Development and Validation of an Auricular Acupuncture Protocol for the Management of Chemotherapy-Induced Nausea and Vomiting in Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
3. Development of the AA Protocol for the Management of CINV
3.1. Planning the Development Process and Reviewing the Published Scientific Evidence
3.2. Drawing upon Existing Theories
3.3. Composing a Team and Establishing Decision-Making Processes
4. Clinical Validation of the AA Protocol for the Management of CINV
4.1. Involving Stakeholders, including Those Who Will Deliver, Use, and Benefit from the Intervention and Carry out Primary Data Collection
4.1.1. Study Design
4.1.2. Sample and Eligibility Criteria
4.2. Recruitment
4.3. Instruments
4.4. Intervention
4.5. Data Collection Procedures
4.6. Data Analysis
4.7. Ethical Considerations
5. Results
5.1. Development of the AA Protocol for the Management of CINV
5.2. Clinical Validation of the AA Protocol for the Management of CINV
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, P.; Robinson, P.D.; Wahib, N.; Cheung, P.; Wong, T.; Cabral, S.; Parker, A.; Cohen, M.; Devine, K.; Gibson, P.; et al. Interventions for the prevention of acute phase chemotherapy-induced nausea and vomiting in adult and pediatric patients: A systematic review and meta-analysis. Support. Care Cancer 2022, 30, 8855–8869. [Google Scholar] [CrossRef] [PubMed]
- Simino, G.P.R.; Reis, I.A.; Acurcio, F.D.A.; Andrade, E.I.G.; Brazil, N.M.L.; Cherchiglia, M.L. Risk factors associated with antineoplastic chemotherapy-induced nausea and vomiting. Rev. Saude Publica 2020, 54, 106–119. [Google Scholar] [CrossRef]
- Qadire, A.M. Chemotherapy-Induced Nausea and Vomiting: Incidence and Management in Jordan. Clin. Nurs. Res. 2018, 27, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.J.; Kwon, K.; Naehrig, D.; Asher, R.; Lacey, J. Characteristics and Symptom Burden of Patients Accessing Acupuncture Services at a Cancer Hospital. Integr. Cancer Ther. 2021, 20, 15347354211002253. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M.; Aapro, M. Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 374, 1356–1367. [Google Scholar] [CrossRef]
- Kong, C.; Han, M.; Zhang, C.; Zhao, Z.; Zhao, Z.; Fang, F.; Zhang, Z.; Huang, F.; Luan, X.; Li, B. Auricular point acupressure improved nausea, vomiting, diarrhea and nutritional status in gastric cancer patients receiving oral S-1 therapy. Int. J. Clin. Exp. Med. 2018, 11, 9200–9209. [Google Scholar]
- Cope, D.G. Clinical Updates in Nausea and Vomiting. Semin. Oncol. Nurs. 2022, 38, 151249. [Google Scholar] [CrossRef]
- Kong, F.; Wang, Z.; Wang, N.; Zhao, L.; Mei, Q.; Yu, Y.; Zhang, D.; Li, X.; Jia, Y. The Clinical Observation of Acupuncture Combined With Antiemetic Drugs in the Prevention and Treatment of CINV in Breast Cancer Patients. Front. Oncol. 2022, 12, 888651–888663. [Google Scholar] [CrossRef]
- Lam, C.S.; Zhou, K.; Loong, H.H.; Chung, V.C.; Ngan, C.K.; Cheung, Y.T. The Use of Traditional, Complementary, and Integrative Medicine in Cancer: Data-Mining Study of 1 Million Web-Based Posts From Health Forums and Social Media Platforms. J. Med. Internet Res. 2023, 25, e45408. [Google Scholar] [CrossRef]
- Höxtermann, M.D.; Haller, H.; Aboudamaah, S.; Bachemir, A.; Dobos, G.; Cramer, H.; Voiss, P. Safety of acupuncture in oncology: A systematic review and meta-analysis of randomized controlled trials. Cancer 2022, 128, 2159–2173. [Google Scholar] [CrossRef]
- Morais, S.F.M.; Turrini, R.N.T. Evaluation of acupuncture and auriculotherapy in the control of chemotherapy-induced nausea and vomiting: A Pilot Study. Rev. Esc. Enferm. USP 2023, 57, e20230191. [Google Scholar] [CrossRef] [PubMed]
