Evaluating Adverse Drug Reactions, Their Reporting Rates and Their Impact on Attitudes Toward Pharmacotherapy Among Female Patients with Schizophrenia: Insights and Implications from a Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics
3.2. Pharmacotherapy
3.3. Adverse Drug Reactions
3.4. Factors Associated with DAI-10
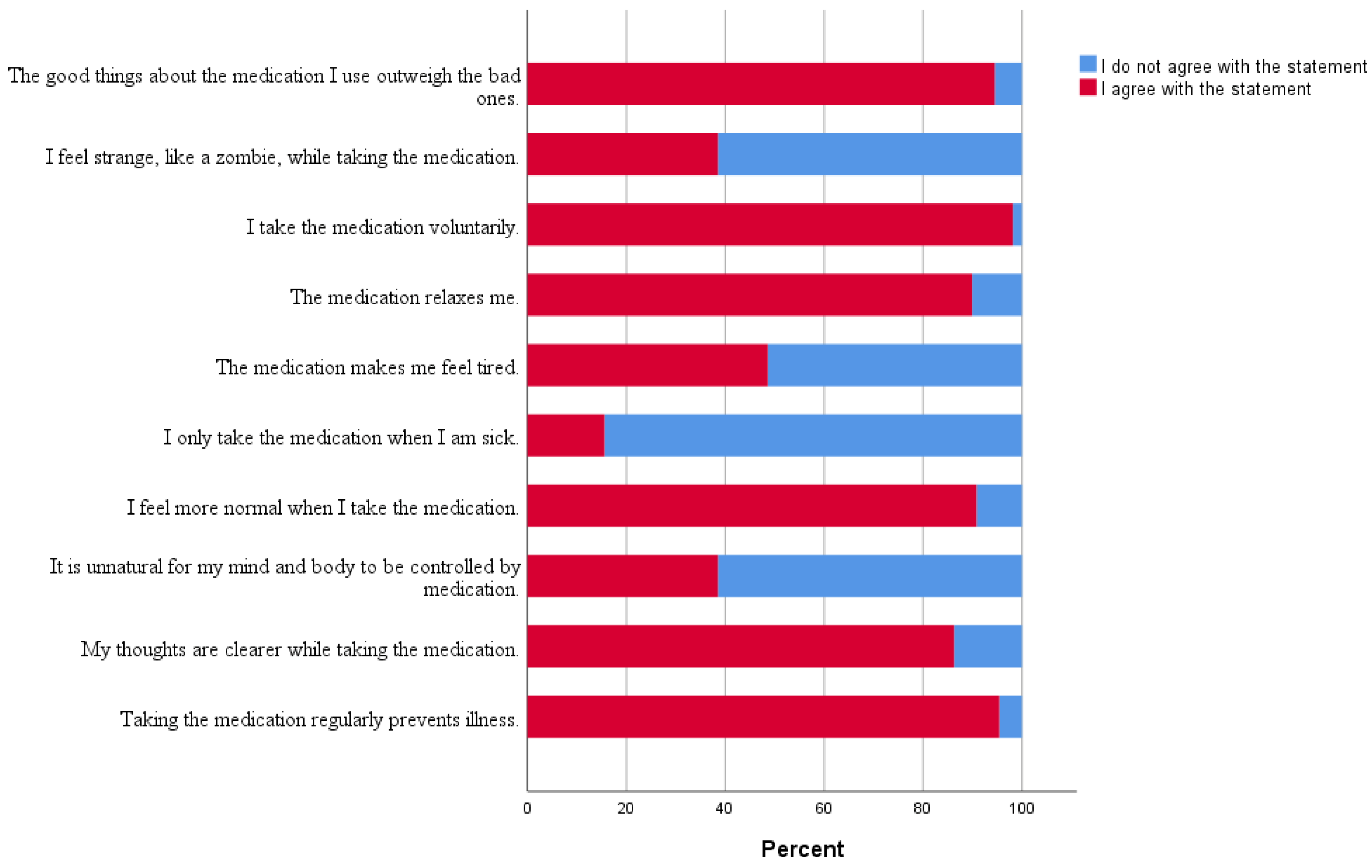
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institute of Mental Health. Available online: www.nimh.nih.gov (accessed on 10 September 2024).
