Health Resort Treatment Improves Functioning and Physical Performance in Long COVID Patients: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Methods and Data Collection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Treatment Protocol
2.5. Methods of Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations and Further Studies Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.J.; Lin, C.W.; Hsiao, M.Y.; Wang, T.G.; Liang, H.W. Long COVID and rehabilitation. J. Formos. Med. Assoc. 2024, 123, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living with Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes. Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, X.; Li, H.; Zhang, H.; Xu, J. Mechanisms of long COVID: An updated review. Chin. Med. J. Pulm. Crit. Care. Med. 2023, 1, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Azcue, N.; Gómez-Esteban, J.C.; Acera, M.; Tijero, B.; Fernandez, T.; Ayo-Mentxakatorre, N.; Pérez-Concha, T.; Murueta-Goyena, A.; Lafuente, J.V.; Prada, Á.; et al. Brain fog of post-COVID-19 condition and Chronic Fatigue Syndrome, same medical disorder? J. Transl. Med. 2022, 20, 569. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recov-ery from post-COVID-19 conditions: The RECOVE trial. J. Appl. Physiol. 2023, 134, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, J.; Passadori, A.; Severac, F.; Dieterlen, A.; Geny, B.; Andrès, E. Effects of Rehabilitation on Long-COVID-19 Patient’s Autonomy, Symptoms and Nutritional Observance. Nutrients 2022, 14, 3027. [Google Scholar] [CrossRef] [PubMed]
- McNarry, M.A.; Berg, R.M.G.; Shelley, J.; Hudson, J.; Saynor, Z.L.; Duckers, J.; Lewis, K.; Davies, G.A.; Mackintosh, K.A. Inspiratory muscle training enhances recovery post-COVID-19: A randomised controlled trial. Eur. Respir. J. 2022, 60, 2103101. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Kaufman, D.L. Oxaloacetate Treatment for Mental and Physical Fatigue in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long-COVID fatigue patients: A non-randomized controlled clinical trial. J. Transl. Med. 2022, 20, 295. [Google Scholar] [CrossRef]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of l-Arginine Plus Vitamin C Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef]
- Hawkins, J.; Hires, C.; Keenan, L.; Dunne, E. Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: A randomized, double-blinded, placebo controlled clinical trial. Complement. Ther. Med. 2022, 67, 102823. [Google Scholar] [CrossRef]
- Santana, K.; França, E.; Sato, J.; Silva, A.; Queiroz, M.; de Farias, J.; Rodrigues, D.; Souza, I.; Ribeiro, V.; Caparelli-Dáquer, E.; et al. Non-invasive brain stimulation for fatigue in post-acute sequelae of SARS-CoV-2 (PASC). Brain. Stimul. 2023, 16, 100–107. [Google Scholar] [CrossRef]