- Quah-Smith, I.; Litscher, G.; Rong, P.; Oleson, T.; Stanton, G.; Pock, A.; Niemtzow, R.; Aung, S.; Nogier, R. Report from the 9th International Symposium on Auriculotherapy Held in Singapore, 10–12 August 2017. Medicines 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Vallim, E.T.A.; Marques, A.d.C.B.; Coelho, R.d.C.F.P.; Guimarães, P.R.B.; Felix, J.V.C.; Kalinke, L.P. Auricular acupressure in the quality of life of women with breast cancer: A randomized clinical trial. Rev. Esc. Enferm. USP 2019, 53, e03525. [Google Scholar] [CrossRef]
- Hou, P.W.; Hsu, H.C.; Lin, Y.W.; Tang, N.Y.; Cheng, C.Y.; Hsieh, C.L. The History, Mechanism, and Clinical Application of Auricular Therapy in Traditional Chinese Medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 495684. [Google Scholar] [CrossRef]
- Mercante, B.; Ginatempo, F.; Manca, A.; Melis, F.; Enrico, P.; Deriu, F. Anatomo-Physiologic Basis for Auricular Stimulation. Med. Acupunct. 2018, 30, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Y.; Mo, C.Y.; Zhao, Q. Research hotspots and trends on acupuncture therapy on vomiting from 1990 to 2022: A bibliometric analysis. Complement. Ther. Med. 2023, 76, 102962. [Google Scholar] [CrossRef] [PubMed]
- Paiva, E.M.C.; Zhu, S.; Chi, Y.; Oliveira, R.A.; Moura, C.C.; Garcia, A.C.M. Auriculotherapy to manage chemotherapy-induced nausea and vomiting in patients with cancer: A systematic review. Progress. Palliat. Care 2023, 31, 100–110. [Google Scholar] [CrossRef]
- Jin, M.; Xie, L.; Mao, N.; Wei, J.; Chen, J.; Chen, X.; Mao, H. The characteristics of registered acupuncture clinical trials enrolling cancer patients. Support. Care Cancer 2022, 30, 10461–10470. [Google Scholar] [CrossRef]
- O’Cathain, A.; Croot, L.; Duncan, E.; Rousseau, N.; Sworn, K.; Turner, K.M.; Yardley, L.; Hoddinott, P. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019, 9, 1. [Google Scholar] [CrossRef]
- MacPherson, H.; Altman, D.G.; Hammerschlag, R.; Youping, L.; Taixiang, W.; White, A.; Moher, D.; STRICTA Revision Group. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT statement. PLoS Med. 2010, 7, e1000261. [Google Scholar] [CrossRef]
- Auricular Acupuncture Point (WFAS STANDARD-002: 2012). World J. Acupunct. Moxibustion. 2013, 23, 12–21. [CrossRef]
- Vieira, T.W.; Sakamoto, V.T.M.; Moraes, L.C.; Blatt, C.R.; Caregnato, R.C.A. Validation methods of nursing protocols: An integrative review. Rev. Bras. Enferm. 2020, 73, e20200050. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, H.C.Q.C.P.; Pena, S.B.; Lopes, J.L.; Lopes, C.T.; Bottura Leite de Barros, A.L. Experts for Validation Studies in Nursing: New Proposal and Selection Criteria. Int. J. Nurs. Knowl. 2016, 27, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Rubio, D.M.; Berg-Weger, M.; Tebb, S.S.; Lee, E.S.; Rauch, S. Objectifying Content Validity: Conducting a Content Validity Study in Social Work Research. Soc. Work. Res. 2003, 27, 94–105. Available online: https://rameliaz.github.io/files/kuliah/matrikulasi-mapsi/rubio.pdf (accessed on 10 November 2023). [CrossRef]
- Revorêdo, L.S.; Maia, R.S.; Torres, G.V.; Maia, E.M.C. The Use of Delphi’s Technique in Health: An Integrative Review of Brazilian Studies. Arq. Ciênc. Saúde 2015, 22, 16–21. Available online: https://repositorio-racs.famerp.br/racs_ol/vol-22-2/O%20uso%20da%20t%C3%A9cnica%20delphi%20em%20sa%C3%BAde%20uma%20revis%C3%A3o%20integrativa%20de%20estudos%20brasileiros.pdf (accessed on 10 November 2023). [CrossRef]
- Fehring, R.J. The Fehring model. In Classification of Nursing Diagnosis: Proceedings of the Tenth Conference; Carroll-Johnson, R.M., Paquette, M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994; pp. 55–57. [Google Scholar]
- EASTERN Cooperative Oncology Group ECOG. Performance Status Scale ECOG. 2016. Available online: https://ecog-acrin.org/resources/ecog-performance-status (accessed on 10 November 2023).
