Reliability and Validity of the Variability Model Testing Procedure for Somatic Dysfunction Assessment: A Comparison with Gait Analysis Parameters in Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Methods
2.2. The 3D-Gait Analysis (3D-GA)
- Cadence: number of steps in a time unit (steps/min);
- Foot off: duration of the stance phase (as a percentage of the gait cycle);
- Step length: longitudinal distance from one foot strike to the next (m);
- Walking speed: mean velocity of progression (m/s);
- Stride length: the distance between two successive placements of the same foot (m).
2.3. Statistical Analysis
3. Results
3.1. Reliability
3.2. Face Validiy
3.3. Content Valifity
3.4. Predictive Quality
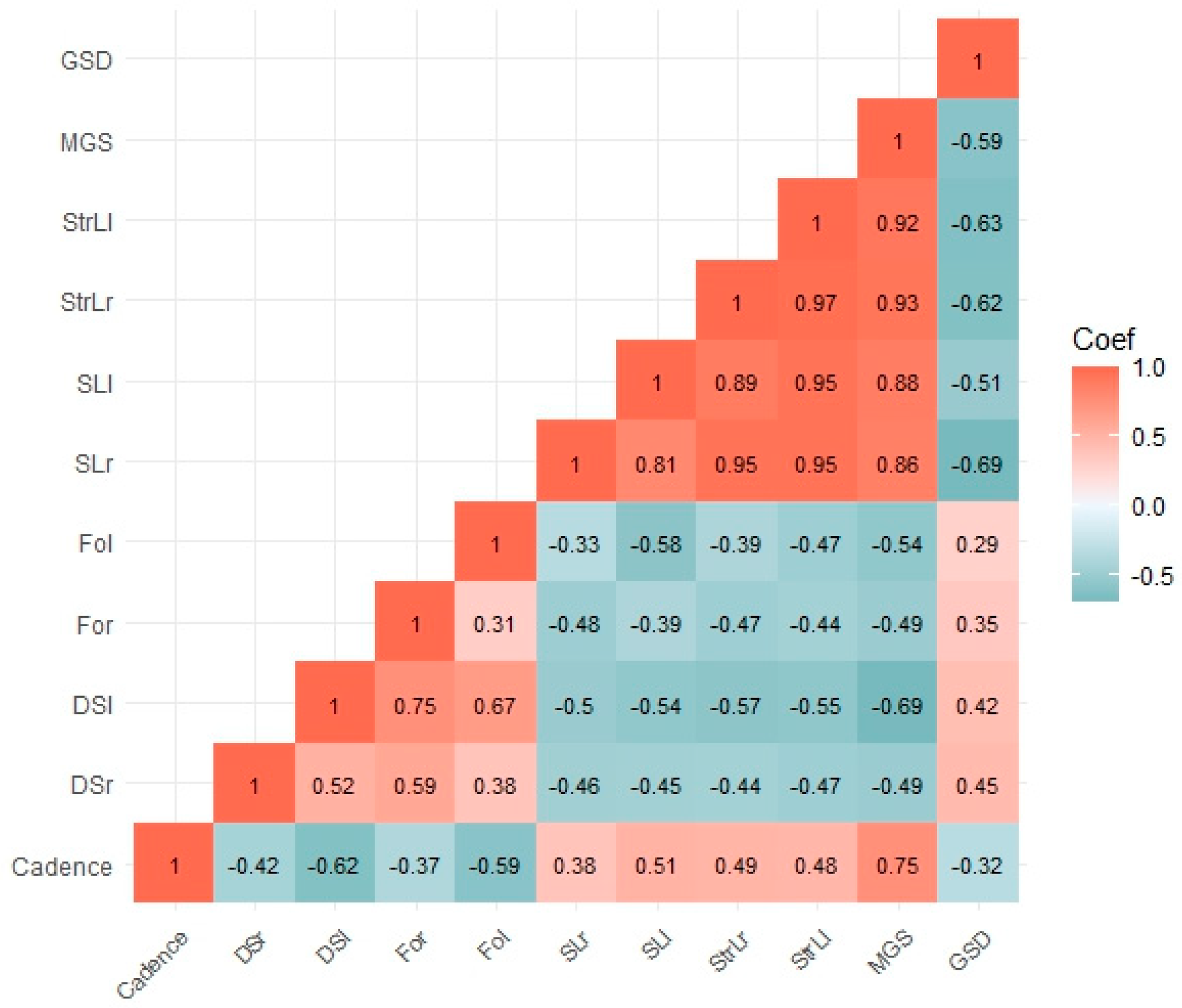
