Four-Dimensional Flow Echocardiography: Blood Speckle Tracking in Congenital Heart Disease: How to Apply, How to Interpret, What Is Feasible, and What Is Missing Still
Abstract
1. Background
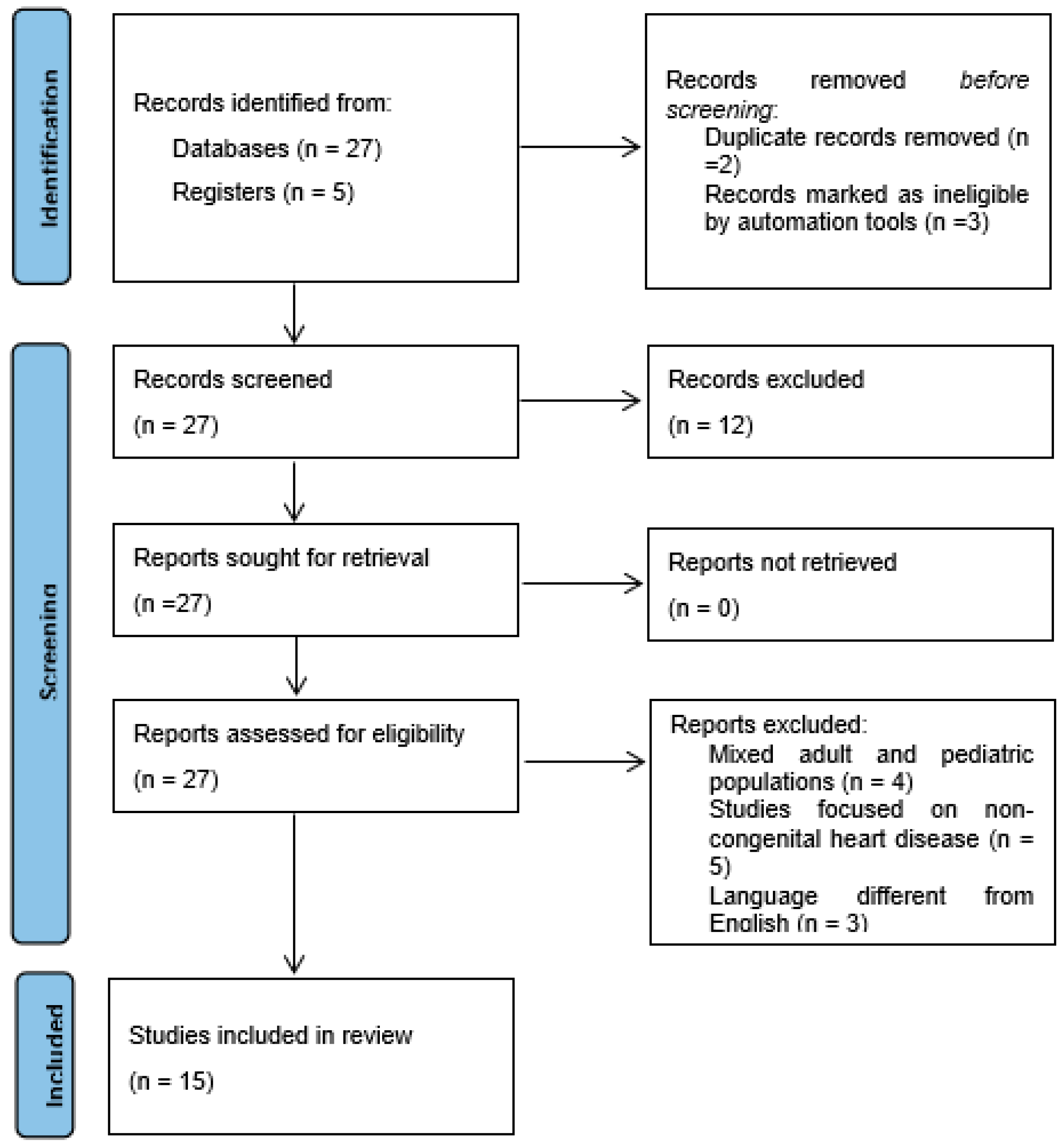
2. Literature Search Criteria
3. Literature Search Results
3.1. Search Results
3.2. Feasibility of BST in Children
3.2.1. BST Imaging Acquisition Technique
3.2.2. Feasibility in Different Conditions
3.2.3. Summary of Current Evidence
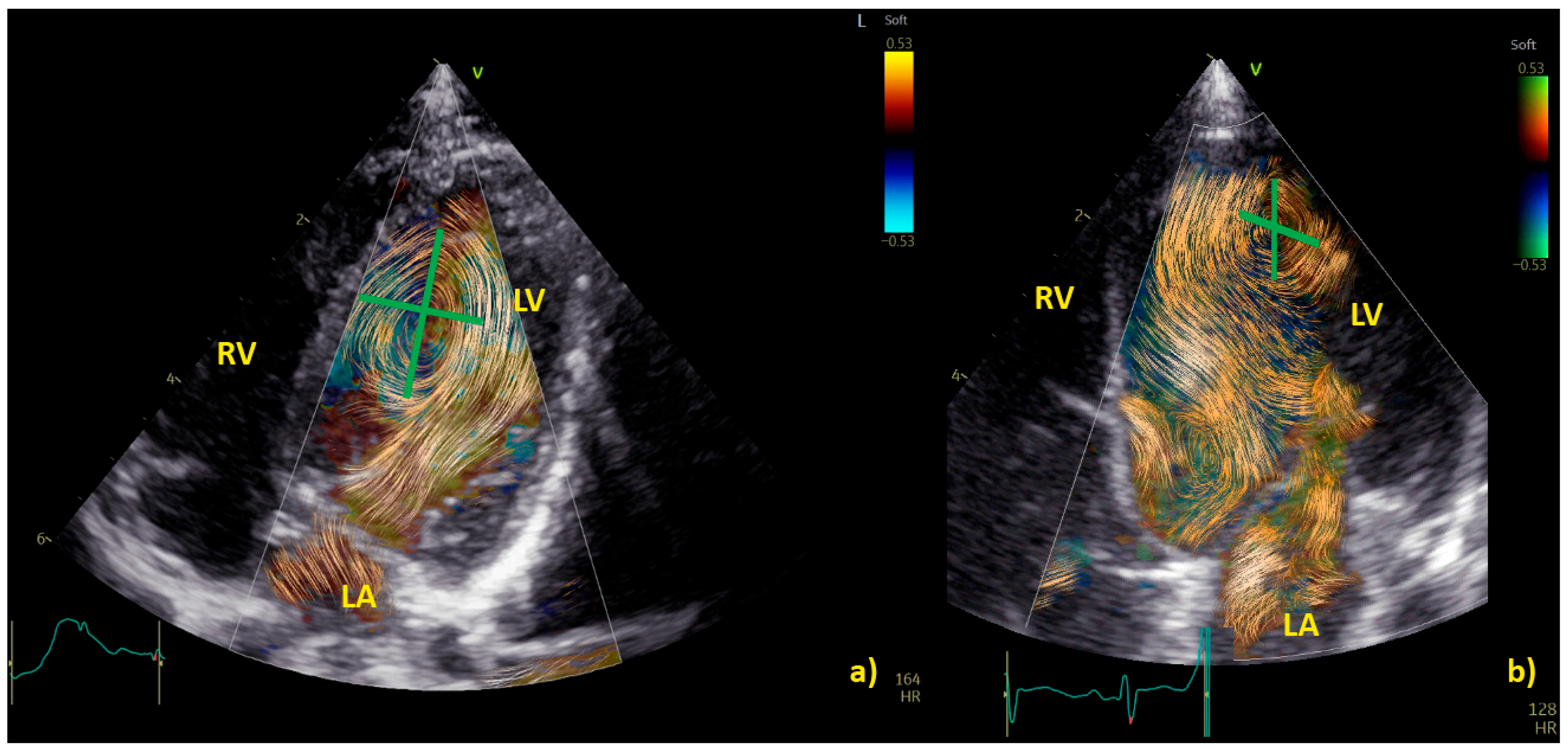
3.3. BST for the Evaluation of Vortex in Ventricular Chambers
3.3.1. Vortex in the Left Ventricle
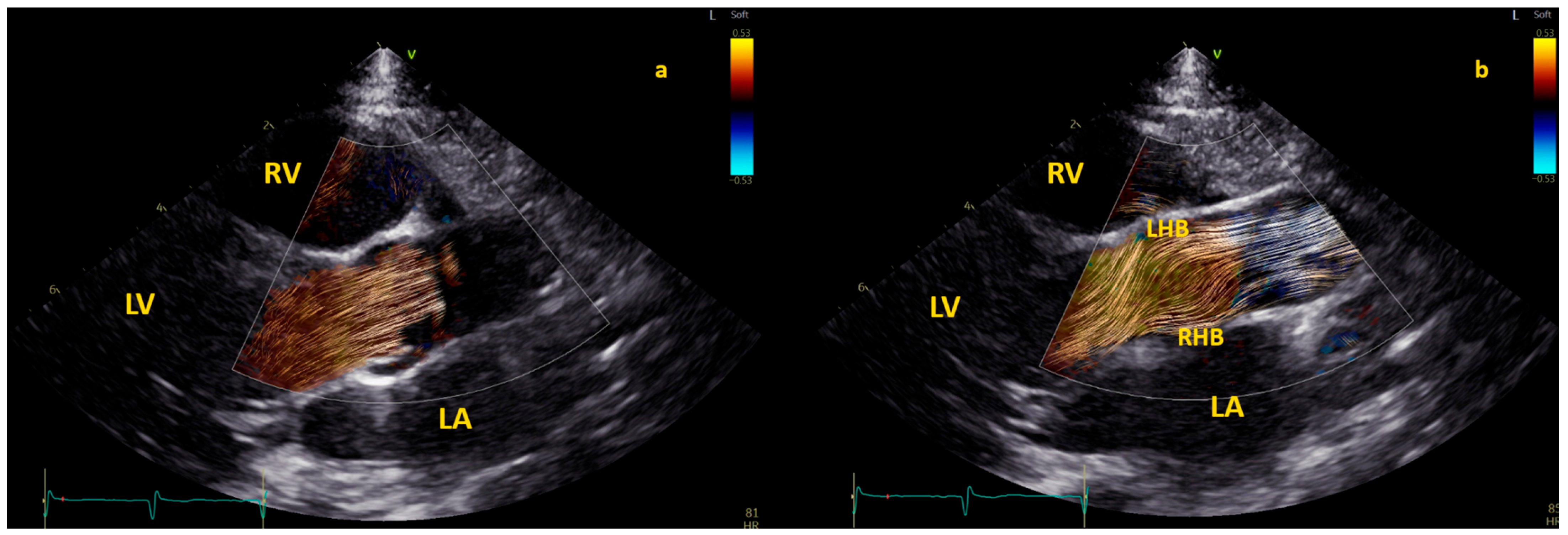
3.3.2. Right Ventricle Flow Dynamics
3.3.3. Summary of the Current Evidence
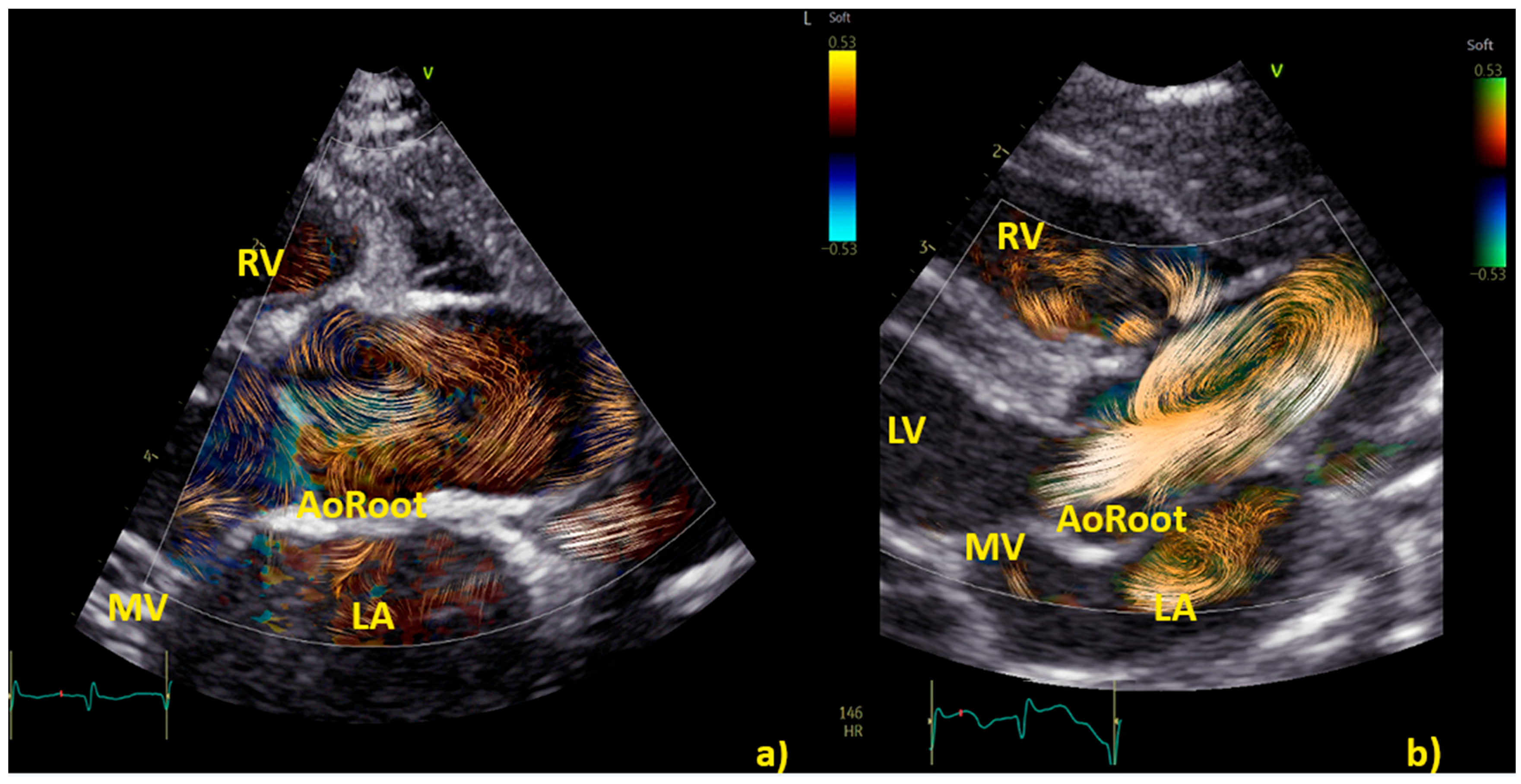
3.4. BST for a Deeper Understanding of Aortic and Pulmonary Flow Dynamics
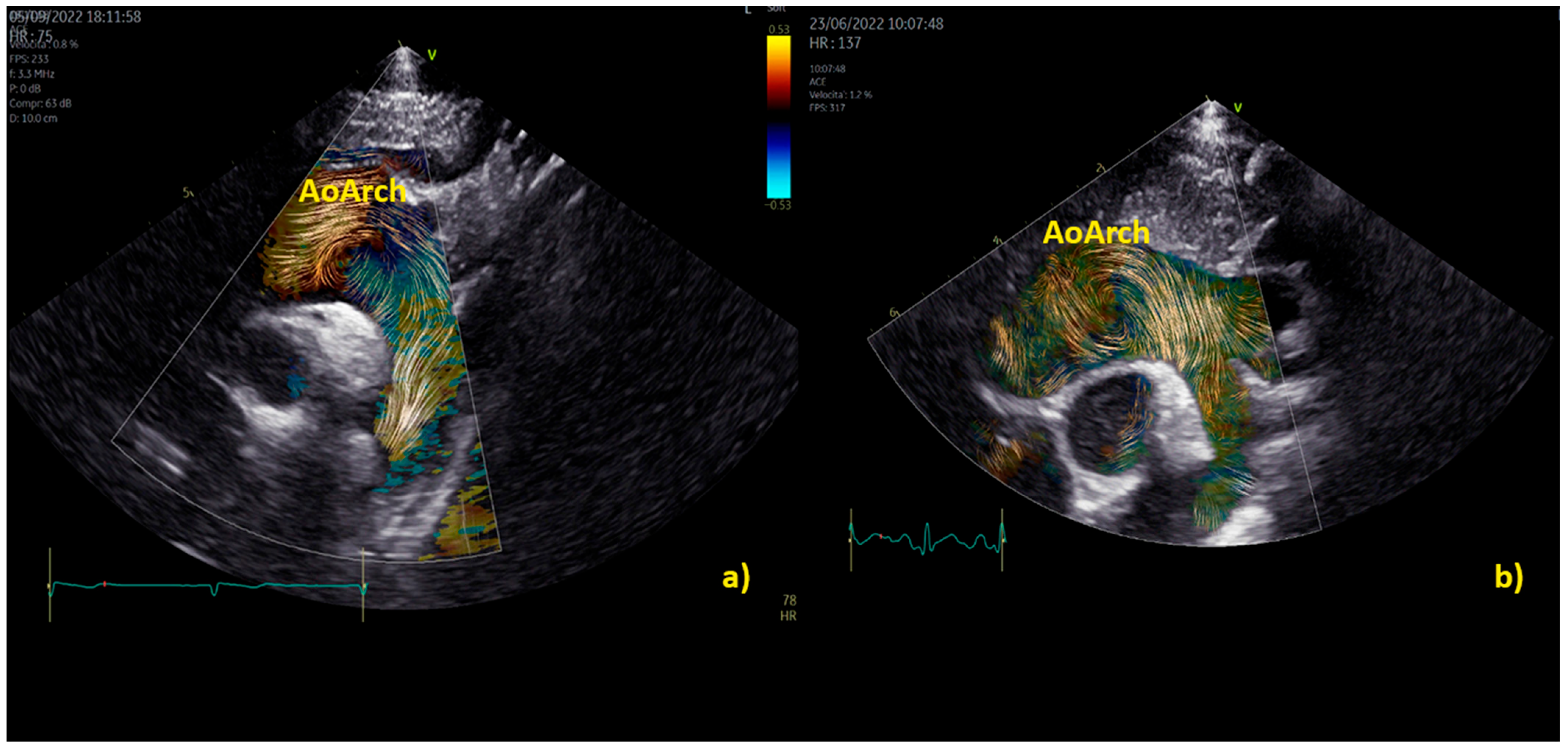
3.4.1. Vortex in the Aorta
3.4.2. Summary of Current Evidence
4. Current Limitations and Future Direction
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rose, M.J.; Rigsby, C.K.; Berhane, H.; Bollache, E.; Jarvis, K.; Barker, A.J.; Schnell, S.; Allen, B.D.; Robinson, J.D.; Markl, M. 4-D flow MRI aortic 3-D hemodynamics and wall shear stress remain stable over short-term follow-up in pediatric and young adult patients with bicuspid aortic valve. Pediatr. Radiol. 2019, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.R.; Pedrizzetti, G.; Tonti, G.; Li, P.; Wei, Z.; Kim, J.K.; Baweja, A.; Liu, S.; Chung, N.; Houle, H.; et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc. Imaging 2008, 1, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Villeman, J.; Baranger, M.K.; Friedberg, C.; Papadacci, A.; Dizeux, A.; Messas, E.; Tanter, M.; Pernot, M.; Mertens, L. Ultrafast Ultrasound Imaging in Pediatric and Adult Cardiology: Techniques, Applications, and Perspectives. JACC Cardiovasc. Imaging 2020, 13, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulos, K.; Budts, W.; Van de Bruaene, A.; Troost, E.; van Melle, J.P. Visualization of the intracavitary blood flow in systemic ventricles of Fontan patients by contrast echocardiography using particle image velocimetry. Cardiovasc. Ultrasound. 