; 12(3):319. https://doi.org/10.3390/healthcare12030319
Chicago/Turabian StyleKamala, Benjamin Anathory, Hege Ersdal, Robert Moshiro, Estomih Mduma, Ulrika Baker, Godfrey Guga, Jan Terje Kvaløy, Dunstan R. Bishanga, Felix Bundala, Boniphace Marwa, and et al. 2024. "Improvements in Obstetric and Newborn Health Information Documentation following the Implementation of the Safer Births Bundle of Care at 30 Facilities in Tanzania" Healthcare 12, no. 3: 319. https://doi.org/10.3390/healthcare12030319
APA StyleKamala, B. A., Ersdal, H., Moshiro, R., Mduma, E., Baker, U., Guga, G., Kvaløy, J. T., Bishanga, D. R., Bundala, F., Marwa, B., Rutachunzibwa, T., Simeo, J., Rutatinisibwa, H. F., Ndungile, Y., Kayera, D., Kalabamu, F. S., & Mdoe, P. (2024). Improvements in Obstetric and Newborn Health Information Documentation following the Implementation of the Safer Births Bundle of Care at 30 Facilities in Tanzania. Healthcare, 12(3), 319. https://doi.org/10.3390/healthcare12030319