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Andrieux, A.; Bartzokis, G.; Cadenhead, K.; Dazzan, P.; Fusar-Poli, P.; Gallinat, J.; Giedd, J.; Grayson, D.R.; Heinrichs, M.; et al. Altering the course of schizophrenia: Progress and perspectives. Nat. Rev. Drug Discov. 2016, 15, 485–515. [Google Scholar] [CrossRef] [PubMed]
- Tasci, B.; Tasci, G.; Ayyildiz, H.; Kamath, A.P.; Barua, P.D.; Tuncer, T.; Dogan, S.; Ciaccio, E.J.; Chakraborty, S.; Acharya, U.R. Automated schizophrenia detection model using blood sample scattergram images and local binary pattern. Multimed. Tools Appl. 2023, 83, 42735–42763. [Google Scholar] [CrossRef]
- Schizophrenia spectrum and other psychotic disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; The American Psychiatric Association (APA): Arlington, VA, USA, 2013; pp. 87–122.
- Lieberman, J.A.; First, M.B. Psychotic Disorders. N. Engl. J. Med. 2018, 379, 270–280. [Google Scholar] [CrossRef]
- Ali, T.; Sisay, M.; Tariku, M.; Mekuria, A.N.; Desalew, A. Antipsychotic-induced extrapyramidal side effects: A systematic review and meta-analysis of observational studies. PLoS ONE 2021, 16, e0257129. [Google Scholar] [CrossRef]
- Herceg, D.; Mimica, N.; Herceg, M.; Puljic, K. Aggression in Women with Schizophrenia Is Associated with Lower HDL Cholesterol Levels. Int. J. Mol. Sci. 2022, 23, 11858. [Google Scholar] [CrossRef]
- Fitzgerald, I.; O’Connell, J.; Keating, D.; Hynes, C.; McWilliams, S.; Crowley, E.K. Metformin in the management of antipsychotic-induced weight gain in adults with psychosis: Development of the first evidence-based guideline using GRADE methodology. Evid. Based Ment. Health 2022, 25, 15–22. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Piras, M.; Dubath, C.; Gholam, M.; Laaboub, N.; Grosu, C.; Gamma, F.; Solida, A.; Plessen, K.J.; von Gunten, A.; Conus, P.; et al. Daily Dose Effects of Risperidone on Weight and Other Metabolic Parameters: A Prospective Cohort Study. J. Clin. Psychiatry 2022, 83, 40889. [Google Scholar] [CrossRef] [PubMed]
- Quality of Life in Patients with Schizophrenia. Available online: https://www.cambridge.org/core/journals/european-psychiatry/article/quality-of-life-in-patients-with-schizophrenia/FFD5BB8BA6EBCACE85FD6390E521134C (accessed on 10 September 2024).
- World Health Organization: Measuring Quality of Life. Available online: https://www.who.int/tools/whoqol (accessed on 10 September 2024).
