Lean Six Sigma Approach to Improve the Management of Patients Undergoing Laparoscopic Cholecystectomy
Abstract
1. Introduction
2. Materials and Methods
- Gender (Male/Female);
- Age (0–29, 30–49, 50–59, 60–69, 70–79, ≥80);
- Hypertension (Yes/No);
- Diabetes (Yes/No);
- Cardiovascular disease (Yes/No);
- Respiratory disease (Yes/No);
- Obesity (Yes/No);
- Liver disease (Yes/No);
- Date and time of admission;
- Date and time of an LC procedure;
- Date and time of discharge.
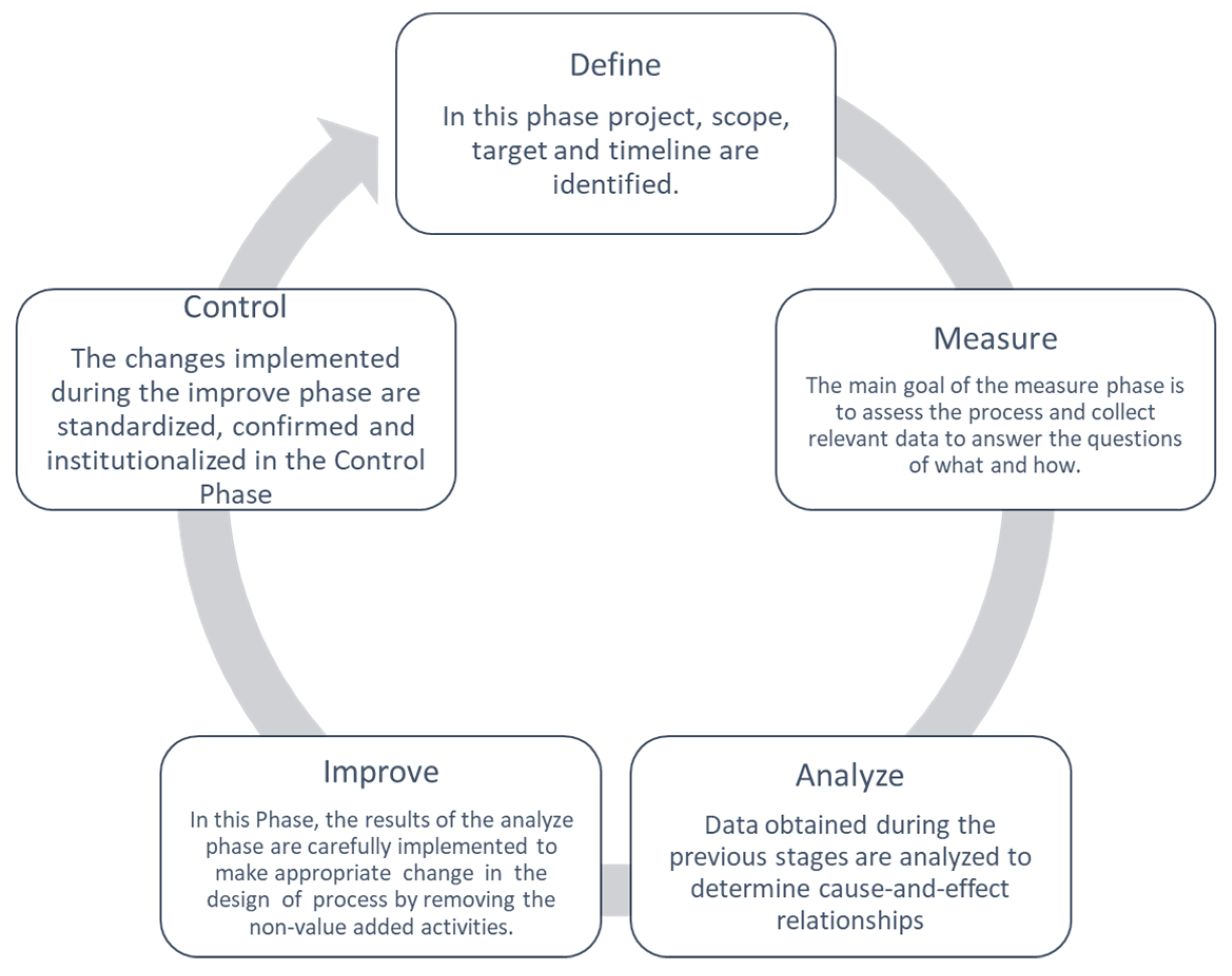
2.1. DMAIC Cycle
2.1.1. Define
- Project title: Lean Six Sigma approach to improve the management of patients undergoing laparoscopic cholecystectomy.