- Kelly, C.M.; Shahrokni, A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J. Oncol. 2016, 2016, 6186543. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, D.; Gao, J.; Tian, S.F. Effect of auricular acupressure combined with ondansetron for chemotherapy induced nausea and vomiting in patients with acute leukemia: Analysis of 100 cases. Int. Med. Health Guid. News 2012, 18, 2357–2359. [Google Scholar]
- Zhong, M.J.; Hu, Z.W.; Huang, L. Auricular acupressure reduces chemotherapy-induced gastrointestinal toxicities: Findings from a clinical study. Chin. J. Inf. TCM 2013, 19, 67–68. [Google Scholar]
- Nassif, M.S.; Iunes, D.H.; Sousa, L.; Costa, I.C.P.; Oliveira, P.E.; Moura, C.C.; Menezes, F.S.; Mantuani, A.P.A.; Chaves, E.C.L. Validation of a laser auriculotherapy protocol for chronic spinal pain. REME Rev. Min. Enferm. 2020, 24, 1350. [Google Scholar] [CrossRef]
- Wood, J.M.; Chapman, K.; Eilers, J. Tools for assessing nausea, vomiting, and retching: A literature review. Cancer Nurs. 2011, 34, 14–24. [Google Scholar] [CrossRef]
- Isidoro, G.M.; Ferreira, A.C.G.; Paiva, E.M.C.; Amaral, J.D.H.F.; Meireles, E.C.A.; Garcia, A.C.M. Escala para Avaliação de Náuseas e Vômitos Relacionados à Quimioterapia: Tradução e Adaptação Transcultural. Rev. Bras. Cancerol. 2022, 68, e-101423. [Google Scholar] [CrossRef]
- Morrow, G.R. Methodology in behavioral and psychosocial cancer research. The assessment of nausea and vomiting. Past problems, current issues and suggestions for future research. Cancer 1984, 53, 2267–2280. [Google Scholar] [CrossRef]
- Morrow, G.R. A Patient Report Measure for the Quantification of Chemotherapy Induced Nausea and Emesis: Psychometric Properties of the Morrow Assessment of Nausea and Emesis (MANE). Br. J. Cancer Suppl. 1992, 19, 72–74. Available online: https://pubmed.ncbi.nlm.nih.gov/1467207/ (accessed on 10 November 2023).
- Zhao, B.; Meng, X.; Sun, J. An Analysis of the Development of Auricular Acupuncture in China in the Past 10 Years. Med. Acupunct. 2018, 30, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, M.; Yekaninejad, M.S.; Varaei, S.; Jalalinia, S.F.; Samimi, M.A.; Sa’atchi, K. The effect of auricular acupressure on nausea and vomiting caused by chemotherapy among breast cancer patients. Complement. Ther. Clin. Pract. 2016, 24, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.; Dai, J.; Xu, Y.; Zhang, R.; Jiang, H.; Yan, X.; Yang, K. Risk of bias tool in systematic reviews/meta-analyses of acupuncture in Chinese journals. PLoS ONE 2011, 6, e28130. [Google Scholar] [CrossRef] [PubMed]
- Bi, H. Observation on the effect of auricular point sticking for vomiting induced by chemotherapy. J. Acupunct. Tuina Sci. 2011, 9, 367–369. [Google Scholar] [CrossRef]
- Nielsen, A.; Gereau, S.; Tick, H. Risks and Safety of Extended Auricular Therapy: A Review of Reviews and Case Reports of Adverse Events. Pain. Med. 2020, 21, 1276–1293. [Google Scholar] [CrossRef]