2012, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Itatani, K.; Miyaji, K.; Ishii, M. Assessment of the vortex flow in the post-stenotic dilatation above the pulmonary valve stenosis in an infant using echocardiography vector flow mapping. Eur. Heart J. 2014, 35, 306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, C.; Zhu, Y.; Zhang, J.; Niu, C.; Liu, D.; Liao, Y.; Zhu, L.; Peng, Q. Analysis of left ventricular fluid dynamics in dilated cardiomyopathy by echocardiographic particle image velocimetry. Echocardiography 2018, 35, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, S.; Nyrnes, S.A.; Torp, H.; Lovstakken, L. Shunt flow evaluation in congenital heart disease based on two-dimensional speckle tracking. Ultrasound Med. Biol. 2014, 40, 2379–2391. [Google Scholar] [CrossRef]
- Henry, M.; Fadnes, S.; Løvstakken, L.L.; Mawad, W.; Mertens, L.; Nyrnes, S.A. Flow Dynamics in Children with Bicuspid Aortic Valve: A Blood Speckle Tracking Study. Ultrasound Med. Biol. 2023, 10, 2354–2360. [Google Scholar] [CrossRef]
- Sørensen, K.; Fadnes, S.; Mertens, L.; Henry, M.; Segers, P.; Løvstakken, L.; Nyrnes, S.A. Assessment of Early Diastolic Intraventricular Pressure Difference in Children by Blood Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 523–532.e3. [Google Scholar] [CrossRef]
- Mawad, W.; Løvstakken, L.; Fadnes, S.; Grønli, T.; Segers, P.; Mertens, L.; Nyrnes, S.A. Right Ventricular Flow Dynamics in Dilated Right Ventricles: Energy Loss Estimation Based on Blood Speckle Tracking Echocardiography—A Pilot Study in Children. Ultrasound Med. Biol. 2021, 47, 1514–1527. [Google Scholar] [CrossRef]