- Question: prolonged post-operative LOS.
- CTQ: post-operative LOS (days).
- Target: Improve the quality process to reduce the CTQ for all patients who undergo LC according to the indication of the Italian government.
- Deliverables: increase in the turnover; decrease in post-operative LOS.
- Timeline:
- ○
- Define: January–March 2015;
- ○
- Measure: April–August 2015;
- ○
- Analyze: September–October 2015;
- ○
- Improve: November–December 2015;
- ○
- Control: January 2016–December 2020.
- In scope: laparoscopic cholecystectomy; transversal to surgery department of “San Giovanni di Dio e Ruggi d’Aragona” of Salerno (Italy).
- Out scope: the departments not enclosed in the process.
- Financial: no financial assistance is required.
- Business need: speeding up the discharge process.
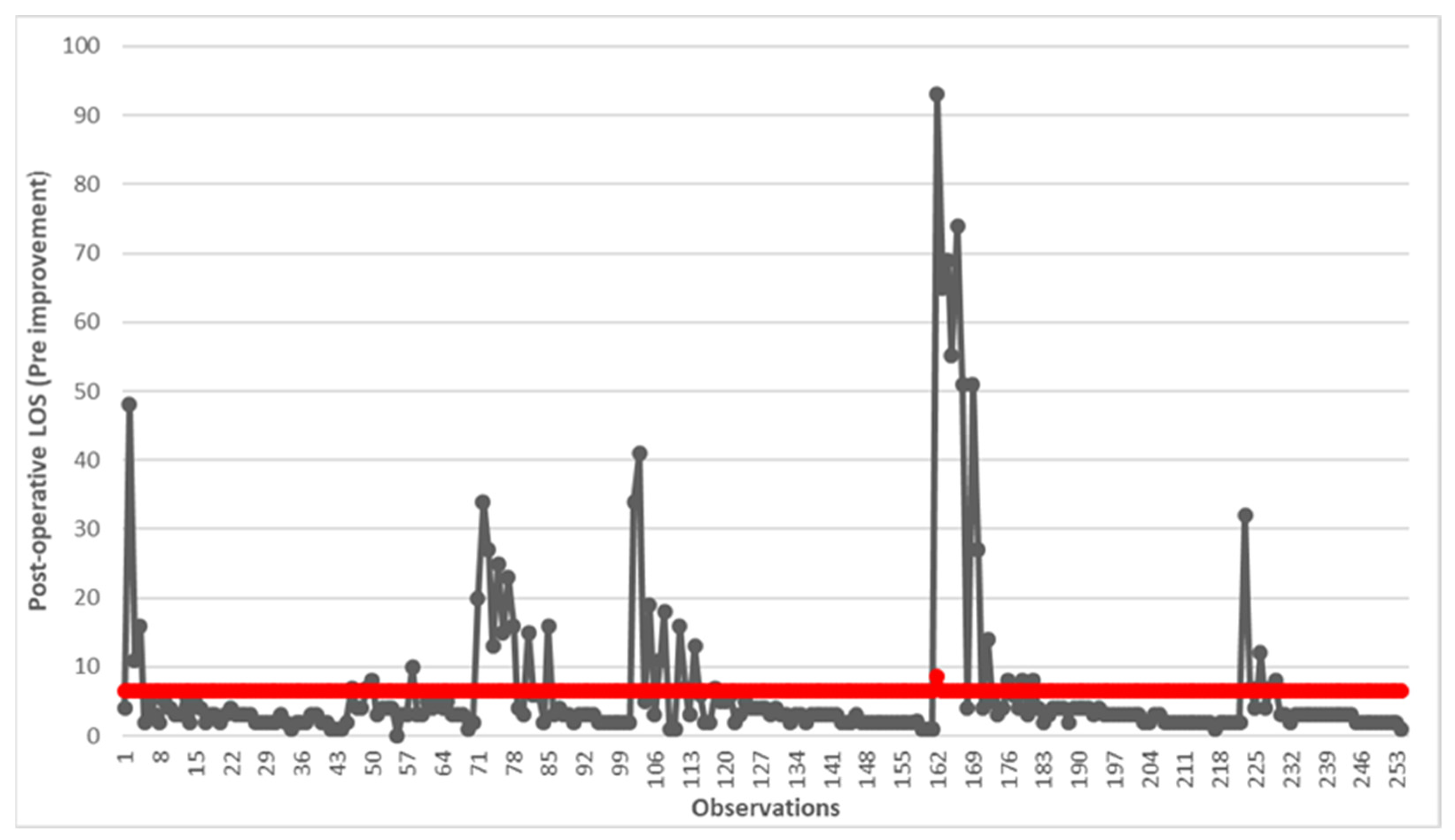
2.1.2. Measure