- Gloeckl, R.; Zwick, R.H.; Fürlinger, U.; Schneeberger, T.; Leitl, D.; Jarosch, I.; Behrends, U.; Scheibenbogen, C.; Koczulla, A.R. Practical Recommendations for Exercise Training in Patients with Long COVID with or without Post-exertional Malaise: A Best Practice Proposal. Sports. Med. Open. 2024, 10, 47. [Google Scholar] [CrossRef]
- Sánchez-García, J.C.; Reinoso-Cobo, A.; Piqueras-Sola, B.; Cortés-Martín, J.; Menor-Rodríguez, M.J.; Alabau-Dasi, R.; Rodríguez-Blanque, R. Long COVID and Physical Therapy: A Systematic Review. Diseases 2023, 11, 163. [Google Scholar] [CrossRef]
- Hawley, S.E.; Bell, Z.W.; Huang, Y.; Gibbs, J.C.; Churchward-Venne, T.A. Evaluation of sex-based differences in resistance exercise training-induced changes in muscle mass, strength, and physical performance in healthy older (≥60 y) adults: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 91, 102023. [Google Scholar] [CrossRef] [PubMed]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients Without Previous Disabilities Recovering From COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Paneroni, M.; Vogiatzis, I.; Bertacchini, L.; Simonelli, C.; Vitacca, M. Predictors of Low Physical Function in Patients With COVID-19 With Acute Respiratory Failure Admitted to a Subacute Unit. Arch. Phys. Med. Rehabil. 2021, 102, 1228–1231. [Google Scholar] [CrossRef]
- Belli, S.; Balbi, B.; Prince, I.; Cattaneo, D.; Masocco, F.; Zaccaria, S.; Bertalli, L.; Cattini, F.; Lomazzo, A.; Dal Negro, F.; et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur. Respir. J. 2020, 56, 2002096. [Google Scholar] [CrossRef]
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary Rehabilitation in Patients Recovering from COVID-19. Respiration 2021, 100, 416–422. [Google Scholar] [CrossRef]
- Baricich, A.; Borg, M.B.; Cuneo, D.; Cadario, E.; Azzolina, D.; Balbo, P.E.; Bellan, M.; Zeppegno, P.; Pirisi, M.; Cisari, C. Midterm functional sequelae and implications in rehabilitation after COVID-19: A cross-sectional study. Eur. J. Phys. Rehabil. Med. 2021, 57, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Hussein, A.A.; Saad, M.; Zayan, H.E.; Abdelsayed, M.; Moustafa, M.; Ezzat, A.R.; Helmy, R.; Abd-Elaal, H.; Aly, K.; Abdelrheem, S.; et al. Post-COVID-19 functional status: Relation to age, smoking, hospitalization, and previous comorbidities. Ann. Thorac. Med. 2021, 16, 260–265. [Google Scholar] [CrossRef]
- Pant, P.; Joshi, A.; Basnet, B.; Shrestha, B.M.; Bista, N.R.; Bam, N.; Das, S.K. Prevalence of Functional Limitation in COVID-19 Recovered Patients Using the Post COVID-19 Functional Status Scale. JNMA J. Nepal. Med. Assoc. 2021, 59, 7–11. [Google Scholar] [CrossRef]
- Leite, L.C.; Carvalho, L.; Queiroz, D.M.; Farias, M.S.Q.; Cavalheri, V.; Edgar, D.W.; Nery, B.R.D.A.; Vasconcelos Barros, N.; Maldaner, V.; Campos, N.G.; et al. Can the post-COVID-19 functional status scale discriminate between patients with different levels of fatigue, quality of life and functional performance? Pulmonology 2022, 28, 220–223. [Google Scholar] [CrossRef]