- Nyrnes, S.A.; Fadnes, S.; Wigen, M.S.; Mertens, L.; Lovstakken, L. Blood Speckle-Tracking Based on High-Frame Rate Ultrasound Imaging in Pediatric Cardiology. J. Am. Soc. Echocardiogr. 2020, 33, 493–503.e5. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.; Nyce, J.; Zhang, J.; Frank, L.H.; Balaras, E.; Vlachos, P.P.; Loke, Y.H. Intracardiac Flow Analysis of the Right Ventricle in Pediatric Patients with Repaired Tetralogy of Fallot Using a Novel Color Doppler Velocity Reconstruction. J. Am. Soc. Echocardiogr. 2023, 36, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Cantinotti, M.; Marchese, P.; Koestenberger, M.; Giordano, R.; Santoro, G.; Assanta, N.; Kutty, S. Intracardiac flow visualization using high-frame rate blood speckle tracking echocardiography: Illustrations from infants with congenital heart disease. Echocardiography 2021, 38, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Daae, A.S.; Wigen, M.S.; Fadnes, S.; Løvstakken, L.; Støylen, A. Intraventricular Vector Flow Imaging with Blood Speckle Tracking in Adults: Feasibility, Normal Physiology and Mechanisms in Healthy Volunteers. Ultrasound Med Biol. 2021, 47, 3501–3513. [Google Scholar] [CrossRef] [PubMed]
- De Waal, K.; Crendal, E.; Boyle, A. Left ventricular vortex formation in preterm infants assessed by blood speckle imaging. Echocardiography 2019, 36, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, N.; Avesani, M.; Sabatino, J.; Ibrahim, A.; Josen, M.; Paredes, J.; Di Salvo, G. Blood speckle imaging: A new echocardiographic approach to study fluid dynamics in congenital heart disease. Int. J. Cardiol. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Xu, R.; Hou, M.; Zhou, D.; Liu, Y.; Xie, L.; Zeng, S. Visualizable intracardiac flow pattern in fetuses with congenital heart defect: Pilot study of blood speckle-tracking echocardiography. Ultrasound Obstet Gynecol. 2023, 62, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Marchese, P.; Cantinotti, M.; Van den Eynde, J.; Assanta, N.; Franchi, E.; Pak, V.; Santoro, G.; Koestenberger, M.; Kutty, S. Left ventricular vortex analysis by high-frame rate blood speckle tracking echocardiography in healthy children and in congenital heart disease. Int. J. Cardiol. Heart Vasc. 2021, 37, 100897. [Google Scholar] [CrossRef]
