Unlicensed/Off-Label Drug Prescriptions at Hospital Discharge in Children: An Observational Study Using Routinely Collected Health Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Collection
2.3. Determining UL/OL Drugs
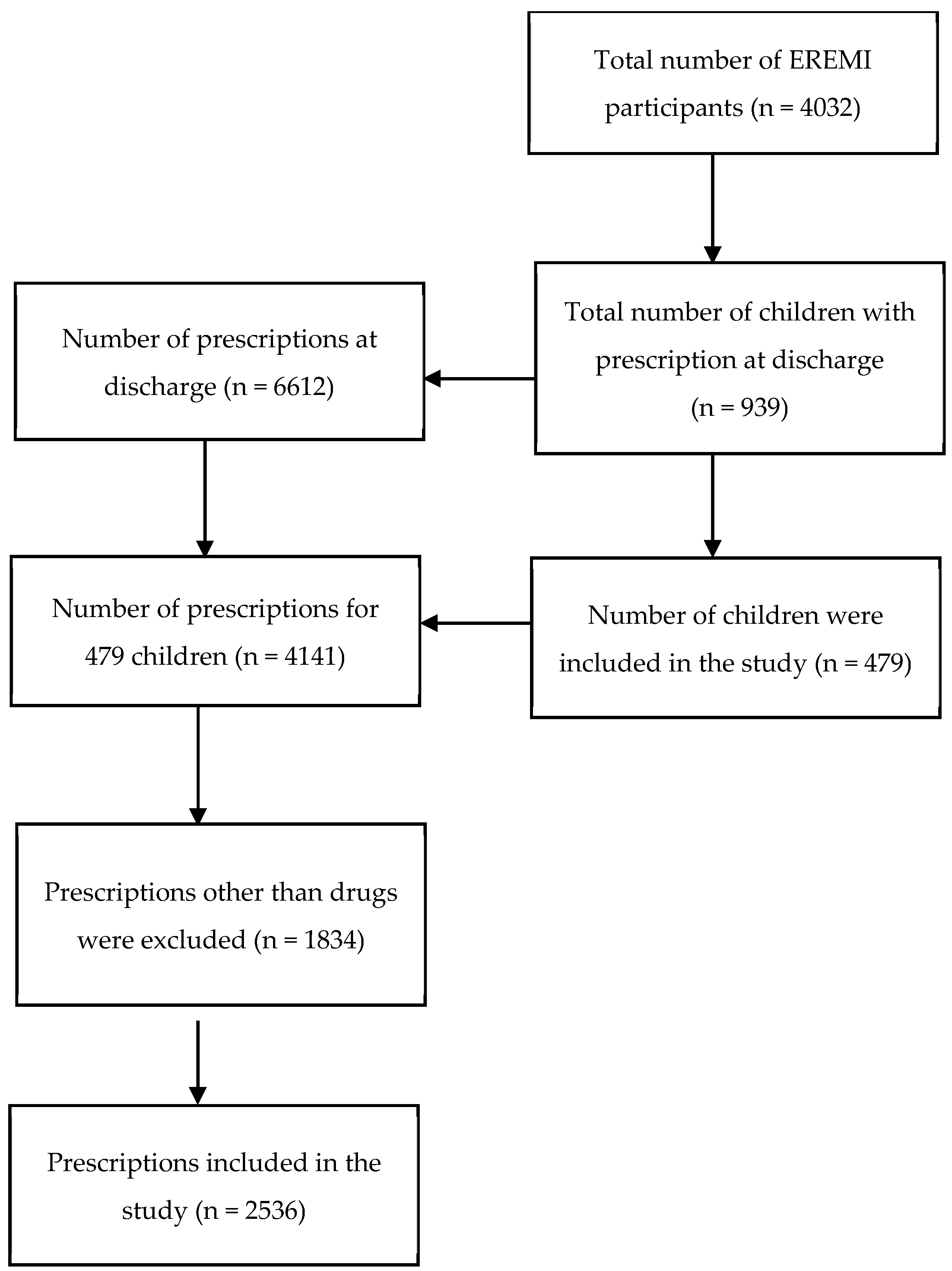
2.4. Sample Size
2.5. Population of the Study
2.6. Data Processing and Statistical Analysis
2.6.1. Epidemiological Characteristics of UL/OL Prescriptions
2.6.2. Subgroup Analyses
2.6.3. Search for Predictors of UL/OL Prescriptions
3. Results
3.1. The Characteristics of the Included Children
3.2. Primary Outcome
4. Discussion
Implications for Practice and Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weda, M.; Hoebert, J.; Vervloet, M.; Puigmart, C.M.; Damen, N.; Marchange, S.; Dijk, L. Study on Off-Label Use of Medicinal Products in the European Union; European Union: Brussels, Belgium, 2017. [Google Scholar]
- Wertheimer, A. Off-label prescribing of drugs for children. Curr. Drug Saf. 2011, 6, 46–48. [Google Scholar] [CrossRef]
- Frattarelli, D.A.; Galinkin, J.L.; Green, T.P.; Johnson, T.D.; Neville, K.A.; Paul, I.M.; Van Den Anker, J.N. Off-label use of drugs in children. Pediatrics 2014, 133, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Nunn, A.; Fielding, K.; Choonara, I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: A prospective study. Acta Paediatr. 1999, 88, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.D.; Craig, J.C.; Caldwell, P.H. Clinical trials in children. Br. J. Clin. Pharmacol. 2015, 79, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Meadows, M. Drug research and children. FDA Consum. 2003, 37, 12–17. [Google Scholar] [PubMed]
- Rodriguez, W.; Selen, A.; Avant, D.; Chaurasia, C.; Crescenzi, T.; Gieser, G.; Di Giacinto, J.; Huang, S.-M.; Lee, P.; Mathis, L.; et al. Improving pediatric dosing through pediatric initiatives: What we have learned. Pediatrics 2008, 121, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.; Rodrigues, A.T.; Roque, F.; Figueiras, A.; Falcão, A.; Herdeiro, M.T. Use of off-label and unlicenced drugs in hospitalised paediatric patients: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 1–13. [Google Scholar] [CrossRef]
- Philips, K.; Zhou, R.; Lee, D.S.; Marrese, C.; Nazif, J.; Browne, C.; Sinnett, M.; Tuckman, S.; Modi, A.; Rinke, M.L. Implementation of a Standardized Approach to Improve the Pediatric Discharge Medication Process. Pediatrics 2021, 147, 2711. [Google Scholar] [CrossRef]
- Bourgeois, F.C.; Olson, K.L.; Mandl, K.D. Patients treated at multiple acute health care facilities: Quantifying information fragmentation. Arch. Intern. Med. 2010, 170, 1989–1995. [Google Scholar] [CrossRef]