- Graham, R.; Mancher, M.; Wolman, M.D.; Greenfield, S.; Steinberg, E. (Eds.) Clinical Practice Guidelines We Can Trust; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef]
- Rauh, S.; Arnold, D.; Braga, S.; Curca, R.; Eckert, R.; Fröbe, A.; Karamouzis, M.; Lakatos, G.; Molitor, J.L. Challenge of implementing clinical practice guidelines. Getting ESMO’s guidelines even closer to the bedside: Introducing the ESMO Practising Oncologists’ checklists and knowledge and practice questions. ESMO Open 2018, 3, e000385. [Google Scholar] [CrossRef]
- Staebler, F. The Role of Acupuncture in the Treatment of Breast Cancer. Eur. J. Orient. Med. 2011, 6, 6–21. [Google Scholar]
- Nair, R.; Aggarwal, R.; Khanna, D. Methods of Formal Consensus in Classification/Diagnostic Criteria and Guideline Development. Semin. Arthritis Rheum. 2011, 41, 95–195. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chien, L.C.; Chiang, Y.C.; Lin, S.W.; Huang, C.K.; Ren, D. Reduction in nausea and vomiting in children undergoing cancer chemotherapy by either appropriate or sham auricular acupuncture points with standard care. J. Altern. Complement. Med. 2012, 18, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, K.; Lanni, T., Jr.; Anderson, M.M.; Patricolo, G.E. Integrative medicine and the oncology patient: Options and benefits. Support. Care Cancer 2018, 26, 2267–2273. [Google Scholar] [CrossRef]
- Contim, C.L.V.; Santo, F.H.E.; Moretto, I.G. Applicability of auriculotherapy in cancer patients: An integrative literature review. Rev. Esc. Enferm. USP 2020, 54, e03609. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.W.; Yu, M.W.; Wang, X.M.; Yang, G.W.; Wang, H.; Zhang, C.X.; Xue, N.; Xu, W.R.; Zhang, Y.; Cheng, P.Y.; et al. Efficacy of acupuncture in the prevention and treatment of chemotherapy-induced nausea and vomiting in patients with advanced cancer: A multi-center, single-blind, randomized, sham-controlled clinical research. Chin. Med. 2020, 15, 57–68. [Google Scholar] [CrossRef]
- Varejão, C.D.S.; Santo, F.H.D.E. Laser Acupuncture for Relieving Nausea and Vomiting in Pediatric Patients Undergoing Chemotherapy: A Single-Blind Randomized Clinical Trial. J. Pediatr. Oncol. Nurs. 2019, 36, 44–54. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Ji, F.Q.; Sun, L.; Jin, L.H. Effect of auricular acupuncture for chemotherapy induced nausea and vomiting in breast cancer patients: Clinical analysis of 40 cases. Chin. J. Tradit. Med. Sci. Technol. 2013, 20, 130–134. [Google Scholar]
- Zhang, C.D.; Fan, Y.P.; Qian, Y.F.; Chen, Z.J. Clinical observation of otopoint pressuremassage to cure nausea and vomit of chemotherapy. Tianjin J. Nurs. 2003, 11, 41–43. [Google Scholar]
| Variables | n (%) |
|---|---|
| Sex | |
| Female | 12 (85.7) |
| Male | 2 (14.3) |
| Time of practical experience | |
| Between 4 and 5 years | 4 (28.6) |
| Between 6 and 10 years | 4 (28.6) |
| Between 11 and 15 years | 2 (14.3) |
| Between 16 and 24 years | 2 (14.3) |
| Over 25 years | 2 (14.3) |
| Time of teaching experience | |
| No experience | 5 (35.7) |