- Cantinotti, M.; Marchese, P.; Scalese, M.; Giordano, R.; Franchi, E.; Assanta, N.; Koestenberger, M.; Barnes, B.T.; Celi, S.; Jani, V.; et al. Characterization of Aortic Flow Patterns by High-Frame-Rate Blood Speckle Tracking Echocardiography in Children. J. Am. Heart Assoc. 2023, 12, e026335. [Google Scholar] [CrossRef]
- Schäfer, M.; Mawad, W. Advanced Imaging Technologies for Assessing Tetralogy of Fallot: Insights into Flow Dynamics. CJC Pediatr. Congenit. Heart Dis. 2023, 2, 380–392. [Google Scholar] [CrossRef]
- Mawad, W.; Fadnes, S.; Løvstakken, L.; Henry, M.; Mertens, L.; Nyrnes, S.A. Pulmonary Hypertension in Children is Associated with Abnormal Flow Patterns in the Main Pulmonary Artery as Demonstrated by Blood Speckle Tracking. CJC Pediatr. Congenit. Heart Dis. 2022, 1, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ghraib, M.; Kremers, D.; Koochesfahani, M.; Kemp, M. Leonardo’s vision of flow visualization. Exp. Fluids 2002, 33, 219–223. [Google Scholar] [CrossRef]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; GutiΩrrez, L.; et al. Aortic flow patterns and wall shear stress maps by 4D-flow cardiovascular magnetic resonance in the assessment of aortic dilatation in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reson. 2018, 20, 28. [Google Scholar] [CrossRef]
- Bissell, M.M.; Loudon, M.; Hess, A.T.; Stoll, V.; Orchard, E.; Neubauer, S.; Myerson, S.G. Differential flow improvements after valve replacements in bicuspid aortic valve disease: A cardiovascular magnetic resonance assessment. J. Cardiovasc. Magn. Reson. 2018, 20, 10. [Google Scholar] [CrossRef]