- Dai, N.; Huang, B.; Gao, T.; Zheng, Y.; Shi, C.; Pu, C.; Yu, X. Initial attitudes toward a drug predict medication adherence in first-episode patients with schizophrenia: A 1-year prospective study in China. BMC Psychiatry 2023, 23, 907. [Google Scholar] [CrossRef]
- Ata, E.E.; Bahadir-Yilmaz, E.; Bayrak, N.G. The impact of side effects on schizophrenia and bipolar disorder patients’ adherence to prescribed medical therapy. Perspect. Psychiatr. Care 2020, 56, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Conus, P.; Eide, P.; Mass, R.; Karow, A.; Moritz, S.; Golks, D.; Naber, D. Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur. Psychiatry 2004, 19, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hogan, T.P.; Awad, A.G.; Eastwood, R. A self-report scale predictive of drug compliance in schizophrenics: Reliability and discriminative validity. Psychol. Med. 1983, 13, 177–183. [Google Scholar] [CrossRef]
- Zhou, J.; Xiang, Y.T.; Li, Q.; Zhu, X.; Li, W.; Ungvari, G.S.; Ng, C.H.; Ongur, D.; Wang, X. Gender differences in attitudes towards antipsychotic medications in patients with schizophrenia. Psychiatry Res. 2016, 245, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef]
- Cotton, S.M.; Lambert, M.; Schimmelmann, B.G.; Foley, D.L.; Morley, K.I.; McGorry, P.D.; Conus, P. Gender differences in premorbid, entry, treatment, and outcome characteristics in a treated epidemiological sample of 661 patients with first episode psychosis. Schizophr. Res. 2009, 114, 17–24. [Google Scholar] [CrossRef]
- Koster, A.; Lajer, M.; Lindhardt, A.; Rosenbaum, B. Gender differences in first episode psychosis. Soc. Psychiatry Psychiatr. Epidemiol. 2008, 43, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.S.; Mao, W.J.; Chan, C.L.; Chen, E.Y.; Conwell, Y. Gender differences in outcomes in people with schizophrenia in rural China: 14-year follow-up study. Br. J. Psychiatry 2015, 206, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bukić, J. Utjecaj Nuspojava Antipsihotika na Zadovoljstvo Liječenjem u Pacijentica sa Shizofrenijom; Sveučilište u Zagrebu, Farmaceutsko-Biokemijski Fakultet: Zagreb, Croatia, 2023. [Google Scholar]
- Hatano, M.; Takeuchi, I.; Yamashita, K.; Morita, A.; Tozawa, K.; Sakakibara, T.; Hajitsu, G.; Hanya, M.; Yamada, S.; Iwata, N.; et al. Satisfaction Survey on Antipsychotic Formulations by Schizophrenia Patients in Japan. Clin. Psychopharmacol. Neurosci. 2021, 19, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, I.; Taavola, H.; Tregunno, P.M.; Mas, P.; Gama, S.; Newbould, V.; Caster, O.; Harmark, L. Characteristics, Quality and Contribution to Signal Detection of Spontaneous Reports of Adverse Drug Reactions Via the WEB-RADR Mobile Application: A Descriptive Cross-Sectional Study. Drug Saf. 2018, 41, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Schmiedl, S.; Rottenkolber, M.; Hasford, J.; Rottenkolber, D.; Farker, K.; Drewelow, B.; Hippius, M.; Salje, K.; Thurmann, P. Self-medication with over-the-counter and prescribed drugs causing adverse-drug-reaction-related hospital admissions: Results of a prospective, long-term multi-centre study. Drug Saf. 2014, 37, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Gaebel, W.; Riesbeck, M.; von Wilmsdorff, M.; Burns, T.; Derks, E.M.; Kahn, R.S.; Rossler, W.; Fleischhacker, W.W. Drug attitude as predictor for effectiveness in first-episode schizophrenia: Results of an open randomized trial (EUFEST). Eur. Neuropsychopharmacol. 2010, 20, 310–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngui, N.; Vasiliadis, H.M.; Tempier, R. Factors associated with adherence over time to antipsychotic drug treatment. Clin. Epidemiol. Glob. Health 2015, 3, 3–9. [Google Scholar] [CrossRef]