- Patterson, T.L.; Mausbach, B.T. Measurement of functional capacity: A new approach to understanding functional differences and real-world behavioral adaptation in those with mental illness. Annu. Rev. Clin. Psychol. 2010, 6, 139–154. [Google Scholar] [CrossRef]
- Maccarone, M.C.; Masiero, S. Spa therapy interventions for post respiratory rehabilitation in COVID-19 subjects: Does the review of recent evidence suggest a role? Environ. Sci. Pollut. Res. Int. 2021, 28, 46063–46066. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.; Maccarone, M.C.; Agostini, F. Health resort medicine can be a suitable setting to recover disabilities in pa-tients tested negative for COVID-19 discharged from hospital? A challenge for the future. Int. J. Biometeorol. 2020, 64, 1807–1809. [Google Scholar] [CrossRef]
- Mratskova, G. Medical resort treatment in post COVID-19 patients with persistent musculoskeletal symptoms. Knowl. Int. J. 2023, 59, 309–315. [Google Scholar]
- Gvozdjáková, A.; Sumbalová, Z.; Kucharská, J.; Rausová, Z.; Kovalčíková, E.; Takácsová, T.; Navas, P.; López-Lluch, G.; Mojto, V.; Palacka, P. Mountain spa rehabilitation improved health of patients with post-COVID-19 syndrome: Pilot study. Environ. Sci. Pollut. Res. Int. 2023, 30, 14200–14211. [Google Scholar] [CrossRef]
- Onik, G.; Knapik, K.; Sieroń, K. Long COVID Cardiopulmonary Symptoms and Health Resort Treatment: A Retrospective Study. J. Clin. Med. 2024, 13, 5563. [Google Scholar] [CrossRef] [PubMed]
- de Facio, C.A.; Guimarães, F.S.; da Cruz, A.G.T.; Bomfim, R.F.; Miranda, S.R.A.P.; Viana, D.R.; Dos Santos Couto Paz, C.C.; Sato, T.O.; Lorenzo, V.A.P.D. Post-COVID-19 functional status scale: Cross-cultural adaptation and measurement properties of the Brazilian Portuguese version. Braz. J. Phys. Ther. 2023, 27, 100503. [Google Scholar] [CrossRef]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- Benkalfate, N.; Eschapasse, E.; Georges, T.; Leblanc, C.; Dirou, S.; Melscoet, L.; Chéné, A.L.; Horeau-Langlard, D.; Bry, C.; Chambellan, A.; et al. Evaluation of the Post-COVID-19 Functional Status (PCFS) Scale in a cohort of patients recovering from hypoxemic SARS-CoV-2 pneumonia. BMJ Open. Respir. Res. 2022, 9, e001136. [Google Scholar] [CrossRef]
- Machado, F.V.C.; Meys, R.; Delbressine, J.M.; Vaes, A.W.; Goërtz, Y.M.J.; van Herck, M.; Houben-Wilke, S.; Boon, G.J.A.M.; Barco, S.; Burtin, C.; et al. Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19. Health. Qual. Life. Outcomes 2021, 19, 40. [Google Scholar] [CrossRef]
- Welch, S.A.; Ward, R.E.; Beauchamp, M.K.; Leveille, S.G.; Travison, T.; Bean, J.F. The Short Physical Performance Battery (SPPB): A Quick and Useful Tool for Fall Risk Stratification Among Older Primary Care Patients. J. Am. Med. Dir. Assoc. 2021, 22, 1646–1651. [Google Scholar] [CrossRef]