- Bradley, E.H.; Curry, L.; Horwitz, L.I.; Sipsma, H.; Thompson, J.W.; Elma, M.; Walsh, M.N.; Krumholz, H.M. Contemporary evidence about hospital strategies for reducing 30-day readmissions: A national study. J. Am. Coll. Cardiol. 2012, 60, 607–614. [Google Scholar] [CrossRef]
- Kripalani, S.; Jackson, A.T.; Schnipper, J.L.; Coleman, E.A. Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists. J. Hosp. Med. 2007, 2, 314–323. [Google Scholar] [CrossRef]
- Berry, J.G.; Ziniel, S.I.; Freeman, L.; Kaplan, W.; Antonelli, R.; Gay, J.; Coleman, E.A.; Porter, S.; Goldmann, D. Hospital readmission and parent perceptions of their child’s hospital discharge. Int. J. Qual. Health Care 2013, 25, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Frei-Jones, M.J.; Field, J.J.; DeBaun, M.R. Risk factors for hospital readmission within 30 days: A new quality measure for children with sickle cell disease. Pediatr. Blood Cancer 2009, 52, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Lampert, M.L.; Kraehenbuehl, S.; Hug, B.L. Drug-related problems: Evaluation of a classification system in the daily practice of a Swiss University Hospital. Pharm. World Sci. 2008, 30, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Keady, S.; Mohamed, F.; Brayley, S.; Thomson, M.; Lloyd, B.W.; Heuschkel, R.; Afzal, N.A. Use of unlicensed and off-label medications in paediatric gastroenterology with a review of the commonly used formularies in the UK. Aliment. Pharmacol. Ther. 2003, 17, 571–575. [Google Scholar] [CrossRef]
- Cras, A.; Conscience, M.-A.; Rajzbaum, G.; Lillo-Le Louët, A.; Lopez, N.; Tersen, I.; Bezie, Y. Off-label prescribing in a French hospital. Pharm. Weekbl. 2007, 29, 97–100. [Google Scholar] [CrossRef]
- Drogou, F.; Netboute, A.; Giai, J.; Dode, X.; Darmon, D.; Kassai, B.; Letrilliart, L. Off-label drug prescriptions in French general practice: A cross-sectional study. BMJ Open 2019, 9, e026076. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, E.; Kassai, B.; Berard, A.; Grenet, G.; Nguyen, K.A. Drug-related risk of hospital readmission in children with chronic diseases, a systematic review. Therapies 2023, 78, 393–408. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Mimouni, Y.; Jaberi, E.; Paret, N.; Boussaha, I.; Vial, T.; Jacqz-Aigrain, E.; Alberti, C.; Guittard, L.; Remontet, L.; et al. Relationship between adverse drug reactions and unlicensed/off-label drug use in hospitalized children (EREMI): A study protocol. Therapies 2021, 76, 675–685. [Google Scholar] [CrossRef]
- Husson, M.C. [Theriaque: Independent-drug database for good use of drugs by health practitioners]. Ann. Pharm. Fr. 2008, 66, 268–277. [Google Scholar] [CrossRef]