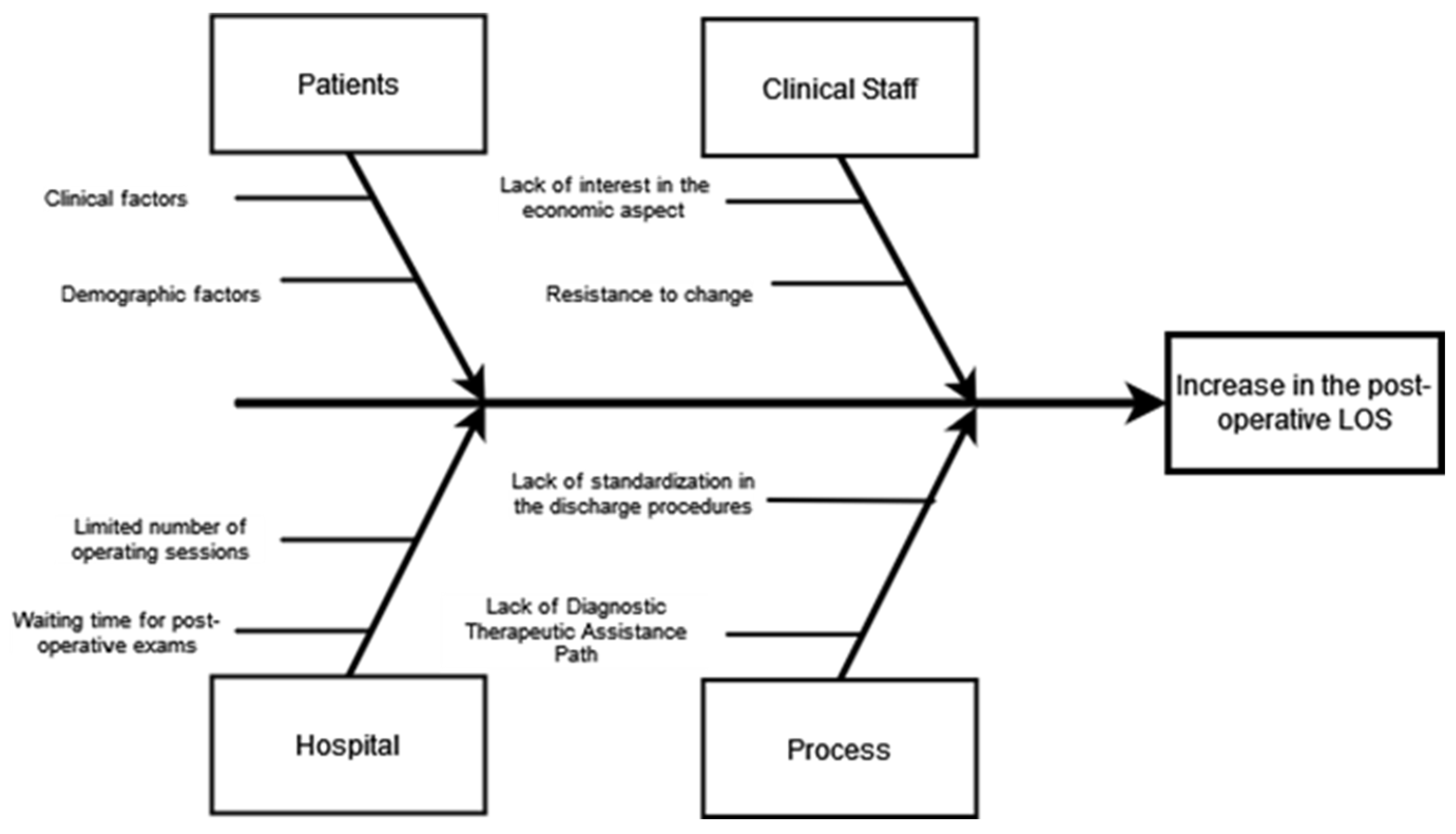
2.1.3. Analyze
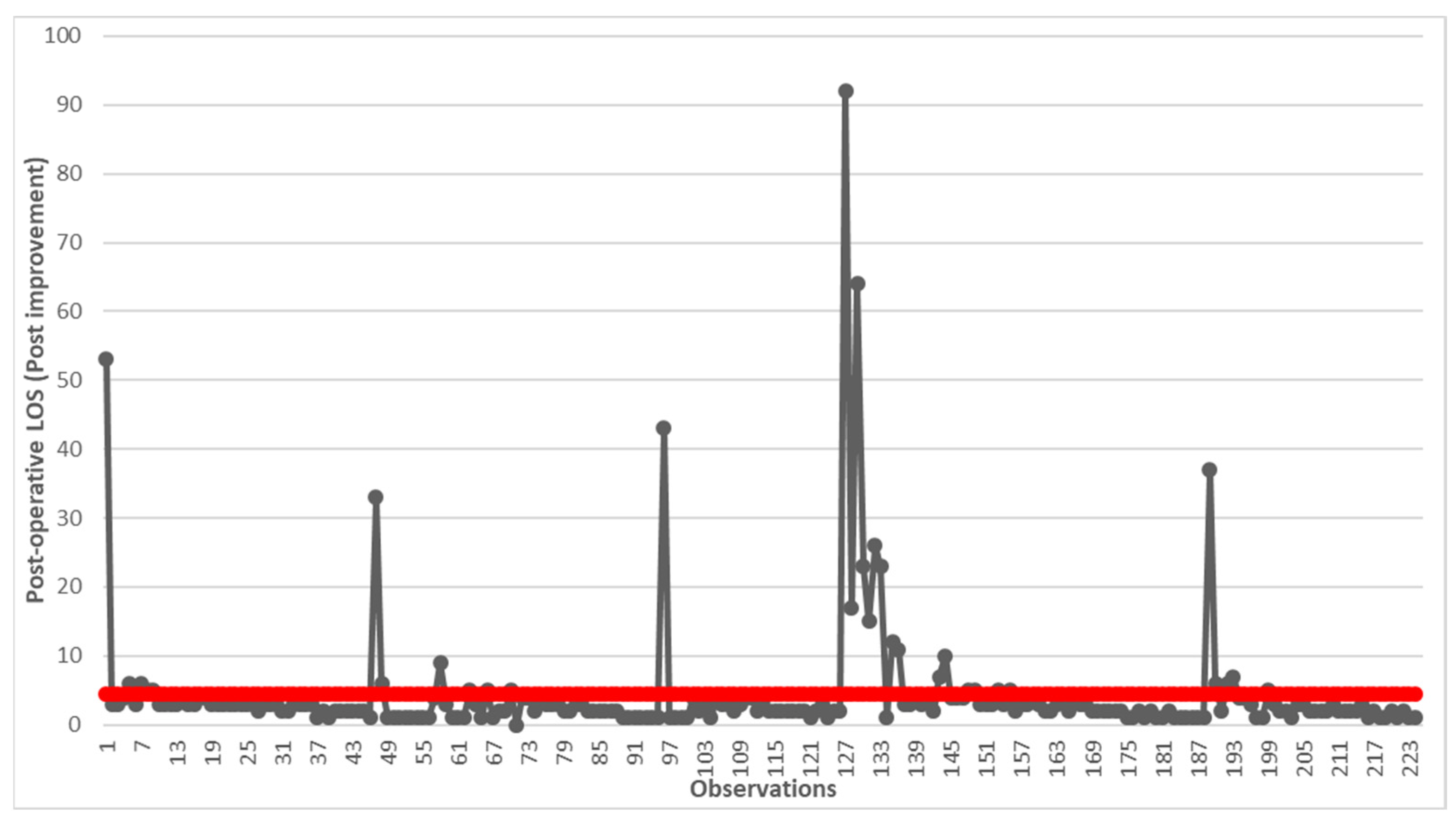
2.1.4. Improve
- Post-operative hematochemical examinations: the time for performing and receiving the result has been reduced thanks to a defined pathway with the laboratories.
- Light feeding after gas canalization.
- Discharge on the first or second day depending on clinical evolution.
- Outpatient surgical check-up 7 days after discharge.