- Lenz, A.; Petersen, J.; Riedel, C.; Weinrich, J.M.; Kooijman, H.; Schoennagel, B.P.; Adam, G.; von Kodolitsch, Y.; Reichenspurner, H.; Girdauskas, E.; et al. 4D flow cardiovascular magnetic resonance for monitoring of aortic valve repair in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reson. 2020, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Daeichin, V.; Bera, D.; Raghunathan, S.; Shabani Motlagh, M.; Chen, Z.; Chen, C.; Noothout, E.; Vos, H.J.; Pertijs, M.; Bosch, J.G.; et al. Acoustic characterization of a miniature matrix transducer for pediatric 3D transesophageal echocardiography. Ultrasound Med. Biol. 2018, 44, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.; Bock, J.; Barker, A.J.; von Knobelsdorff-Brenkenhoff, F.; Wallis, W.; Korvink, J.G.; Bissell, M.M.; Schulz-Menger, J.; Markl, M. 4D flow magnetic resonance imaging in bicuspid aortic valve disease demonstrates altered distribution of aortic blood flow helicity. Magn. Reson. Med. 2014, 71, 1542–1553. [Google Scholar] [CrossRef]
- Allen, B.D.; van Ooij, P.; Barker, A.J.; Carr, M.; Gabbour, M.; Schnell, S.; Jarvis, K.B.; Carr, J.C.; Markl, M.; Rigsby, C.; et al. Thoracic aorta 3D hemodynamics in pediatric and young adult patients with bicuspid aortic valve. J. Magn. Reson. Imaging 2015, 42, 954–963. [Google Scholar] [CrossRef]
- Cantinotti, M.; Kutty, S.; Franchi, E.; Paterni, M.; Scalese, M.; Iervasi, G.; Koestenberger, M. Pediatric echocardiographic nomograms: What has been done and what still needs to be done. Trends Cardiovasc. Med. 2017, 27, 336–349. [Google Scholar] [CrossRef]
- Cantinotti, M.; Scalese, M.; Giordano, R.; Assanta, N.; Marchese, P.; Franchi, E.; Viacava, C.; Koestenberger, M.; Jani, V.; Kutty, S. A statistical comparison of reproducibility in current pediatric two-dimensional echocardiographic nomograms. Pediatr. Res. 2021, 89, 579–590. [Google Scholar] [CrossRef]
- Chubb, H.; Simpson, J.M. The use of Z-scores in paediatric cardiology. Ann. Pediatr. Cardiol. 2012, 5, 179–184. [Google Scholar] [PubMed]