- Zacker, C.; Puckett, J.T.; Kamal-Bahl, S. Real-World Adherence and Discontinuation of Oral Antipsychotics and Associated Factors in a National Sample of US Medicare Beneficiaries with Schizophrenia. ClinicoEcon. Outcomes Res. 2024, 16, 567–579. [Google Scholar] [CrossRef]
- San, L.; Bernardo, M.; Gomez, A.; Martinez, P.; Gonzalez, B.; Pena, M. Socio-demographic, clinical and treatment characteristics of relapsing schizophrenic patients. Nord. J. Psychiatry 2013, 67, 22–29. [Google Scholar] [CrossRef]
- Masand, P.S.; Narasimhan, M. Improving adherence to antipsychotic pharmacotherapy. Curr. Clin. Pharmacol. 2006, 1, 47–56. [Google Scholar] [CrossRef]
- Velligan, D.I.; Weiden, P.J.; Sajatovic, M.; Scott, J.; Carpenter, D.; Ross, R.; Docherty, J.P. The expert consensus guideline series: Adherence problems in patients with serious and persistent mental illness. J. Clin. Psychiatry 2009, 70 (Suppl. 4), 1–46, quiz 7–8. [Google Scholar]
- Semahegn, A.; Torpey, K.; Manu, A.; Assefa, N.; Tesfaye, G.; Ankomah, A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 17. [Google Scholar] [CrossRef]
- Bjedov, S.; Ciglar, M.; Malekovic, H. Attitudes of Croatian patients with severe mental illness towards long-acting injectable antipsychotics. Psychiatr. Danub. 2016, 28, 278–283. [Google Scholar] [PubMed]
- Weiden, P.; Rapkin, B.; Mott, T.; Zygmunt, A.; Goldman, D.; Horvitz-Lennon, M.; Frances, A. Rating of medication influences (ROMI) scale in schizophrenia. Schizophr. Bull. 1994, 20, 297–310. [Google Scholar] [CrossRef]
- Widschwendter, C.G.; Kemmler, G.; Rettenbacher, M.A.; Yalcin-Siedentopf, N.; Hofer, A. Subjective well-being, drug attitude, and changes in symptomatology in chronic schizophrenia patients starting treatment with new-generation antipsychotic medication. BMC Psychiatry 2018, 18, 212. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, J.; Feng, Y.; Fang, H.; Jiang, T.; Zhao, L.; Wang, Q.; Yang, Y. Attitude and influencing factors of patients with schizophrenia toward long-acting injections: A community-based cross-sectional investigation in China. Front. Public. Health 2022, 10, 951544. [Google Scholar] [CrossRef]
- Irani, F.; Dankert, M.; Siegel, S.J. Patient and family attitudes toward schizophrenia treatment. Curr. Psychiatry Rep. 2004, 6, 283–288. [Google Scholar] [CrossRef]
- Nielsen, R.E.; Lindstrom, E.; Nielsen, J.; Levander, S. DAI-10 is as good as DAI-30 in schizophrenia. Eur. Neuropsychopharmacol. 2012, 22, 747–750. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N (%) |
|---|---|
| Age | |
| 20–29 | 5 (4.6) |
| 30–39 | 15 (13.8) |
| 40–49 | 30 (27.5) |
| 50–59 | 44 (40.4) |
| >60 | 15 (12.7) |
| Relationship status | |
| Single | 37 (33.9) |
| In a relationship | 8 (7.3) |
| Married | 29 (26.6) |
| Divorced | 22 (20.2) |
| Widow | 13 (11.9) |
| Employment status | |
| Unemployed | 60 (55.0) |
| Employed | 49 (45.0) |
| Drug | N (%) |
|---|---|
| Phenothiazines | |