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Clinical Investigation of Medicinal Products in the Pediatric Population, ICH-E112004 Data. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_,Guideline.pdf (accessed on 1 October 2022).
- LaPolla, F.W.Z. Excel for data visualization in academic health sciences libraries: A qualitative case study. J. Med. Libr. Assoc. 2020, 108, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 November 2022).
- Akram, G. Profiling psychotropic discharge medication from a children’s psychiatric ward. Int. J. Clin. Pharm. 2015, 37, 753–757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, I.C.K.; Basra, N.; Yeung, V.W.; Cope, J. Supply problems of unlicensed and off-label medicines after discharge. Arch. Dis. Child. 2006, 91, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, C.; Behringer, J.; Walther, M.; Egger, R.; Kohler, H. BEA-005 Unlicensed and Off-Label Drug Prescription at Discharge from a Swiss Children’s Hospital. Eur. J. Hosp. Pharm. Sci. Pract. 2013, 20, A223. [Google Scholar] [CrossRef][Green Version]
- Langerová, P.; Vrtal, J.; Urbánek, K. Incidence of unlicensed and off-label prescription in children. Ital. J. Pediatr. 2014, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Teigen, A.; Wang, S.; Truong, B.T.; Bjerknes, K. Off-label and unlicensed medicines to hospitalised children in Norway. J. Pharm. Pharmacol. 2017, 69, 432–438. [Google Scholar] [CrossRef]
- Joret-Descout, P.; Prot-Labarthe, S.; Brion, F.; Bataille, J.; Hartmann, J.-F.; Bourdon, O. Off-label and unlicensed utilisation of medicines in a French paediatric hospital. Int. J. Clin. Pharm. 2015, 37, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Riou, S.; Plaisant, F.; Maucort Boulch, D.; Kassai, B.; Claris, O.; Nguyen, K.-A. Unlicensed and off-label drug use: A prospective study in French NICU. Acta Paediatr. 2015, 104, e228–e231. [Google Scholar] [CrossRef]
- Chalumeau, M.; Tréluyer, J.M.; Salanave, B.; Assathiany, R.; Chéron, G.; Crocheton, N.; Rougeron, C.; Mares, M.; Bréart, G.; Pons, G. Off label and unlicensed drug use among French office based paediatricians. Arch. Dis. Child. 2000, 83, 502–505. [Google Scholar] [CrossRef]
- Moulis, F.; Durrieu, G.; Lapeyre-Mestre, M. Off-label and unlicensed drug use in children population. Therapies 2018, 73, 135–149. [Google Scholar] [CrossRef]
- Pereira Gomes, V.; Melo da Silva, K.; Oliveira Chagas, S.; dos Santos Magalhães, I.R. Off-label and unlicensed utilization of drugs in a Brazilian pediatric hospital. Farm. Hosp. 2015, 39, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Cibulskis, C.C.; Giardino, A.P.; Moyer, V.A. Care transitions from inpatient to outpatient settings: Ongoing challenges and emerging best practices. Hosp. Pract. 2011, 39, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.D.T. Global Pediatric Drug Development. Curr. Ther. Res. Clin. Exp. 2019, 90, 135–142. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Carroll, K.A.; Onishi, T.; Matsumaru, N.; Brasseur, D.; Nakamura, H. Improvement of Pediatric Drug Development: Regulatory and Practical Frameworks. Clin. Ther. 2016, 38, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Vignot, S.; Daynes, P.; Bacon, T.; Vial, T.; Montagne, O.; Albin, N.; Emmerich, J.; Ratignier-Carbonneil, C.; Martin, D.; Maison, P. Collaboration Between Health-Care Professionals, Patients, and National Competent Authorities Is Crucial for Prevention of Health Risks Linked to the Inappropriate Use of Drugs: A Position Paper of the ANSM (Agence Nationale de Sécurité du Médicament et des Produits de Santé). Front. Pharmacol. 2021, 12, 635841. [Google Scholar] [CrossRef]
- Becker, C.; Zumbrunn, S.; Beck, K.; Vincent, A.; Loretz, N.; Müller, J.; Amacher, S.A.; Schaefert, R.; Hunziker, S. Interventions to Improve Communication at Hospital Discharge and Rates of Readmission: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2119346. [Google Scholar] [CrossRef]
- Winterstein, A.G.; Sauer, B.C.; Hepler, C.D.; Poole, C. Preventable drug-related hospital admissions. Ann. Pharmacother. 2002, 36, 1238–1248. [Google Scholar] [CrossRef]
- Lagnaoui, R.; Moore, N.; Fach, J.; Longy-Boursier, M.; Bégaud, B. Adverse drug reactions in a department of systemic diseases-oriented internal medicine: Prevalence, incidence, direct costs and avoidability. Eur. J. Clin. Pharmacol. 2000, 56, 181–186. [Google Scholar] [CrossRef]
| <27 Days | 28 Days–23 Months | 2–11 Years | 12–18 Years | Total | |
|---|---|---|---|---|---|
| Demographics | |||||
| Number of children discharged (%) | 2 (0.4) | 138 (27.2) | 298 (58.9) | 68 (13.4) | 479 |
| Gender M 1/F 2 | 2/0 | 81/55 | 159/125 | 32/30 | 265/194 |
| Median weight at discharge (kg) (IQR 3) | 4.1 (4.1–4.1) | 8.6 (7.2–9.7) | 19.8 (14.4–27.8) | 39 (32.5–46.6) | 19.9 (12.3–32.1) |
| Mean weight at discharge (kg) (SD 4) | 4.1 (0) | 8.7 (3) | 22.4 (11.7) | 39.7 (13.4) | 23.7 (14.5) |
| Prescription characteristics | |||||
| Number of prescriptions | 4 | 391 | 1642 | 499 | 2536 |
| Number of products used | 3 | 93 | 265 | 147 | 309 |
| Number of prescriptions per child. Median (IQR) | 2 | 2 (1–4) | 3 (1–6) | 4 (2–9) | 3 (1–6) |
| <27 Days | 28 Days–23 Months | 2–11 Years | 12–18 Years | Total | |
|---|---|---|---|---|---|
| Number of OL 1 prescriptions (%) | 2 (50) | 134 (34.3) | 660 (40.2) | 195 (39.1) | 993 (39.2) |
| Number of UL 2 prescriptions (%) | 0 | 11 (2.8) | 36 (2.3) | 10 (2) | 57 (2.3) |
| Number of children with OL or UL prescriptions (%) | 2 (100) | 70 (51) | 208 (70) | 53 (78) | 323 (74) |