- Bergland, A.; Strand, B.H. Norwegian reference values for the Short Physical Performance Battery (SPPB): The Tromsø Study. BMC Geriatr. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.; Mendonca, S.; Booth, S.; Ewing, G.; Gardener, A.C.; Farquhar, M. Are within-person Numerical Rating Scale (NRS) ratings of breathlessness ’on average’ valid in advanced disease for patients and for patients’ informal carers? BMJ Open. Respir. Res. 2017, 4, e000235. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain. Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Besnier, F.; Bérubé, B.; Malo, J.; Gagnon, C.; Grégoire, C.A.; Juneau, M.; Simard, F.; L’Allier, P.; Nigam, A.; Iglésies-Grau, J.; et al. Cardiopulmonary Rehabilitation in Long-COVID-19 Patients with Persistent Breathlessness and Fatigue: The COVID-Rehab Study. Int. J. Environ. Res. Public Health 2022, 19, 4133. [Google Scholar] [CrossRef] [PubMed]
- Oldenmenger, W.H.; de Raaf, P.J.; de Klerk, C.; van der Rijt, C.C. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: A systematic review. J. Pain. Symptom Manage. 2013, 45, 1083–1093. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef]
- Collier, D.; Garip, G. The hidden pandemic: A qualitative study on how middle-aged women make sense of managing their long COVID symptoms. Health Psychol. Rep. 2023, 11, 269–281. [Google Scholar] [CrossRef]
- Rahimi, F.; Saadat, M.; Hessam, M.; Ravanbakhsh, M.; Monjezi, S. Post-COVID-19 physical and cognitive impairments and associations with quality of life: A cross-sectional study. Front. Sports. Act. Living 2024, 6, 1246585. [Google Scholar] [CrossRef]
- Carmona-Cervelló, M.; León-Gómez, B.B.; Dacosta-Aguayo, R.; Lamonja-Vicente, N.; Montero-Alía, P.; Molist, G.; Ayet, A.; Chacón, C.; Costa-Garrido, A.; López-Lifante, V.M.; et al. Long COVID: Cognitive, balance, and retina manifestations. Front. Med. 2024, 11, 1399145. [Google Scholar] [CrossRef]
- Dzięcioł-Anikiej, Z.; Dakowicz, A.; Dzięcioł, J.; Kopko, S.; Moskal-Jasińska, D.; Gawlikowska-Sroka, A.; Kuryliszyn-Moskal, A.; Kostro, A.M. Balance Disorders in People with History of COVID-19 in Light of Posturographic Tests. J. Clin. Med. 2023, 12, 4461. [Google Scholar] [CrossRef]
- Kowal, M.; Morgiel, E.; Winiarski, S.; Gieysztor, E.; Madej, M.; Sebastian, A.; Madziarski, M.; Wedel, N.; Proc, K.; Madziarska, K.; et al. Effect of COVID-19 on Musculoskeletal Performance in Gait and the Timed-Up and Go Test. J. Clin. Med. 2023, 12, 4184. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Legarra-Gorgoñon, G.; Oscoz-Ochandorena, S.; García-Alonso, Y.; García-Alonso, N.; Oteiza, J.; Ernaga Lorea, A.; Correa-Rodríguez, M.; Izquierdo, M. Reduced muscle strength in patients with long-COVID-19 syndrome is mediated by limb muscle mass. J. Appl. Physiol. 2023, 134, 50–58. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Ribeiro Silva, C.; Ohara, D.G.; Matos, A.P.; Pinto, A.C.P.N.; Pegorari, M.S. Short Physical Performance Battery as a Measure of Physical Performance and Mortality Predictor in Older Adults: A Comprehensive Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 10612. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Pérez-Sousa, M.A.; Venegas-Sanabria, L.C.; Cano-Gutierrez, C.A.; Hernández-Quiñonez, P.A.; Rincón-Pabón, D.; García-Hermoso, A.; Zambom-Ferraresi, F.; Sáez de Asteasu, M.L.; Izquierdo, M. Normative Values for the Short Physical Performance Battery (SPPB) and Their Association with Anthropometric Variables in Older Colombian Adults. The SABE Study, 2015. Front. Med. 2020, 7, 52. [Google Scholar] [CrossRef]
- Taboada, M.; Cariñena, A.; Moreno, E.; Rodríguez, N.; Domínguez, M.J.; Casal, A.; Riveiro, V.; Diaz-Vieito, M.; Valdés, L.; Álvarez, J.; et al. Post-COVID-19 functional status six-months after hospitalization. J. Infect. 2021, 82, 31–33. [Google Scholar] [CrossRef]
- Sylvester, S.V.; Rusu, R.; Chan, B.; Bellows, M.; O’Keefe, C.; Nicholson, S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: A review. Curr. Med. Res. Opin. 2022, 38, 1391–1399. [Google Scholar] [CrossRef]
- Reger, M.; Kutschan, S.; Freuding, M.; Schmidt, T.; Josfeld, L.; Huebner, J. Water therapies (hydrotherapy, balneotherapy or aqua therapy) for patients with cancer: A systematic review. J. Cancer. Res. Clin. Oncol. 2022, 148, 1277–1297. [Google Scholar] [CrossRef]
- Gálvez, I.; Fioravanti, A.; Ortega, E. Spa therapy and peripheral serotonin and dopamine function: A systematic review. Int. J. Biometeorol. 2024, 68, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.; Maccarone, M.C.; Magro, G. Balneotherapy and human immune function in the era of COVID-19. Int. J. Biome-Teorol. 2020, 64, 1433–1434. [Google Scholar] [CrossRef]
- Bai, R.; Li, C.; Xiao, Y.; Sharma, M.; Zhang, F.; Zhao, Y. Effectiveness of spa therapy for patients with chronic low back pain: An updated systematic review and meta-analysis. Medicine 2019, 98, e17092. [Google Scholar] [CrossRef]
- Yanaoka, T.; Numata, U.; Nagano, K.; Kurosaka, S.; Kawashima, H. Effects of different intermittent pneumatic compression stimuli on ankle dorsiflexion range of motion. Front. Physiol. 2022, 13, 1054806. [Google Scholar] [CrossRef] [PubMed]
- Wiecha, S.; Jarocka, M.; Wiśniowski, P.; Cieśliński, M.; Price, S.; Makaruk, B.; Kotowska, J.; Drabarek, D.; Cieśliński, I.; Sacewicz, T. The efficacy of intermittent pneumatic compression and negative pressure therapy on muscle function, soreness and serum indices of muscle damage: A randomized controlled trial. BMC Sports. Sci. Med. Rehabil. 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Wheibe, E.; Dalkin, B.H.; Meltzer, H.C.; Russ-Sellers, R.; Grier, J.T. The Multisystem effects of Long COVID Syndrome and Potential Benefits of Massage Therapy in Long COVID Care. Int. J. Ther. Massage. Bodyw. 2024, 17, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Zwolińska, J.; Gąsior, M. Effects of complex spa therapy in patients with osteoarthritis of the spine receiving treatments in health resorts in south-eastern Poland. Sci. Rep. 2022, 12, 14663. [Google Scholar] [CrossRef]