| Promazine | 14 (10.4) |
| Fluphenazine | 8 (5.9) |
| Butyrophenones | |
| Haloperidol | 14 (10.4) |
| Benzamides | |
| Sulpiride | 4 (2.9) |
| Tricyclics | |
| Clozapine | 16 (11.8) |
| Quetiapine | 12 (8.8) |
| Olanzapine | 21 (15.5) |
| Benzisoxazoles | |
| Risperidone | 13 (9.6) |
| Paliperidone | 18 (13.3) |
| Phenylpiperazines | |
| Aripiprazole | 15 (11.1) |
| Total | 135 (100) |
| Adverse Drug Reactions | N (%) |
|---|---|
| Decreased libido | 2 (3.7) |
| Extrapyramidal symptoms (tremor, dystonia) | 15 (28.3) |
| Hypersalivation | 6 (11.3) |
| Amenorrhea | 1 (1.9) |
| Increase in body weight | 15 (28.3) |
| Sedation | 7 (13.2) |
| Headache | 2 (3.7) |
| Instability | 1 (1.9) |
| Hyperprolactinemia | 1 (1.9) |
| Lack of concentration | 1 (1.9) |
| Constipation | 1 (1.9) |
| Depression | 1 (1.9) |
| Total: | 53 |
| Independent Variable | B [95% CI] | β | p-Value | R2 |
|---|---|---|---|---|
| Age | 0.271 [0.091; 0.452] | 0.089 | 0.003 | 0.008 |
| Number of Used Medications | −0.675 [−0.804; −0.0545] | −0.295 | <0.001 | 0.087 |
| Adverse Drug Reaction Presence | −1.382 [−1.765; −0.988] | −0.209 | <0.001 | 0.044 |
| Duration of Medication Usage | 0.006 [0.000; 0.000] | 0.064 | 0.035 | 0.004 |
| Medication Route of Administration | 0.488 [0.014; 0.882] | 0.061 | 0.043 | 0.004 |
| Presence of First-Generation Antipsychotics | −1.340 [−1.752; −0.928] | −0.190 | <0.001 | 0.036 |
| Variable | B [95% CI] | β | p-Value | R2 | ΔR2 |
|---|---|---|---|---|---|
| Number of used medications | −0.641 [−1.093; −0.256] | −0.295 | 0.002 | 0.122 | 0.035 |
| Adverse drug reaction presence * | −1.236 [−2.430; −0.042] | −0.187 | 0.0043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukić, J.; Herceg, D.; Modun, D.; Krce, I.; Leskur, D.; Durdov, T.; Herceg, M.; Šešelja Perišin, A.; Rušić, D. Evaluating Adverse Drug Reactions, Their Reporting Rates and Their Impact on Attitudes Toward Pharmacotherapy Among Female Patients with Schizophrenia: Insights and Implications from a Cross-Sectional Study. Healthcare 2024, 12, 2595. https://doi.org/10.3390/healthcare12242595
Bukić J, Herceg D, Modun D, Krce I, Leskur D, Durdov T, Herceg M, Šešelja Perišin A, Rušić D. Evaluating Adverse Drug Reactions, Their Reporting Rates and Their Impact on Attitudes Toward Pharmacotherapy Among Female Patients with Schizophrenia: Insights and Implications from a Cross-Sectional Study. Healthcare. 2024; 12(24):2595. https://doi.org/10.3390/healthcare12242595
Chicago/Turabian StyleBukić, Josipa, Dora Herceg, Darko Modun, Ivana Krce, Dario Leskur, Toni Durdov, Miroslav Herceg, Ana Šešelja Perišin, and Doris Rušić. 2024. "Evaluating Adverse Drug Reactions, Their Reporting Rates and Their Impact on Attitudes Toward Pharmacotherapy Among Female Patients with Schizophrenia: Insights and Implications from a Cross-Sectional Study" Healthcare 12, no. 24: 2595. https://doi.org/10.3390/healthcare12242595
APA StyleBukić, J., Herceg, D., Modun, D., Krce, I., Leskur, D., Durdov, T., Herceg, M., Šešelja Perišin, A., & Rušić, D. (2024). Evaluating Adverse Drug Reactions, Their Reporting Rates and Their Impact on Attitudes Toward Pharmacotherapy Among Female Patients with Schizophrenia: Insights and Implications from a Cross-Sectional Study. Healthcare, 12(24), 2595. https://doi.org/10.3390/healthcare12242595