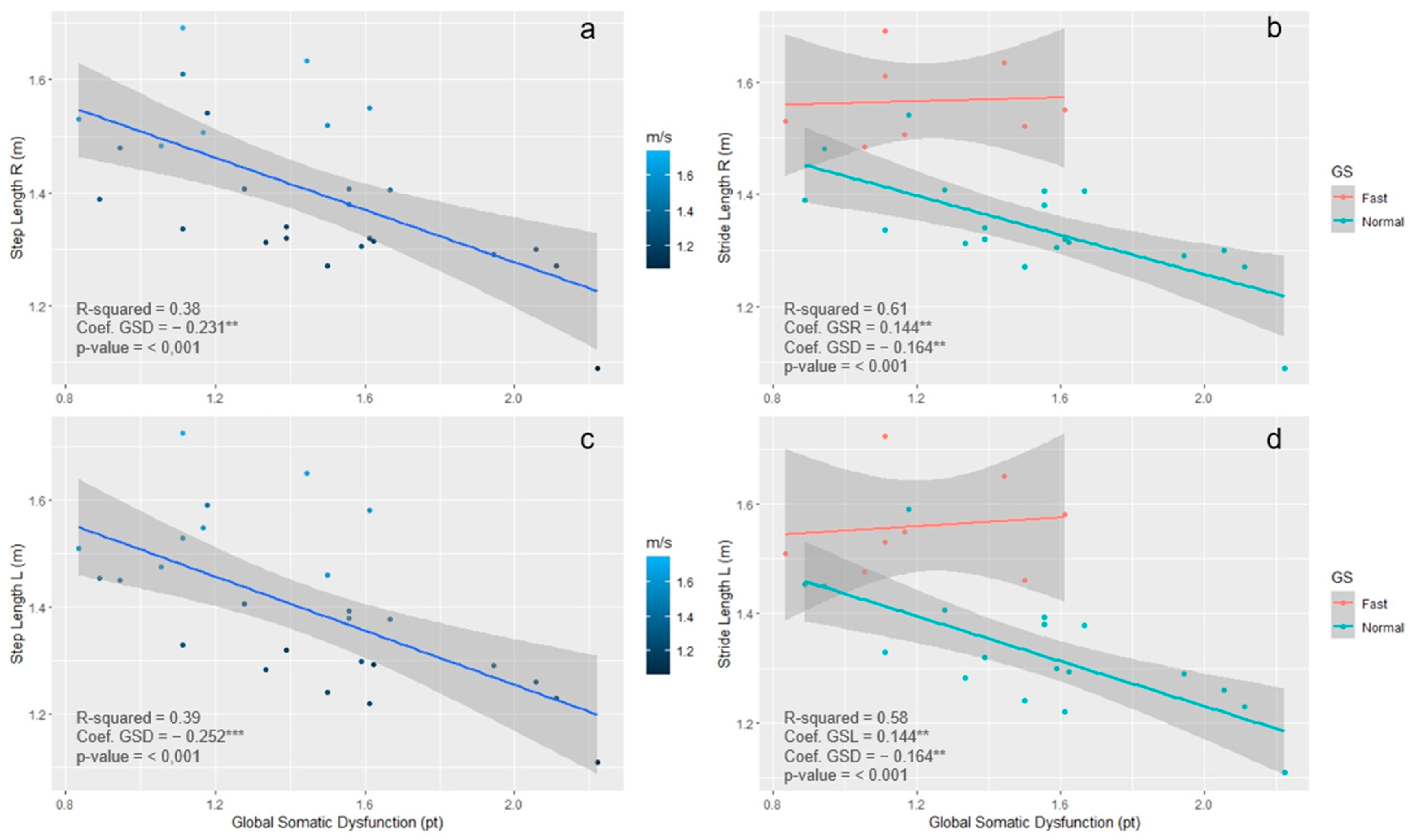
3.5. Concurrent Validity
4. Discussion
Considerations and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Procedure of Investigation

Appendix A.2. Somatic Dysfunction Definition
Appendix A.3. Variability Model Definition
Appendix A.4. Somatic Dysfunction Classification According to VM
- Grade 0—No SD: symmetric variability of motion in the NZ present (symmetric motion);
- Grade 1—Mild SD: asymmetric variability of mobility in the NZ (asymmetric motion);
- Grade 2—Moderate SD: asymmetric variability of motion in the NZ + altered tissue texture or tenderness;
- Grade 3—Severe SD: asymmetric variability of motion in the NZ + altered tissue texture + tenderness.