| Between 3 and 5 years | 2 (14.3) |
| Between 6 and 10 years | 3 (21.4) |
| Between 11 and 15 years | 2 (14.3) |
| Between 16 and 24 years | 2 (14.3) |
| Research experience with published articles on auricular acupuncture (AA) and/or acupuncture in relevant journals | |
| Yes | 13 (92.9) |
| No | 1 (7.1) |
| Participation in research groups in the field of AA and/or acupuncture | |
| Yes | 13 (92.9) |
| No | 1 (7.1) |
| Master’s degree with a dissertation in AA and/or acupuncture | |
| Yes | 7 (50.0) |
| No | 7 (50.0) |
| PhD with a thesis in the field of AA and/or acupuncture | |
| Yes | 5 (35.7) |
| No | 9 (64.3) |
| Course/specialization or residency in the field of AA and/or acupuncture | |
| Yes | 13 (92.0) |
| No | 1 (7.1) |
| Item Description | Final Version of the Proposed Protocol | Agreement Index (%) |
|---|---|---|
| Acupuncture style | Auricular acupuncture (AA) based on the precepts of traditional Chinese medicine (TCM) | 92.9 |
| Reasoning for treatment provided, based on historical context, literature sources, and/or consensus methods, with references where suitable | AA is a specialized approach to TCM/acupuncture which uses the auricular microsystem for the treatment, prevention, and diagnosis of health conditions [36]. It is currently used in more than 249 areas of the health sciences, including oncology [36]. AA’s physiological mechanism of action is still under study [14]. However, it is believed to be neurologically based on stimulation of the trigeminal and vagus nerves, thus generating neuromodulation in the central nervous system (CNS). In addition, the stimulation of auricular points may be related to an increase in vagal tone, as well as regulations in the gastrointestinal, endocrine, cardiovascular, and respiratory systems [14,15]. | 85.7 |
| Extent to which treatment may vary | All patients will receive the same treatment protocol in all five sessions. The final version of the protocol will be based on the results of a previous systematic review [17], on content validation by specialists in the field, and on clinical validation. | 85.7 |
| Number of device insertions per subject and per session | Seven auricular seeds will be applied to each subject per session. | 100 |
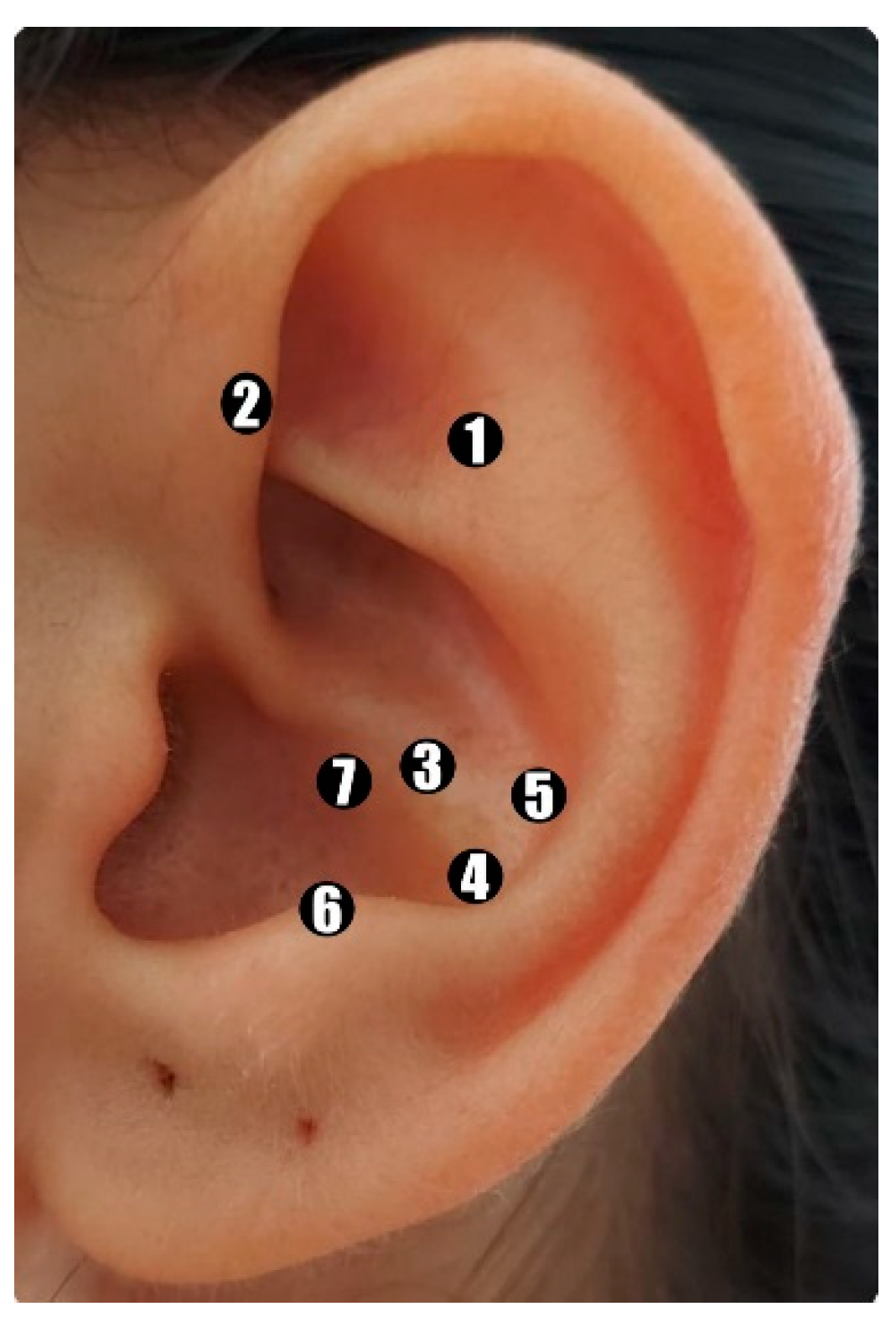
| Names (or location if no standard name) of points used (uni-/bilateral) | Shénmén (TF4) shenmen, Jiaogan (AH6a) sympathetic nerve, Wèi (CO4) stomach, Pí (CO13) spleen, Gän (CO12) liver, Pízhìxià (AT4) subcortex, and bënmén (C03) cardia. These points will be applied unilaterally, alternating ears each session. | 100 |
| Depth of insertion | Not applicable. | 100 |
| Response sought | Patients will be instructed to press the seeds at three different times, in the morning (3 times), in the afternoon (3 times), and in the evening (3 times), and whenever they feel nauseous or vomit, for approximately 30 s at each point, until the ear becomes slightly hyperemic [39] or until they feel slight discomfort or pain [29,37]—with a sensation of “deqi”. | 100 |