2.2. Control
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agresta, F.; Campanile, F.C.; Vettoretto, N.; Silecchia, G.; Bergamini, C.; Maida, P.; Lombari, P.; Narilli, P.; Marchi, D.; Carrara, A.; et al. Laparoscopic cholecystectomy: Consensus conference-based guidelines. Langenbeck’s Arch. Surg. 2015, 400, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Matsevych, O.; Koto, M.; Balabyeki, M.; Aldous, C. Trauma laparoscopy: When to start and when to convert? Surg. Endosc. 2018, 32, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Fan, S.-T.; Lai, E.C.S.; Lo, C.-M.; Chu, K.-M. Factors Affecting Conversion of Laparoscopic Cholecystectomy to Open Surgery. Arch. Surg. 1996, 131, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.; Seabold, E.; Herzing, K.; Markert, R.; Gans, A.; Ekeh, A.P. Laparoscopic cholecystectomy in the Acute Care Surgery model: Risk factors for complications. Trauma Surg. Acute Care Open 2019, 4, e000312. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Bhatti, U.F.; Petrosyan, M.; Washington, G.; Nizam, W.; Williams, M.; Tran, D.; Cornwell, E.E.; Fullum, T.M. The heavy price of conversion from laparoscopic to open procedures for emergent cholecystectomies. Am. J. Surg. 2019, 217, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Watanabe, M.; Kusachi, S.; Matsukiyo, H.; Saito, T.; Kodama, H.; Kiribayashi, T.; Enomoto, T.; Nakamura, Y.; Okamoto, Y.; et al. Risk factors for conversion of laparoscopic cholecystectomy to open surgery associated with the severity characteristics according to the Tokyo guidelines. Surg. Today 2014, 44, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Harboe, K.M.; Bardram, L. The quality of cholecystectomy in Denmark: Outcome and risk factors for 20,307 patients from the national database. Surg. Endosc. 2010, 25, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.S.; Nijsse, B.A.; Sokal, S.M.; Chang, Y.; Berger, D.L. Surgical outcomes of open cholecystectomy in the laparoscopic era. Am. J. Surg. 2009, 197, 781–784. [Google Scholar] [CrossRef]
- Pogorelic, Z.; Aralica, M.; Jukic, M.; Zitko, V.; Despot, R.; Juric, I. Gallbladder Disease in Children: A 20-year Single-center Experience. Indian Pediatr. 2019, 56, 384–386. [Google Scholar] [CrossRef]
- Akhtar-Danesh, G.-G.; Doumouras, A.G.; Bos, C.; Flageole, H.; Hong, D. Factors Associated with Outcomes and Costs after Pediatric Laparoscopic Cholecystectomy. JAMA Surg. 2018, 153, 551–557. [Google Scholar] [CrossRef]
- Khoo, A.K.; Cartwright, R.; Berry, S.; Davenport, M. Cholecystectomy in English Children: Evidence of an Epidemic (1997–2012). J. Pediatr. Surg. 2014, 49, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.R.; Kovacic, K.; Yan, K.; Simpson, P.; Sood, M.R. Trends of Cholecystectomies for Presumed Biliary Dyskinesia in Children in the United States. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Gadacz, T.R. Update on laparoscopic cholecystectomy, including a clinical pathway. Surg. Clin. N. Am. 2000, 80, 1127–1150. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, A.G.; Solej, M.; Enrico, S.; Falcone, A.; Catalano, S.; Pozzi, G.; Marola, S.; Martino, V. Elective and emergency laparoscopic cholecystectomy in the elderly: Our experience. BMC Surg. 2013, 13, S21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, K.-R.; Ibrahim, S.; Tan, N.-C.; Lim, S.-H.; Tay, K.-H. Risk Factors for Conversion to Open Surgery in Patients with Acute Cholecystitis Undergoing Interval Laparoscopic Cholecystectomy. Ann. Acad. Med. Singap. 2007, 36, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Schietroma, M.; Carlei, F.; Liakos, C.; Rossi, M.; Carloni, A.; Enang, G.N.; Pistoia, M.A. Laparoscopic versus open cholecystectomy. An analysis of clinical and financial aspects. Panminerva Medica 2001, 43, 239–242. [Google Scholar] [PubMed]