- Khaltaev, N.; Solimene, U.; Vitale, F.; Zanasi, A. Balneotherapy and hydrotherapy in chronic respiratory disease. J. Thorac. Dis. 2020, 12, 4459–4468. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, H.; Kusaka, Y.; Hirai, T.; Inoue, H.; Agishi, Y.; Schuh, A. Climatotherapy in Japan: A pilot study. Int. J. Biometeorol. 2017, 61, 2141–2143. [Google Scholar] [CrossRef]
- Scurati, R.; Papini, N.; Giussani, P.; Alberti, G.; Tringali, C. The Challenge of Long COVID-19 Management: From Disease Molecular Hallmarks to the Proposal of Exercise as Therapy. Int. J. Mol. Sci. 2022, 23, 12311. [Google Scholar] [CrossRef]
- Ghiotto, L.; Muollo, V.; Tatangelo, T.; Schena, F.; Rossi, A.P. Exercise and physical performance in older adults with sarcopenic obesity: A systematic review. Front. Endocrinol. 2022, 13, 913953. [Google Scholar] [CrossRef]
- Tsekoura, M.; Fousekis, K.; Billis, E.; Dionyssiotis, Y.; Tsepis, E. Cross-cultural adaptation of the Greek version of post-COVID-19 Functional Status Scale: Assessment of non-hospitalised post-COVID-19 survivors. Eur. J. Transl. Myol. 2023, 33, 11328. [Google Scholar] [CrossRef]
- Western, M.J.; Malkowski, O.S. Associations of the Short Physical Performance Battery (SPPB) with Adverse Health Outcomes in Older Adults: A 14-Year Follow-Up from the English Longitudinal Study of Ageing (ELSA). Int. J. Environ. Res. Public. Health 2022, 19, 16319. [Google Scholar] [CrossRef]
- Prossegger, J.; Huber, D.; Grafetstätter, C.; Pichler, C.; Weisböck-Erdheim, R.; Iglseder, B.; Wewerka, G.; Hartl, A. Effects of moderate mountain hiking and balneotherapy on community-dwelling older people: A randomized controlled trial. Exp. Gerontol. 2019, 122, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Román, P.Á.; Rentero-Blanco, M.; Laredo-Aguilera, J.A.; García-Pinillos, F. Effect of a 12-day balneotherapy programme on pain, mood, sleep, and depression in healthy elderly people. Psychogeriatrics 2015, 15, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kardeş, S.; Karagülle, M.; Geçmen, İ.; Adıgüzel, T.; Yücesoy, H.; Karagülle, M.Z. Outpatient balneological treatment of osteoarthritis in older persons: A retrospective study. Z. Gerontol. Geriatr. 2019, 52, 164–171. (In English) [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S. Evaluation of the Role of Balneotherapy in Rehabilitation Medicine. J. Nippon. Med. Sch. 2018, 85, 196–203. [Google Scholar] [CrossRef]
- Wang, P.C.; Song, Q.C.; Chen, C.Y.; Su, T.C. Cardiovascular physiological effects of balneotherapy: Focused on seasonal differences. Hypertens. Res. 2023, 46, 1650–1661. [Google Scholar] [CrossRef]
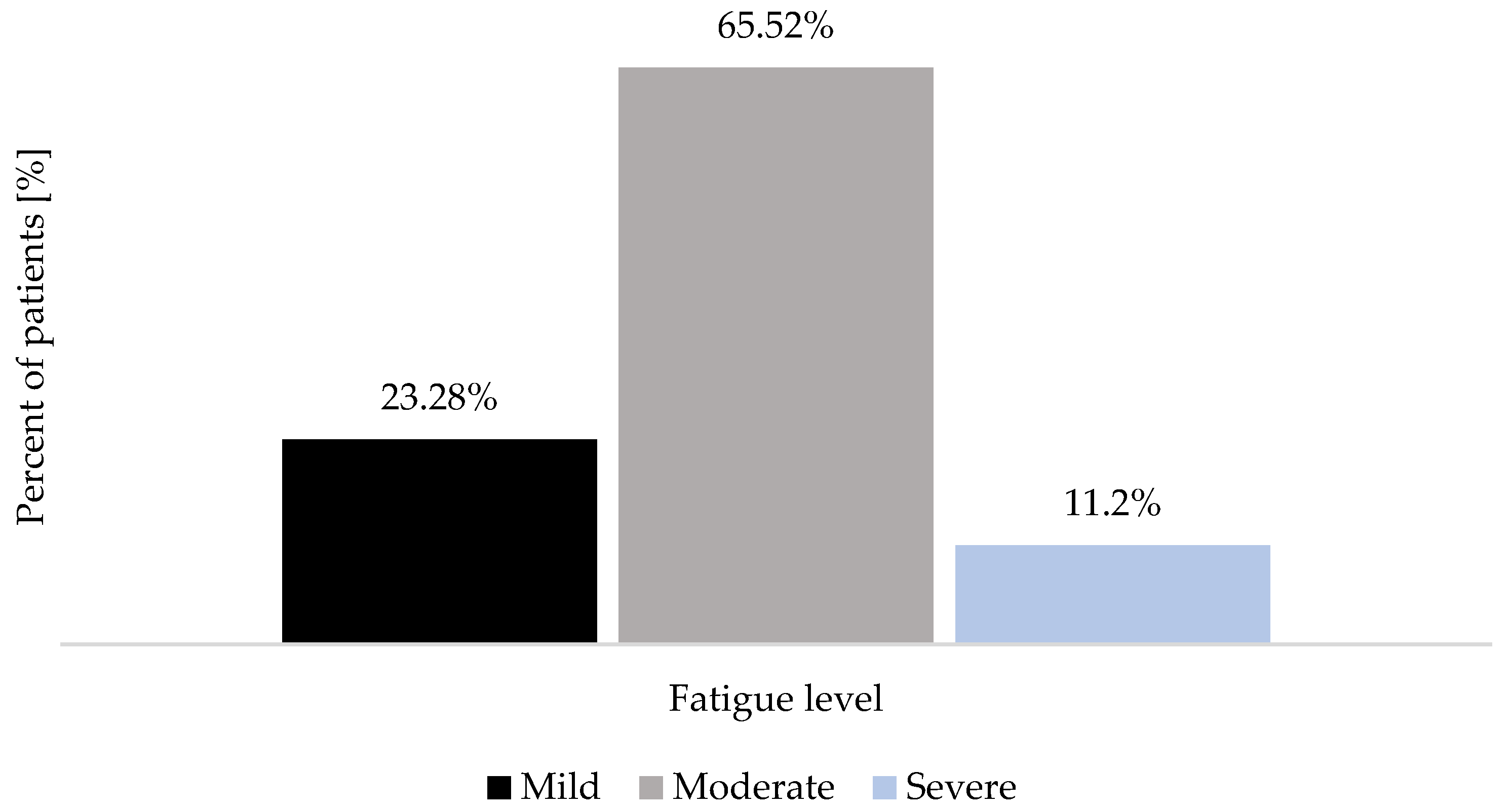
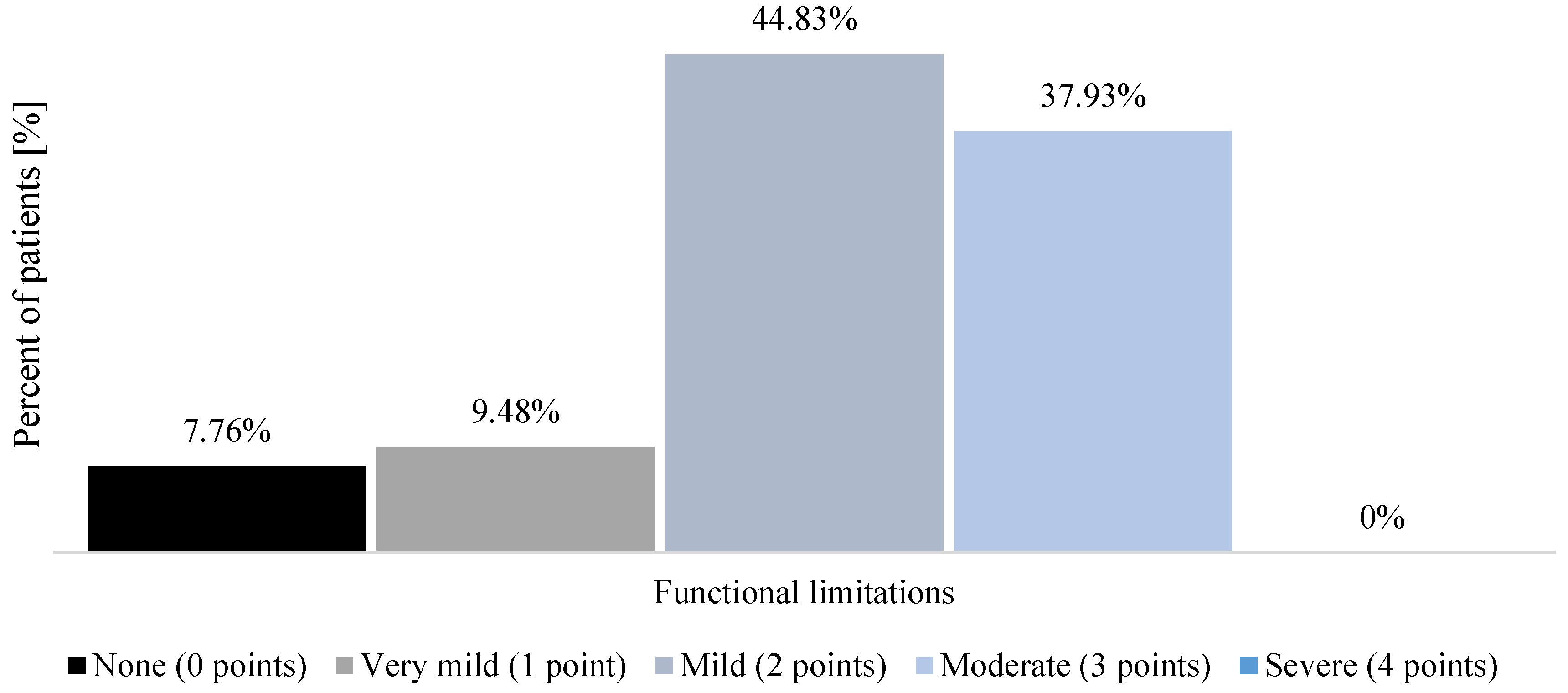
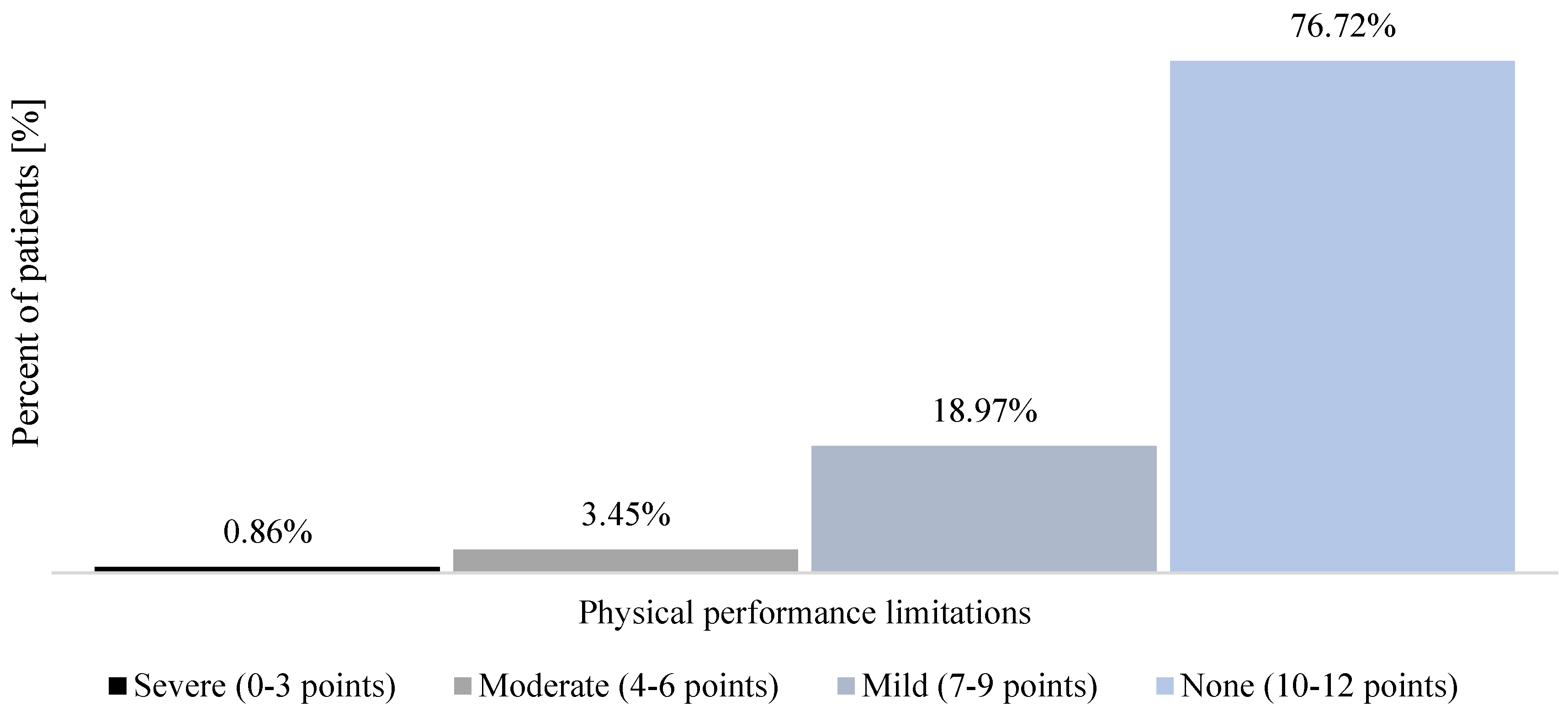