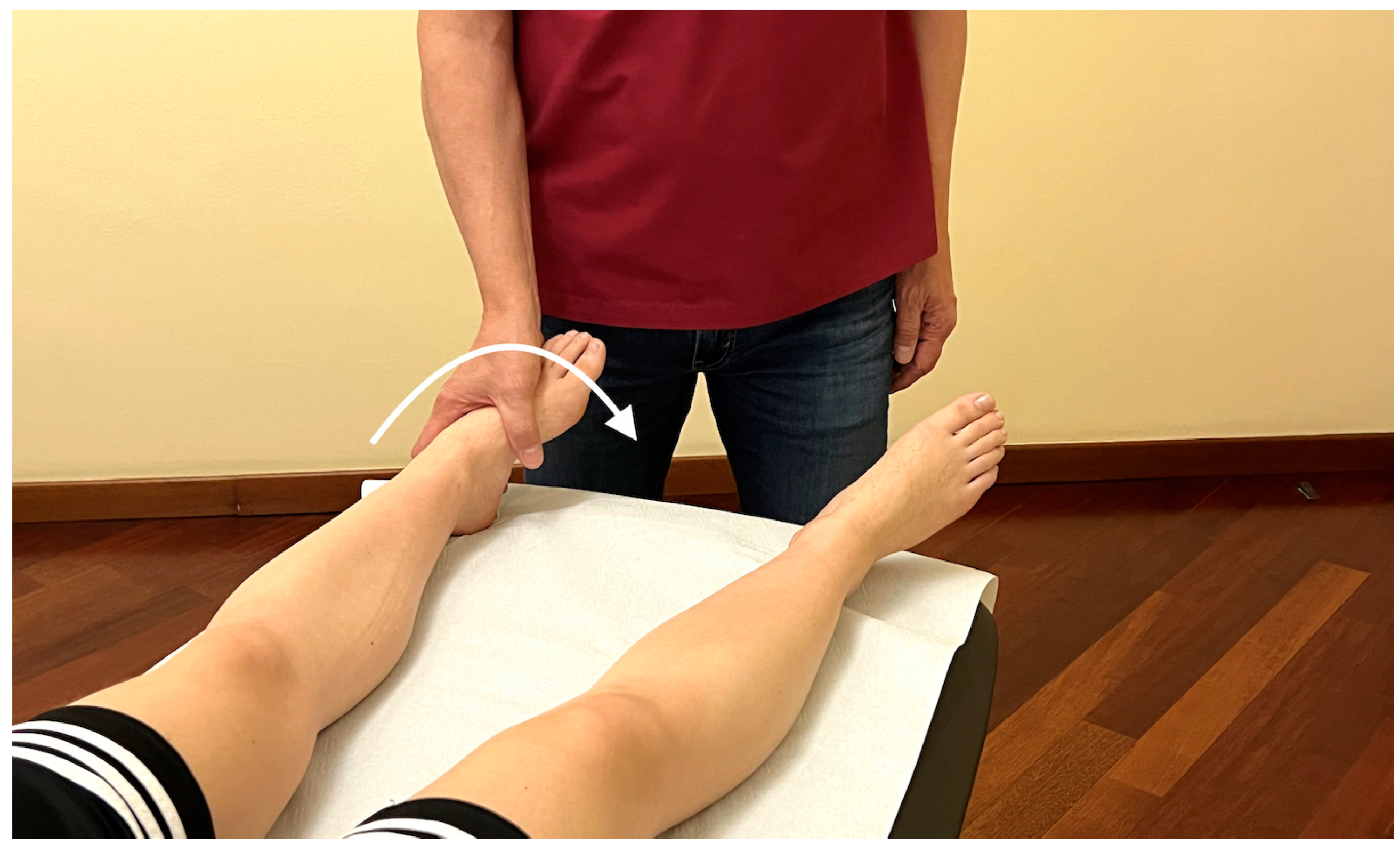
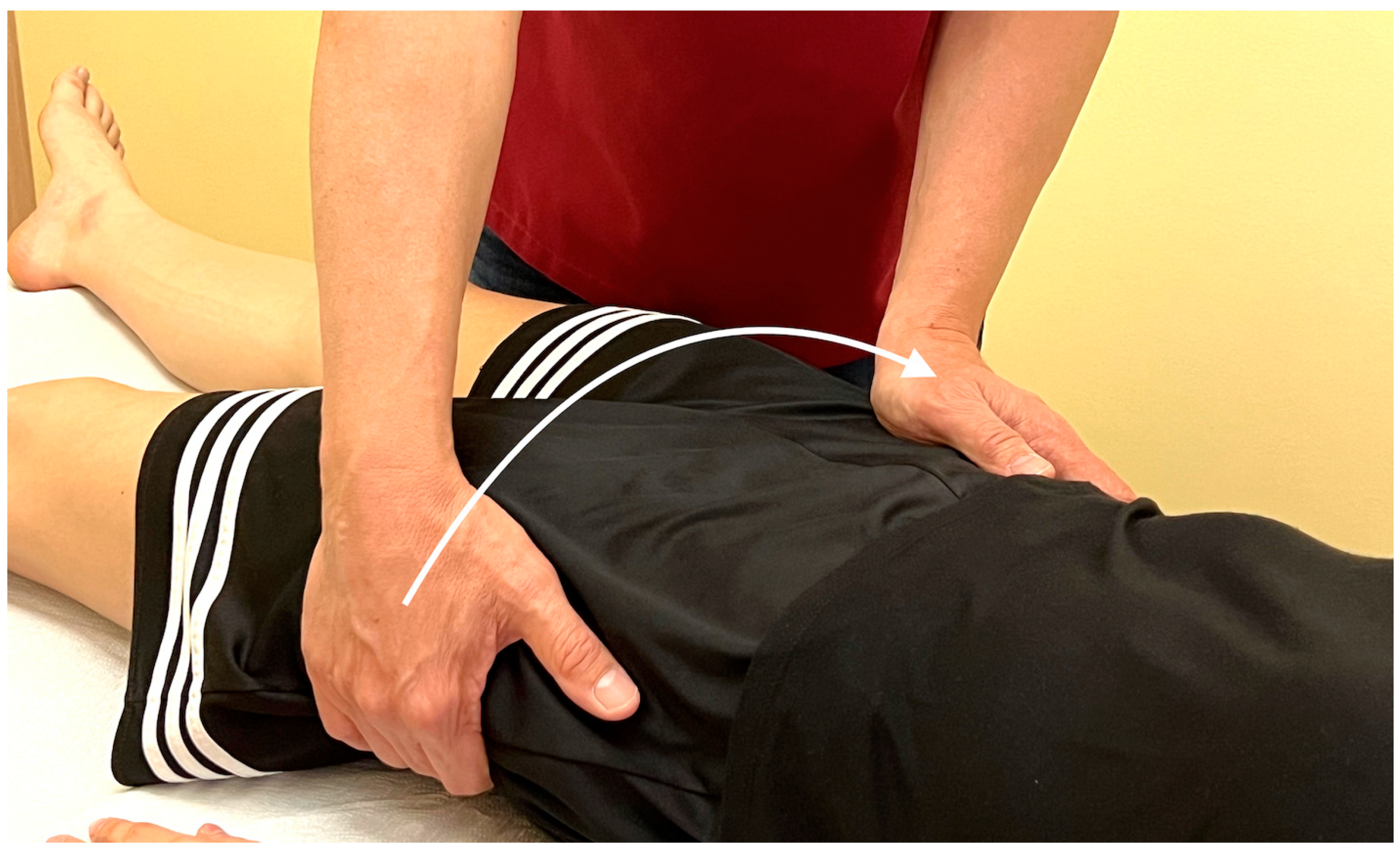
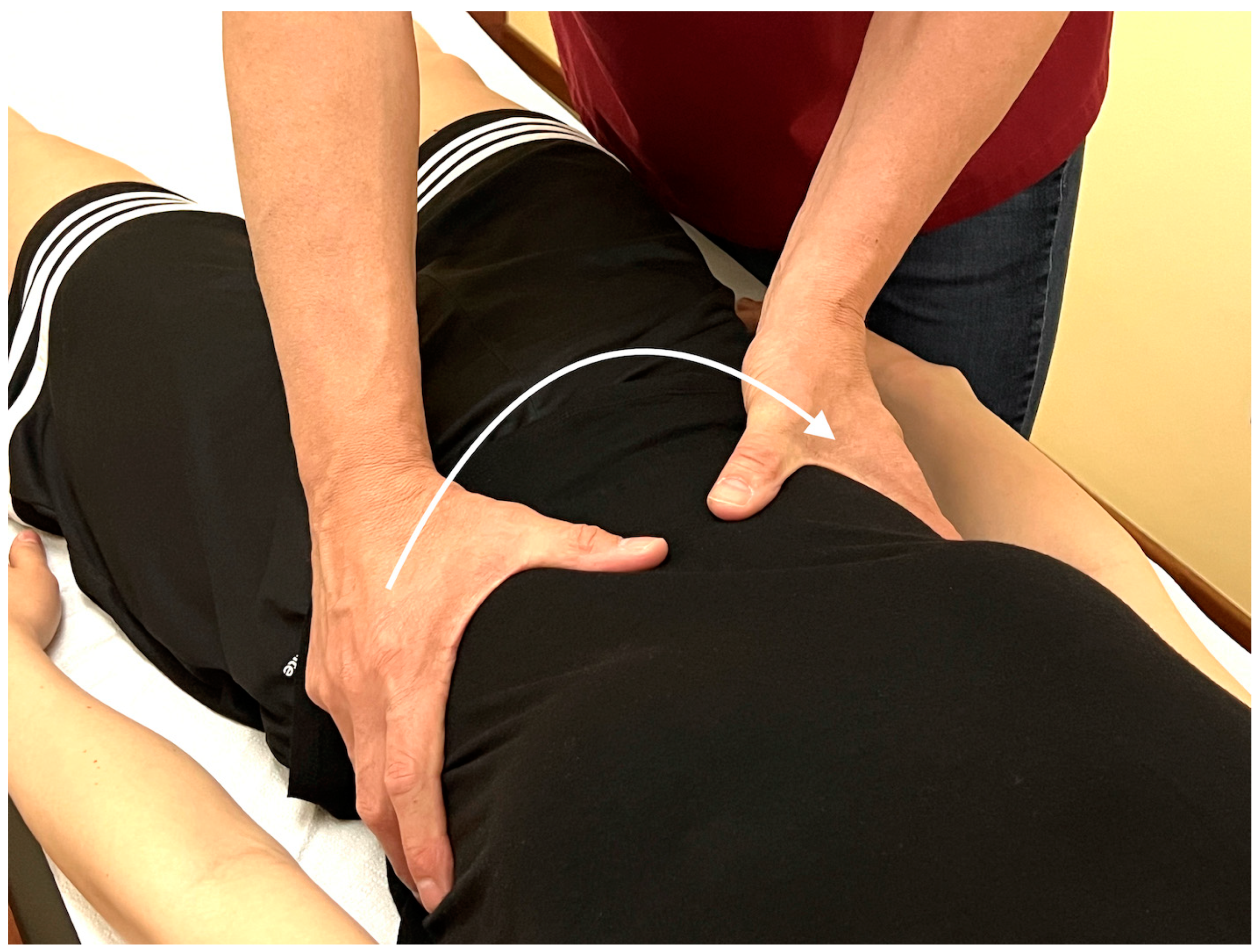
Appendix A.5. Palpatory Procedure

References
- Osteopathic International Alliance (OIA). Osteopathy and Osteopathic Medicine: A Global View of Practice, Patients, Education and the Contribution to Healthcare Delivery; Osteopathic International Alliance: Chicago, IL, USA, 2013; Available online: https://oialliance.org/wp-content/uploads/2014/01/OIA-Stage-2-Report.pdf (accessed on 25 November 2023).
- World Health Organization. Benchmarks for Training in Traditional/Complementary and Alternative Medicine: Benchmarks for Training in Osteopathy; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44356 (accessed on 25 November 2023).
- Lunghi, C.; Consorti, G.; Tramontano, M.; Esteves, J.E.; Cerritelli, F. Perspectives on tissue adaptation related to allostatic load: Scoping review and integrative hypothesis with a focus on osteopathic palpation. J. Bodyw. Mov. Ther. 2020, 24, 212–220. [Google Scholar] [CrossRef] [PubMed]
- American Association of Colleges of Osteopathic Medicine (AACOM). Glossary of Osteopathic Terminology, 2nd ed.; American Association of Colleges of Osteopathic Medicine: Maryland, MD, USA, 2017. [Google Scholar]