- Shea, J.A.; Berlin, J.A.; Bachwich, D.R.; Staroscik, R.N.; Malet, P.F.; McGuckin, M.; Schwartz, J.S.; Escarce, J.J. Indications for and Outcomes of Cholecystectomy. Ann. Surg. 1998, 227, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Michel, J.M.; Vonlanthen, R.; Becker, K.; Kocher, T.; Krähenbühl, L. Laparoscopic cholecystectomy in acute cholecystitis: Indication, technique, risk and outcome. Langenbeck’s Arch. Surg. 2005, 390, 373–380. [Google Scholar] [CrossRef]
- Livingston, E.H.; Rege, R.V. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am. J. Surg. 2004, 188, 205–211. [Google Scholar] [CrossRef]
- Alponat, A.; Kum, C.K.; Koh, B.C.; Rajnakova, A.; Goh, P.M. Predictive Factors for Conversion of Laparoscopic Cholecystectomy. Mol. Med. 1997, 21, 629–633. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Torrance, G.W.; O’brien, B.J.; Stoddart, G.L. Methods for the Economic Evaluation of Health Care Programmes, 3rd ed.; Oxford University Press (OUP): Evans Road Cary, NC, USA, 2005. [Google Scholar]
- Braga, M.; Vignali, A.; Gianotti, L.; Zuliani, W.; Frasson, M.; Di Serio, C.; Di Carlo, V. Laparoscopic versus open colorectal surgery cost-benefit analysis in a single-center ran-domized trial. Ann. Surg. 2005, 242, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Safran, D.G.; Landon, B.E.; Landrum, M.B.; He, Y.; Mechanic, R.E.; Day, M.P.; Chernew, M.E.; Navathe, A.S.; Emanuel, E.J.; et al. The ‘Alternative Quality Contract,’ Based on A Global Budget, Lowered Medical Spending And Improved Quality. Health Aff. 2012, 31, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Berwick, D.M.; Hackbarth, A.D. Eliminating waste in US health care. J. Am. Med. Assoc. 2012, 307, 1513–1516. [Google Scholar]
- Casale, A.S.; Paulus, R.A.; Selna, M.J.; Doll, M.C.; Bothe, A.E., Jr.; McKinley, K.E.; Berry, S.A.; Davis, D.E.; Gilfillan, R.J.; Hamory, B.H.; et al. “ProvenCareSM”: A provider-driven pay-for-performance program for acute episodic cardiac surgical care. Ann. Surg. 2007, 246, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health; National Agency for Regional Health Services (AGENAS). PNE Programma Nazionale Esi-ti—Edizione 2020; Italian Ministry of Health: Rome, Italy, 2020.
- Decree of the Ministry of Health n ° 70 of 2 April 2015 (Italy). Available online: https://www.gazzettaufficiale.it/eli/id/2015/06/04/15G00084/sg (accessed on 6 December 2023).
- Dash, S.; Shakyawar, S.K.; Sharma, M.; Kaushik, S. Big data in healthcare: Management, analysis and future prospects. J. Big Data 2019, 6, 54. [Google Scholar] [CrossRef]
- Chiarini, A.; Bracci, E. Implementing Lean Six Sigma in healthcare: Issues from Italy. Public Money Manag. 2013, 33, 361–368. [Google Scholar] [CrossRef]
- Ahmed, S.; Manaf, N.H.; Islam, R. Effects of Lean Six Sigma application in healthcare services: A literature review. Rev. Environ. Health 2013, 28, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lighter, D.E. The application of Lean Six Sigma to provide high-quality, reliable pediatric care. Int. J. Pediatr. Adolesc. Med. 2014, 1, 8–10. [Google Scholar] [CrossRef]
- Bhat, S.; Gijo, E.V.; Jnanesh, N.A. Productivity and performance improvement in the medical records department of a hospital: An application of Lean Six Sigma. Int. J. Product. Perform. Manag. 2016, 65, 98–125. [Google Scholar] [CrossRef]