| Women (n = 66) | Men (n = 50) | |||||
|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | |
| Age [years] | 43 | 77 | 64.91 ± 8.63 | 42 | 79 | 63.54 ± 8.84 |
| Body weight [kg] | 55 | 120 | 81.32 ± 15.27 | 70 | 120 | 90.44 ± 12.38 |
| Body height [m] | 1.5 | 1.78 | 1.62 ± 0.06 | 1.61 | 1.95 | 1.75 ± 0.07 |
| BMI [kg/m2] | 21.45 | 44.08 | 30.97 ± 5.46 | 23.99 | 39.79 | 29.7 ± 3.55 |
| Systolic blood pressure [mmHg] | 105 | 160 | 139.41 ± 14.25 | 115 | 165 | 140.44 ± 11.8 |
| Diastolic blood pressure [mmHg] | 55 | 95 | 79.2 ± 8.22 | 66 | 95 | 81.52 ± 6.54 |
| Treatment duration [day] | 19 | 47 | 24.97 ± 6.83 | 17 | 46 | 23.78 ± 5.78 |
| Short Physical Performance Battery (SPPB) [Points] | Pre-Treatment Measurement | Post-Treatment Measurement | p Value 1 | ||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
| Whole group (n = 116) | 3 | 12 | 10.41 ± 1.84 | 6 | 12 | 11.57 ± 0.94 | p < 0.00001 |
| Women (n = 66) | 3 | 12 | 10.02 ± 1.89 | 6 | 12 | 11.35 ± 1.14 | p < 0.00001 |
| Men (n = 50) | 4 | 12 | 10.92 ± 1.65 | 10 | 12 | 11.86 ± 0.45 | p < 0.00001 |
| p value 2 | p < 0.01 | p < 0.01 | |||||
| Post-COVID-19 Functional Status (PCFS) [points] | Pre-Treatment Measurement | Post-Treatment Measurement | p Value 1 | ||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
| Whole group (n = 116) | 0 | 3 | 2.13 ± 0.88 | 0 | 2 | 1.23 ± 0.62 | p < 0.00001 |
| Women (n = 66) | 0 | 3 | 2.26 ± 0.73 | 0 | 2 | 1.26 ± 0.54 | p < 0.00001 |
| Men (n = 50) | 0 | 3 | 1.96 ± 1.03 | 0 | 2 | 1.2 ± 0.73 | p < 0.00001 |
| p value 2 | p > 0.05 | p > 0.05 | |||||
| Fatigue [points] | Pre-Treatment Measurement | Post-Treatment Measurement | p Value 1 | ||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
| Whole group (n = 116) | 0 | 10 | 4.83 ± 2.38 | 0 | 6 | 2.15 ± 1.31 | p < 0.00001 |
| Women (n = 66) | 0 | 9 | 5.12 ± 2.13 | 0 | 6 | 2.24 ± 1.23 | p < 0.00001 |
| Men (n = 50) | 0 | 10 | 4.46 ± 2.63 | 0 | 6 | 2.02 ± 1.42 | p < 0.00001 |
| p value 2 | p > 0.05 | p > 0.05 | |||||
| Δ Short Physical Performance Battery (SPPB) [Points] | |||
| Min | Max | Mean ± SD | |
| Women (n = 66) | −4 | 1 | −1.33 ± 1.42 |
| Men (n = 50) | −8 | 0 | −0.94 ± 1.48 |
| p value | p > 0.05 | ||
| Δ Post-COVID-19 Functional Status (PCFS) [points] | |||
| Min | Max | Mean ± SD | |
| Women (n = 66) | 0 | 2 | 1.00 ± 0.74 |
| Men (n = 50) | 0 | 2 | 0.76 ± 0.75 |
| p value | p > 0.05 | ||
| Δ fatigue [points] | |||
| Min | Max | Mean ± SD | |
| Women (n = 66) | 0 | 6 | 2.88 ± 1.5 |
| Men (n = 50) | 0 | 6 | 2.44 ± 1.7 |
| p value | p > 0.05 | ||
| Short Physical Performance Battery (SPPB) [Points] | Pre-Treatment Measurement | Post-Treatment Measurement | p Value 1 | ||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
| Group I (n = 35) | 4 | 12 | 10.94 ± 1.81 | 10 | 12 | 11.80 ± 0.53 | p < 0.01 |
| Group II (n = 40) | 7 | 12 | 10.53 ± 1.54 | 8 | 12 | 11.55 ± 0.96 | p < 0.0001 |
| Group III (n = 41) | 3 | 12 | 9.83 ± 2.01 | 6 | 12 | 11.39 ± 1.14 | p < 0.0001 |