| Device stimulation | Manual seed acupressure. | 92.9 |
| Device retention time | Patients will be instructed to keep the seeds fixed in their ears for a period of seven days. In the event that they are removed before this period (intentionally or unintentionally), patients will be instructed to inform the researcher. | 92.9 |
| Device type | Vaccaria seeds | 85.7 |
| Number of treatment sessions | Five sessions | 92.9 |
| Frequency and duration of treatment sessions | Once a week, for approximately 20 min each session. The entire treatment will last approximately five weeks. | 100 |
| Details of the other interventions administered | No other interventions will be applied. | 100 |
| Setting and context of treatment, including instructions to practitioners, and information and explanations to patients | Only a qualified interventionist will apply the intervention. After attaching the seeds, participants will be instructed to press each point for 30 s, three times a day, in the morning, afternoon, and evening, and whenever they feel nauseous or vomit [38]. In addition, they will be instructed to keep the devices inserted until the next AA session, for a period of seven days, or to remove them in the event of discomfort, allergic processes, and/or itching [40]. They will also be instructed on how to maintain hygiene and how to keep the seeds in the ear, as well as how to address the possible discomfort resulting from the procedure. These guidelines will be reinforced with each new session. To reinforce the necessary care, patients will receive a leaflet containing guidelines on how to maintain the stitches, the scheduled days for the AA sessions and evaluations, as well as a contact number of the researcher in charge. | 92.90 |
| Description of participating acupuncturists | Academic qualifications: Nurse. Specialization in classical systemic acupuncture, with 1200 h/Minimum of two years of experience; 80 h AA course/Minimum of four years of experience. | 100 |
| Variables | n (%) |
|---|---|
| Sex | |
| Male | 5 (25) |
| Female | 15 (75) |
| Marital status | |
| Single | 4 (20) |
| Married | 11 (55) |
| Divorced | 2 (10) |
| Widowed | 3 (15) |
| Race | |
| White | 18 (90) |
| Black | 2 (10) |
| Frequency of chemotherapy sessions | |
| Weekly | 9 (45) |
| Fortnightly | 4 (20) |
| Three weeks and a weekly break | 4 (20) |
| 21 days | 3 (15) |
| Duration of chemotherapy treatment | |
| Less than six months | 9 (45) |
| Between six months and one year | 3 (15) |
| Between one and six years | 2 (10) |
| Over three years | 6 (30) |
| Type of cancer | |
| Breast | 4 (20) |
| Multiple myeloma | 4 (20) |
| Colon | 2 (10) |
| Prostate | 3 (15) |
| Esophagus | 2 (10) |
| Ovary | 2 (10) |
| Rectum | 2 (10) |
| Stomach | 1 (5) |
| Chemotherapy protocol | |
| Carboplatin and paclitaxel | 4 (20) |
| Folfiri (fluoruracil, calcium folinate, and irinotecan) | 3 (15) |
| Bortezomib | 2 (10) |
| Nordic flox (5-fluorouracil, leucovorin combined with oxaliplatin) | 1 (5) |