- Mandahawi, N.; Al Araidah, O.; Boran, A.; Khasawneh, M. Application of Lean Six Sigma tools to minimise length of stay for ophthalmology day case surgery. Int. J. Six Sigma Compet. Advant. 2011, 6, 156. [Google Scholar] [CrossRef]
- Improta, G.; Balato, G.; Ricciardi, C.; Russo, M.A.; Santalucia, I.; Triassi, M.; Cesarelli, M. Lean Six Sigma in healthcare: Fast track surgery for patients undergoing prosthetic hip replacement surgery. TQM J. 2019, 31, 526–540. [Google Scholar] [CrossRef]
- Trunfio, T.A.; Scala, A.; Borrelli, A.; Sparano, M.; Triassi, M.; Improta, G. Application of the Lean Six Sigma approach to the study of the LOS of patients who undergo laparoscopic cholecystectomy at the San Giovanni di Dio and Ruggi d’Aragona University Hospital. In Proceedings of the ICMHI 2021: 2021 5th International Conference on Medical and Health Informatics, Kyoto, Japan, 14–16 May 2021. [Google Scholar]
- Scala, A.; Ponsiglione, A.M.; Loperto, I.; Della Vecchia, A.; Borrelli, A.; Russo, G.; Triassi, M.; Improta, G. Lean Six Sigma Approach for Reducing Length of Hospital Stay for Patients with Femur Fracture in a University Hospital. Int. J. Environ. Res. Public Health 2021, 18, 2843. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; O’Connell, E.; Rogers, A.C.; Sorensen, J.; McNamara, D.A. Systematic review and meta-analysis of factors which reduce the length of stay associated with elective laparoscopic cholecystectomy. HPB 2021, 23, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Soria, V.; Pellicer, E.; Flores, B.; Carrasco, M.; Candel, M.F.; Aguayo, J.L. Evaluation of the Clinical Pathway for Laparoscopic Cholecystectomy. Am. Surg. 2005, 71, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Takifuji, K.; Tani, M.; Onishi, H.; Yamaue, H. Effectiveness of the clinical pathway to decrease length of stay and cost for laparoscopic surgery. Surg. Endosc. 2002, 16, 1594–1597. [Google Scholar] [CrossRef]
- Ko-Iam, W.; Sandhu, T.; Paiboonworachat, S.; Pongchairerks, P.; Chotirosniramit, A.; Chotirosniramit, N.; Chandacham, K.; Jirapongcharoenlap, T.; Junrungsee, S. Predictive Factors for a Long Hospital Stay in Patients Undergoing Laparoscopic Cholecystectomy. Int. J. Hepatol. 2017, 2017, 5497936. [Google Scholar] [CrossRef]
- Strömberg, J.; Hammarqvist, F.; Sadr-Azodi, O.; Sandblom, G. Cholecystectomy in Patients with Liver Cirrhosis. Gastroenterol. Res. Pract. 2015, 2015, 783823. [Google Scholar] [CrossRef]
- Morimoto, Y.; Mizuno, H.; Akamaru, Y.; Yasumasa, K.; Noro, H.; Kono, E.; Yamasaki, Y. Predicting prolonged hospital stay after laparoscopic cholecystectomy. Asian J. Endosc. Surg. 2015, 8, 289–295. [Google Scholar] [CrossRef]
- Chong, J.U.; Lee, J.H.; Yoon, Y.C.; Kwon, K.H.; Cho, J.Y.; Kim, S.-J.; Kim, J.K.; Kim, S.H.; Choi, S.B.; Kim, K.S. Influencing factors on postoperative hospital stay after laparoscopic cholecystectomy. Korean J. Hepato-Biliary-Pancreat. Surg. 2016, 20, 12–16. [Google Scholar] [CrossRef]
- Damani, S.A.R.; Haider, S.; Bilal, H.; Perveen, S. Comparison of operative time and length of hospital stay in laparoscopic cholecystectomy in acute verses chronic cholecystitis. J. Ayub Med. Coll. Abbottabad 2015, 27, 102–104. [Google Scholar]
- Henrique, D.B.; Filho, M.G. A systematic literature review of empirical research in Lean and Six Sigma in healthcare. Total Qual. Manag. Bus. Excel. 2020, 31, 429–449. [Google Scholar] [CrossRef]
- Kuy, S.; Sosa, J.A.; Roman, S.A.; Desai, R.; Rosenthal, R.A. Age matters: A study of clinical and economic outcomes following cholecystectomy in elderly Americans. Am. J. Surg. 2011, 201, 789–796. [Google Scholar] [CrossRef] [PubMed]