| p value 2 | p < 0.01 | p > 0.05 | |||||
| Post hoc test | Group I > Group III (p = 0.005) | ||||||
| Post-COVID-19 Functional Status (PCFS) [points] | Pre-Treatment Measurement | Post-Treatment Measurement | p Value 1 | ||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
| Group I (n = 35) | 0 | 3 | 2.17 ± 0.89 | 0 | 2 | 1.26 ± 0.66 | p < 0.00001 |
| Group II (n = 40) | 0 | 3 | 2.15 ± 0.95 | 0 | 2 | 1.25 ± 0.63 | p < 0.00001 |
| Group III (n = 41) | 0 | 3 | 2.07 ± 0.82 | 0 | 2 | 1.2 ± 0.6 | p < 0.00001 |
| p value 2 | p > 0.05 | p > 0.05 | |||||
| Fatigue [points] | Pre-Treatment Measurement | Post-Treatment Measurement | p Value 1 | ||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
| Group I (n = 35) | 0 | 10 | 5.43 ± 2.38 | 0 | 6 | 2.49 ± 1.38 | p < 0.00001 |
| Group II (n = 40) | 0 | 9 | 4.8 ± 2.26 | 0 | 4 | 2.13 ± 1.18 | p < 0.00001 |
| Group III (n = 41) | 0 | 8 | 4.37 ± 2.4 | 0 | 6 | 1.88 ± 1.35 | p < 0.00001 |
| p value 2 | p > 0.05 | p > 0.05 | |||||
| Δ Short Physical Performance Battery (SPPB) [Points] | p Value | Post Hoc Test | |||
| Min | Max | Mean ± SD | |||
| Group I (n = 35) | −8 | 1 | −0.86 ± 1.63 | p < 0.05 | Group I > Group III(p = 0.03) |
| Group II (n = 40) | −4 | 2 | −1.03 ± 1.12 | ||
| Group III (n = 41) | −4 | 1 | −1.56 ± 1.52 | ||
| Δ Post-COVID-19 Functional Status (PCFS) [points] | p value | Post Hoc Test | |||
| Min | Max | Mean ± SD | |||
| Group I (n = 35) | 0 | 2 | 0.91 ± 0.78 | p > 0.05 | - |
| Group II (n = 40) | 0 | 2 | 0.9 ± 0.71 | ||
| Group III (n = 41) | 0 | 2 | 0.88 ± 0.78 | ||
| Δ fatigue [points] | p value | Post Hoc Test | |||
| Min | Max | Mean ± SD | |||
| Group I (n = 35) | 0 | 6 | 2.94 ± 1.57 | p > 0.05 | - |
| Group II (n = 40) | 0 | 6 | 2.67 ± 1.62 | ||
| Group III (n = 41) | 0 | 6 | 2.49 ± 1.61 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onik, G.; Knapik, K.; Dąbrowska-Galas, M.; Sieroń, K. Health Resort Treatment Improves Functioning and Physical Performance in Long COVID Patients: A Retrospective Study. Healthcare 2024, 12, 2344. https://doi.org/10.3390/healthcare12232344
Onik G, Knapik K, Dąbrowska-Galas M, Sieroń K. Health Resort Treatment Improves Functioning and Physical Performance in Long COVID Patients: A Retrospective Study. Healthcare. 2024; 12(23):2344. https://doi.org/10.3390/healthcare12232344
Chicago/Turabian StyleOnik, Grzegorz, Katarzyna Knapik, Magdalena Dąbrowska-Galas, and Karolina Sieroń. 2024. "Health Resort Treatment Improves Functioning and Physical Performance in Long COVID Patients: A Retrospective Study" Healthcare 12, no. 23: 2344. https://doi.org/10.3390/healthcare12232344
APA StyleOnik, G., Knapik, K., Dąbrowska-Galas, M., & Sieroń, K. (2024). Health Resort Treatment Improves Functioning and Physical Performance in Long COVID Patients: A Retrospective Study. Healthcare, 12(23), 2344. https://doi.org/10.3390/healthcare12232344