| Doxorubicin and cyclophosphamide | 2 (10) |
| Folfoxiri (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) | 1 (5) |
| Gemcitabine | 2 (10) |
| Cisplatin, vincriatin, and filgrastim | 1 (5) |
| Leuprorelin combined with bicalutamide prostate and with cyclophosphamide and leuprorelin | 1 (5) |
| Leuprorelin and zoledronic acid | 1 (5) |
| Cisplatin and Gemcitabine | 1 (5) |
| Vinorelbine and trastuzumab | 1 (5) |
| Variables | Pretest | Post-Test | p |
|---|---|---|---|
| n (%) | n (%) | ||
| Presented nausea after the last chemotherapy session | 20 (100) | 11 (55) | 0.004 * |
| How often have you felt nauseous after the chemotherapy session? | |||
| Never | 0 | 9 (45) | 0.001 ** |
| 1 to 3 times | 6 (30) | 9 (45) | |
| 4 to 6 times | 3 (15) | 0 | |
| 7 to 9 times | 0 | 0 | |
| 9 times or more | 11 (55) | 2 (10) | |
| How was your worst nausea feeling after your last chemotherapy session? | |||
| I did not feel nauseous | 0 | 9 (45) | 0.001 ** |
| Very light | 0 | 2 (10) | |
| Light | 2 (10) | 4 (20) | |
| Moderate | 3 (15) | 2 (10) | |
| Strong | 8 (40) | 2 (10) | |
| Very strong | 6 (30) | 1 (5) | |
| Unbearable | 1 (5) | 0 | |
| When was your worst nausea feeling? | |||
| I did not feel nauseous | 0 | 9 (45) | 0.002 ** |
| During chemotherapy | 1 (5) | 0 | |
| 0 to 4 h after chemotherapy | 3 (15) | 2 (10) | |
| 5 to 8 h after chemotherapy | 2 (10) | 0 | |
| 9 to 12 h after chemotherapy | 0 | 0 | |
| 13 to 24 h after chemotherapy | 0 | 0 | |
| Over 24 h after chemotherapy | 9 (45) | 7 (35) | |
| The feeling of nausea remained the same the entire time | 5 (25) | 2 (10) | |
| Experienced vomiting after the last chemotherapy session | 13 (65) | 7 (35.0) | 0.031 * |
| How many times have you vomited after the chemotherapy session? | |||
| Never | 6 (30) | 13 (65) | 0.007 ** |
| 1 to 3 times | 10 (50) | 5 (25) | |
| 4 to 6 times | 1 (5) | 1 (5) | |
| 7 to 9 times | 1 (5) | 0 | |
| 9 times or more | 2 (10) | 1 (5) | |
| What was the worst instance of vomiting you have experienced? | |||
| I did not vomit | 6 (30) | 13 (65) | 0.052 ** |
| Very light | 0 | 1 (5) | |
| Light | 4 (20) | 2 (10) | |
| Moderate | 3 (15) | 0 | |
| Strong | 3 (15) | 3 (15) | |
| Very strong | 4 (20) | 1 (5) | |
| Unbearable | 0 | 0 | |
| When was the worst instance of vomiting you have experienced? | |||
| I did not vomit | 6 (30) | 12 (60) | 0.021 ** |
| During chemotherapy | 0 | 0 | |
| 0 to 4 h after chemotherapy | 1 (5) | 2 (10) | |
| 5 to 8 h after chemotherapy | 0 (0) | 0 | |
| 9 to 12 h after chemotherapy | 0 (0) | 0 | |
| 13 to 24 h after chemotherapy | 1 (5) | 0 | |
| Over 24 h after chemotherapy | 10 (50) | 5 (25) | |
| The feeling of vomiting remained the same the entire time | 2 (10) | 1 (5) | |
| Experienced nausea before chemotherapy? | 7 (35) | 3 (15) | 0.219 * |
| How was your feeling of nausea before chemotherapy? | |||
| I did not feel nauseous | 13 (65) | 17 (85) | 0.034 ** |
| Very light | 0 | 0 | |
| Light | 1 (5) | 3 (15) | |
| Moderate | 0 | 0 | |