| Variable | Category | Post-Operative LOS * (Mean ± Std Dev **) | N | p-Value |
|---|---|---|---|---|
| Age | 0–29 | 10.000 ± 20.008 | 13 | 0.382 |
| 30–49 | 6.655 ± 9.913 | 55 | ||
| 50–59 | 6.250 ± 12.128 | 68 | ||
| 60–69 | 6.592 ± 13.681 | 71 | ||
| 70–79 | 6.122 ± 8.838 | 41 | ||
| over 80 | 4.833 ± 4.535 | 6 | ||
| Gender | Man | 6.780 ± 12.910 | 104 | 0.886 |
| Women | 6.430 ± 11.380 | 150 | ||
| Hypertension | No | 6.727 ± 11.063 | 143 | 0.527 |
| Yes | 6.369 ± 13.174 | 111 | ||
| Diabetes | No | 6.638 ± 11.953 | 213 | 0.540 |
| Yes | 6.220 ± 12.433 | 41 | ||
| Cardiovascular disease | No | 6.694 ± 12.935 | 180 | 0.426 |
| Yes | 6.270 ± 9.453 | 74 | ||
| Respiratory disease | No | 6.242 ± 11.174 | 231 | 0.283 |
| Yes | 9.870 ± 18.450 | 23 | ||
| Obesity | No | 6.634 ± 12.368 | 227 | 0.386 |
| Yes | 6.037 ± 8.555 | 27 | ||
| Liver disease | No | 6.304 ± 11.414 | 227 | 0.516 |
| Yes | 8.815 ± 16.272 | 27 |
| Variable | Category | Pre-Improvement (Mean ± Std Dev *) | Post-Improvement (Mean ± Std Dev *) | Difference of the Mean (%) | p-Value |
|---|---|---|---|---|---|
| All | All | 134 | 23.80 | 5 | 262 |
| Age | 0–29 | 10.000 ± 20.008 | 1.750 ± 0.500 | −82.50 | 0.008 |
| 30–49 | 6.655 ± 9.913 | 7.159 ± 17.430 | +7.57 | 0.002 | |
| 50–59 | 6.250 ± 12.128 | 4.235 ± 6.752 | −32.24 | 0.193 | |
| 60–69 | 6.592 ± 13.681 | 2.950 ± 3.730 | −55.25 | 0.0001 | |
| 70–79 | 6.122 ± 8.838 | 4.038 ± 7.082 | −34.04 | 0.086 | |
| over 80 | 4.833 ± 4.535 | 5.307 ± 5.907 | +9.80 | 0.858 | |
| Gender | Man | 6.780 ± 12.910 | 3.906 ± 7.049 | −42.39 | 0.001 |
| Women | 6.430 ± 11.380 | 4.836 ± 10.825 | −24.79 | 0.0007 | |
| Hypertension | No | 6.727 ± 11.063 | 4.396 ± 9.134 | −34.65 | 0.0001 |
| Yes | 6.369 ± 13.174 | 4.504 ± 9.731 | −29.28 | 0.017 | |
| Diabetes | No | 6.638 ± 11.953 | 4.511 ± 9.846 | −32.04 | 0.0001 |
| Yes | 6.220 ± 12.433 | 9.900 ± 15.933 | −16.48 | 0.044 | |
| Cardiovascular disease | No | 6.694 ± 12.935 | 4.380 ± 8.381 | −34.56 | 0.0001 |
| Yes | 6.270 ± 9.453 | 4.000 ± 8.367 | −36.20 | 0.012 | |
| Respiratory disease | No | 6.242 ± 11.174 | 4.513 ± 9.984 | −27.69 | 0.0001 |
| Cardiological Disorders | Yes | 9.870 ± 18.450 | 4.269 ± 5.204 | −56.74 | 0.119 |
| Obesity | No | 6.634 ± 12.368 | 4.465 ± 9.490 | −32.69 | 0.0001 |
| Yes | 6.037 ± 8.555 | 4.522 ± 9.686 | −28.30 | 0.125 | |
| Liver disease | No | 6.304 ± 11.414 | 4.432 ± 9.644 | −29.69 | 0.0001 |
| Yes | 8.815 ± 16.272 | 5.700 ± 7.718 | −35.33 | 0.245 | |
| Hypertension | No | 6.727 ± 11.063 | 4.396 ± 9.134 | −34.65 | 0.0001 |
| Yes | 6.369 ± 13.174 | 4.504 ± 9.731 | −29.28 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, A.; Improta, G. Lean Six Sigma Approach to Improve the Management of Patients Undergoing Laparoscopic Cholecystectomy. Healthcare 2024, 12, 292. https://doi.org/10.3390/healthcare12030292
Scala A, Improta G. Lean Six Sigma Approach to Improve the Management of Patients Undergoing Laparoscopic Cholecystectomy. Healthcare. 2024; 12(3):292. https://doi.org/10.3390/healthcare12030292
Chicago/Turabian StyleScala, Arianna, and Giovanni Improta. 2024. "Lean Six Sigma Approach to Improve the Management of Patients Undergoing Laparoscopic Cholecystectomy" Healthcare 12, no. 3: 292. https://doi.org/10.3390/healthcare12030292
APA StyleScala, A., & Improta, G. (2024). Lean Six Sigma Approach to Improve the Management of Patients Undergoing Laparoscopic Cholecystectomy. Healthcare, 12(3), 292. https://doi.org/10.3390/healthcare12030292