| Drug Name | Total Prescriptions N 1, 2536 | Percentage (95% CI 2) | Labeling Status |
|---|---|---|---|
| Salbutamol | 222 | 8.8 (7.7–9.9) | Licensed |
| Paracetamol | 217 | 8.6 (7.5–9.7) | Licensed |
| Prednisolone | 129 | 5.1 (4.3–6.0) | Licensed |
| Amoxicillin and clavulanic acid | 86 | 3.4 (2.7–4.2) | Licensed |
| Esomeprazole | 64 | 2.5 (1.9–3.2) | Licensed |
| Sulfamethoxazole and trimethoprim | 63 | 2.5 (1.9–3.2) | Licensed |
| Vitamin D | 61 | 2.4 (1.8–3.1) | Licensed |
| Macrogol | 60 | 2.4 (1.8–3.0) | Licensed |
| Pancreatin | 58 | 2.3 (1.7–2.9) | Licensed |
| Vitamin A | 56 | 2.2 (1.7–2.9) | Licensed |
| Mometasone | 49 | 2.0 (1.4–2.5) | Licensed |
| Vitamin (ADEC) | 44 | 1.7 (1.3–2.3) | Licensed |
| Generic Name | Trade Name | Percentage (95% CI 1) | OL 2 Characteristics |
|---|---|---|---|
| Amoxicillin and clavulanic acid | Augmentine pdr 3 for oral susp. 4 250 mg/5 mL—tbl. 5 1 G—cap. 6 500 mg | 2.8 (2.2–3.5) | Weight < 40 kg |
| Macrogol | Forlax 10 g, pdr for oral solution in sachet | 0.9 (0.6–1.4) | Age < 8 years old |
| Esomeprazole | Inexium tbl. 20 mg | 0.7 (0.4–1.1) | Age < 18 years old |
| Vitamin D | Uvedose 100,000 UI oral sol. 7 (ampoule) | 0.6 (0.4–1) | Age < 5 years old |
| Sulfamethoxazole and trimethoprim | Bactrim forte tbl. 800 + 160 mg | 0.6 (0.4–1) | Age < 18 years old |
| Name | Percentage (95% CI 1) |
|---|---|
| Vitamin A | 0.5 (0.3–0.9) |
| Melatonin | 0.2 (0.0–0.4) |
| Enalapril | 0.1 (0.0–0.3) |
| Ursodesoxycholic acid | 0.08 (0.0–03) |
| Fludrocortisone | 0.08 (0.0–0.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaberi, E.; Boussaha, I.; Dode, X.; Grenet, G.; Kassai, B.; Nguyen, K.A. Unlicensed/Off-Label Drug Prescriptions at Hospital Discharge in Children: An Observational Study Using Routinely Collected Health Data. Healthcare 2024, 12, 208. https://doi.org/10.3390/healthcare12020208
Jaberi E, Boussaha I, Dode X, Grenet G, Kassai B, Nguyen KA. Unlicensed/Off-Label Drug Prescriptions at Hospital Discharge in Children: An Observational Study Using Routinely Collected Health Data. Healthcare. 2024; 12(2):208. https://doi.org/10.3390/healthcare12020208
Chicago/Turabian StyleJaberi, Elham, Inesse Boussaha, Xavier Dode, Guillaume Grenet, Behrouz Kassai, and Kim An Nguyen. 2024. "Unlicensed/Off-Label Drug Prescriptions at Hospital Discharge in Children: An Observational Study Using Routinely Collected Health Data" Healthcare 12, no. 2: 208. https://doi.org/10.3390/healthcare12020208
APA StyleJaberi, E., Boussaha, I., Dode, X., Grenet, G., Kassai, B., & Nguyen, K. A. (2024). Unlicensed/Off-Label Drug Prescriptions at Hospital Discharge in Children: An Observational Study Using Routinely Collected Health Data. Healthcare, 12(2), 208. https://doi.org/10.3390/healthcare12020208