- World Health Organization. ICD-11: International Classification of Diseases. 11th Revision; World Health Organization: Geneva, Switzerland, 2019; Available online: https://icd.who.int/ (accessed on 25 November 2023).
- Tramontano, M.; Tamburella, F.; Dal Farra, F.; Bergna, A.; Lunghi, C.; Innocenti, M.; Cavera, F.; Savini, F.; Manzo, V.; D’Alessandro, G. International Overview of Somatic Dysfunction Assessment and Treatment in Osteopathic Research: A Scoping Review. Healthcare 2021, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Bergna, A.; Vismara, L.; Parravicini, G.; Dal Farra, F. A new perspective for Somatic Dysfunction in Osteopathy: The Variability Model. J. Bodyw. Mov. Ther. 2020, 24, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fryer, G. Somatic dysfunction: An osteopathic conundrum. Int. J. Osteopath. Med. 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Moran, R. Somatic dysfunction—Conceptually fascinating, but does it help us address health needs? Int. J. Osteopath. Med. 2016, 22, 1–2. [Google Scholar] [CrossRef]
- Degenhardt, B.F.; Johnson, J.C.; Snider, K.T.; Snider, E.J. Maintenance and improvement of interobserver reliability of osteopathic palpatory tests over a 4-month period. J. Am. Osteopath. Assoc. 2010, 110, 579–586. [Google Scholar]