- Sade, L.E.; Joshi, S.S.; Cameli, M.; Cosyns, B.; Delgado, V.; Donal, E.; Edvardsen, T.; Carvalho, R.F.; Manka, R.; Podlesnikar, T.; et al. Current clinical use of speckle-tracking strain imaging: Insights from a worldwide survey from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]

| Author | Population | Analysis | Software |
|---|---|---|---|
| Nyrnes et al. 2020 [11], Norwegian University and St. Olavs University Hospital Trondheim, Norway | 102 subjects (age 21 weeks to 11.5 years) | Feasibility of BST in CHD and reference velocities | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) Research Software |
| Cantinotti et al. 2021 [13], Massa, Italy | 20 neonates and children with CHD | Feasibility of BST in CHD | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) |
| Borrelli et al. 2021 [16], London (UK) and Padua (Italy) | 7 CHD 3 healthy controls | Feasibility of BST in CHD | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) |
| Marchese et al. 2021 [18], Massa, Italy | 193 healthy children (median age 6.33 years, IQR 2.9–10.2 years) 60 CHD (median age 1.28 years, IQR 0.2–6.82 years) | LV vortex characteristics | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) |
| Mawad W, 2021 [17], Toronto (Canada) and Trondheim (Norway) | 57 TF post-repair with severe PR (age 41, 21–74 months) 11 ASD (age 66, 33–99 months) 25 healthy controls (age 42, 26–76 months) | Energy loss in the RV | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) Research Software |
| Sorensen et al. 2023 [14], Helse Møre and Romsdal (norway) and Toronto (Canada) | 138 healthy controls (2 days–17.36 years 10 DCM (0.50–14.92 years), 21 HCM (1.25–17.50 years) | IVPD | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) Research Software |
| Cantinotti et al. 2023 [19], Massa, Italy | 82 healthy children (Age 8.2, 5.6–11.0 years) | Aortic flow patterns | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) |