| Strong | 2 (10) | 0 | |
| Very strong | 3 (15) | 0 | |
| Unbearable | 1 (5) | 0 | |
| How long before your chemotherapy session did you feel nauseous? | |||
| I did not feel nauseous before the last chemotherapy session | 13 (65) | 17 (85) | 0.059 ** |
| I felt nauseous 1 to 3 h before the last chemotherapy session | 1 (5) | 2 (10) | |
| I felt nauseous 4 to 6 h before the last chemotherapy session | 1 (5) | 0 | |
| I felt nauseous 7 to 9 h before the last chemotherapy session | 0 | 0 | |
| I felt nauseous 9 h before the last chemotherapy session | 5 (25) | 1 (5) | |
| Vomited before the last chemotherapy session | 3 (15) | 0 | 0.250 * |
| What was the worst vomiting experience you had before your last chemotherapy session? | |||
| I did not vomit | 17 (85) | 20 (100) | 0.083 ** |
| Very light | 0 | 0 | |
| Light | 1 (5) | 0 | |
| Moderate | 0 | 0 | |
| Strong | 1 (5) | 0 | |
| Very strong | 1 (5) | 0 | |
| Unbearable | 0 (0) | 0 | |
| How long before your last chemotherapy session did you vomit? | |||
| I did not vomit before the last chemotherapy session | 18 (90) | 20 (100) | 0.157 ** |
| I vomited 1 to 3 h before the last chemotherapy session | 1 (5) | 0 | |
| I vomited 4 to 6 h before the last chemotherapy session | 0 (0) | 0 | |
| I vomited 7 to 9 h before the last chemotherapy session | 0 (0) | 0 | |
| I vomited 9 h before the last chemotherapy session | 1 (0) | 0 | |
| Have you taken any medication for nausea and/or vomiting after your last chemotherapy session? | 16 (80) | 15 (75) | 1.000 * |
| Did this medication help? | |||
| Yes | 10 (50) | 11 (55) | 0.405 ** |
| A little | 1 (5) | 3 (15) | |
| Very little | 2 (10) | 0 | |
| No | 3 (15) | 2 (10) | |
| I did not use any medication | 4 (20) | 4 (20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, E.M.d.C.; Moura, C.d.C.; Nogueira, D.A.; Garcia, A.C.M. Development and Validation of an Auricular Acupuncture Protocol for the Management of Chemotherapy-Induced Nausea and Vomiting in Cancer Patients. Healthcare 2024, 12, 218. https://doi.org/10.3390/healthcare12020218
Paiva EMdC, Moura CdC, Nogueira DA, Garcia ACM. Development and Validation of an Auricular Acupuncture Protocol for the Management of Chemotherapy-Induced Nausea and Vomiting in Cancer Patients. Healthcare. 2024; 12(2):218. https://doi.org/10.3390/healthcare12020218
Chicago/Turabian StylePaiva, Eliza Mara das Chagas, Caroline de Castro Moura, Denismar Alves Nogueira, and Ana Cláudia Mesquita Garcia. 2024. "Development and Validation of an Auricular Acupuncture Protocol for the Management of Chemotherapy-Induced Nausea and Vomiting in Cancer Patients" Healthcare 12, no. 2: 218. https://doi.org/10.3390/healthcare12020218
APA StylePaiva, E. M. d. C., Moura, C. d. C., Nogueira, D. A., & Garcia, A. C. M. (2024). Development and Validation of an Auricular Acupuncture Protocol for the Management of Chemotherapy-Induced Nausea and Vomiting in Cancer Patients. Healthcare, 12(2), 218. https://doi.org/10.3390/healthcare12020218