- Seffinger, M.A.; Najm, W.I.; Mishra, S.I.; Adams, A.; Dickerson, V.M.; Murphy, L.S.; Reinsch, S. Reliability of Spinal Palpation for Diagnosis of Back and Neck Pain. Spine 2004, 29, E413–E425. [Google Scholar] [CrossRef]
- Hutchison, A.M.; Evans, R.; Bodger, O.; Pallister, I.; Topliss, C.; Williams, P.; Vannet, N.; Morris, V.; Beard, D. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013, 19, 112–117. [Google Scholar] [CrossRef]
- Kumar, S.; Panjabi, M.M. In Vivo Axial Rotations and Neutral Zones of the Thoracolumbar Spine. J. Spinal Disord. 1995, 8, 253–263. [Google Scholar] [CrossRef]
- Panjabi, M.M. Clinical spinal instability and low back pain. J. Electromyogr. Kinesiol. 2003, 13, 371–379. [Google Scholar] [CrossRef]
- Petty, N.J.; Maher, C.; Latimer, J.; Lee, M. Manual examination of accessory movements–seeking R1. Man. Ther. 2002, 7, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, R.E.; Russell Smith, A. The science of spinal motion palpation: A review and update with implications for assessment and intervention. J. Man. Manip. Ther. 2013, 21, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The Stabilizing System of the Spine. Part II. Neutral Zone and Instability Hypothesis. J. Spinal Disord. 1992, 5, 390–397. [Google Scholar] [CrossRef]