| Henry M et al. 2023 [8], Toronto (Canada) and Trondheim (Norway) | 38 healthy children (age 1.91, 0.11–5.6 years) 14 BAV (age 4.72, 1.08–7.7 years) | vorticity, vector complexity, energy loss in the Ao and RV | Vivid E-9 system (GE Vingmed Ultrasound, Horten, Norway) Research Software |
| Healthy (n = 118) | Healthy Age-Matched (n = 48) | CHD (n = 43) | P° | |
|---|---|---|---|---|
| Age, years | 6.84 (2.94–10.5) | 1.53 (0.37–6.84) | 0.99 (0.10–6.82) | 0.473 |
| Height, cm | 121 (89.2–142) | 80.0 (62.5–114) | 75.0 (58.5–116) | 0.613 |
| Weight, kg | 25.0 (14.3–37.2) | 11.0 (6.55–24.0) | 7.95 (4.22–19.0) | 0.327 |
| Distance to apex, mm | 21.2 (16.0–28.0) | 17.9 (13.7–21.0) | 16.5 (12.1–23.9) | 0.766 |
| Distance to apex/distance from apex to mitral valve, % | 39 (32–49) | 38 (30–51) | 39 (30–48) | 0.742 |
| Distance to IVS, mm | 11.0 (8.42–13.5) | 9.41(6.49–13.4) | 8.70 (6.10–13.3) | 0.526 |
| Distance to IVS/distance from IVS to LV free wall, % | 31 (25–41) | 37 (27–45) | 34 (25–40) | 0.309 |
| Height/BSA, mm/m2 | 10.7 (7.95–15.4) | 15.3 (10.3–20.5) | 18.6 (11.4–28.3) | 0.142 |
| Height/LVEDA, mm/cm2 | 0.75 (0.54–1.31) | 0.74 (0.50–1.03) | 0.51 (0.40–0.73) | 0.429 |
| Width/BSA, mm/m2 | 9.18 (6.86–11.9) | 12.4 (9.25–15.6) | 16.7 (10.2–22.5) | 0.051 |
| Width/LVEDA, mm/cm2 | 0.66 (0.48–0.98) | 0.57 (0.44–0.81) | 0.43 (0.34–0.57) | 0.235 |
| Sphericity Index | 1.20 (1.05–1.39 | 1.24 (1.06–1.50) | 1.18 (1.00–1.33) | 0.437 |
| Area/BSA, cm2/m2 | 0.67 (0.51–0.95) | 0.82 (0.63–1.08) | 1.01 (0.75–1.64) | 0.096 |
| Area/LVEDA, cm2/cm2 | 0.03 (0.02–0.05) | 0.04 (0.03–0.06) | 0.04 (0.03–0.08) | 0.349 |
| Diastole | Systole | |
|---|---|---|
| Controls (n = 25) Age: 42 (26–76) mo Weight:15.8 (12.3–23.6) kg | 1.34 (0.55–2.06) (mJ/m) Site: TV | 0.17 (0.10–0.48) (mJ/m) Site: RVOT |
| ASD (n = 11) Age: 66 (33–99) mo Weight: 17.9 (10.5–27.3) kg | 2.86 (1.47–3.65) * (mJ/m) Site: TV | 0.44 (0.29–0.68) (mJ/m) Site: RVOT |
| r-TOF (n = 21) Age: 41 (21–74) mo Weight: 12.2 (10.0–19.5) kg | 1.93 (1.46–2.74) * (mJ/m) Site: TV, Apical (where TR and PR colloid) | 0.29 (0.07–0.51) (mJ/m) Site: RVOT |
| Control (n = 24) | Bicuspid Aortic Valve (n = 14) | p | |
|---|---|---|---|
| Age (years) | 1.91 (0.11–5.6) | 4.72 (1.08–7.7) | 0.08 |
| Weight (kg) | 12.3 (4.0–22.4) | 16.85 (7.9–23.9) | 0.19 |
| Aortic regurgitation | 1 moderate, 5 mild 8 no or trivial | ||
| Peak velocity (m/s) | 0.97 (0.8–1.2) | 1.9 (1.4–2.5) | <0.001 |
| Peak diastolic kinetic energy (J/m) | 0.08 ± 0.05 | 0.18 ± 0.08 | <0.001 |
| Energy loss (mW/m) | 6.8 ± 4.7 | 28.7 ± 15.11 | <0.001 |
| Vorticity (Hz) | 22.7 ± 7.8 | 39.8 ± 11.6 | <0.001 |
| Vector complexity | 0.57 ± 0.18 | 0.70 ± 0.12 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantinotti, M.; Marchese, P.; Franchi, E.; Santoro, G.; Assanta, N.; Giordano, R. Four-Dimensional Flow Echocardiography: Blood Speckle Tracking in Congenital Heart Disease: How to Apply, How to Interpret, What Is Feasible, and What Is Missing Still. Healthcare 2024, 12, 263. https://doi.org/10.3390/healthcare12020263
Cantinotti M, Marchese P, Franchi E, Santoro G, Assanta N, Giordano R. Four-Dimensional Flow Echocardiography: Blood Speckle Tracking in Congenital Heart Disease: How to Apply, How to Interpret, What Is Feasible, and What Is Missing Still. Healthcare. 2024; 12(2):263. https://doi.org/10.3390/healthcare12020263
Chicago/Turabian StyleCantinotti, Massimiliano, Pietro Marchese, Eliana Franchi, Giuseppe Santoro, Nadia Assanta, and Raffaele Giordano. 2024. "Four-Dimensional Flow Echocardiography: Blood Speckle Tracking in Congenital Heart Disease: How to Apply, How to Interpret, What Is Feasible, and What Is Missing Still" Healthcare 12, no. 2: 263. https://doi.org/10.3390/healthcare12020263
APA StyleCantinotti, M., Marchese, P., Franchi, E., Santoro, G., Assanta, N., & Giordano, R. (2024). Four-Dimensional Flow Echocardiography: Blood Speckle Tracking in Congenital Heart Disease: How to Apply, How to Interpret, What Is Feasible, and What Is Missing Still. Healthcare, 12(2), 263. https://doi.org/10.3390/healthcare12020263