- Tarantino, A.G.; Vismara, L.; Buffone, F.; Bianchi, G.; Bergna, A.; Vanoni, M.; Tabbi, C.; Bresesti, I.; Agosti, M. Model-Base Estimation of Non-Invasive Ventilation Weaning of Preterm Infants Exposed to Osteopathic Manipulative Treatment: A Propensity-Score-Matched Cohort Study. Healthcare 2022, 10, 2379. [Google Scholar] [CrossRef] [PubMed]
- Vismara, L.; Cimolin, V.; Buffone, F.; Bigoni, M.; Clerici, D.; Cerfoglio, S.; Galli, M.; Mauro, A. Brain Asymmetry and Its Effects on Gait Strategies in Hemiplegic Patients: New Rehabilitative Conceptions. Brain Sci. 2022, 12, 798. [Google Scholar] [CrossRef]
- Krebs, D.E.; Edelstein, J.E.; Fishman, S. Reliability of Observational Kinematic Gait Analysis. Phys. Ther. 1985, 65, 1027–1033. [Google Scholar] [CrossRef]
- Gage, J.R. Gait analysis. An essential tool in the treatment of cerebral palsy. Clin. Orthop. Relat. Res. 1993, 288, 126–134. [Google Scholar] [CrossRef]
- Patterson, K.K.; Nadkarni, N.K.; Black, S.E.; McIlroy, W.E. Gait symmetry and velocity differ in their relationship to age. Gait Posture 2012, 35, 590–594. [Google Scholar] [CrossRef]
- Carse, B.; Meadows, B.; Bowers, R.; Rowe, P. Affordable clinical gait analysis: An assessment of the marker tracking accuracy of a new low-cost optical 3D motion analysis system. Physiotherapy 2013, 99, 347–351. [Google Scholar] [CrossRef]
- Roberts, M.; Mongeon, D.; Prince, F. Biomechanical parameters for gait analysis: A systematic review of healthy human gait. Phys. Ther. Rehabil. 2017, 4, 6. [Google Scholar] [CrossRef]
- Perry, J. Gait Analysis: Normal and Pathological Function; Slack Thorofare: West Deptford, NJ, USA, 1992. [Google Scholar]
- Simon, S.R. Quantification of human motion: Gait analysis—Benefits and limitations to its application to clinical problems. J. Biomech. 2004, 37, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.M.; Bartonek, Å.; Haglund-Åkerlind, Y.; Saraste, H. Centre of mass motion during gait in persons with myelomeningocele. Gait Posture 2003, 18, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Comfortable and maximum walking speed of adults aged 20–79 years: Reference values and determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Arslanova, I.; Takamuku, S.; Gomi, H.; Haggard, P. Multidigit tactile perception I: Motion integration benefits for tactile trajectories presented bimanually. J. Neurophysiol. 2022, 128, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, B.F.; Snider, K.T.; Snider, E.J.; Johnson, J.C. Interobserver reliability of osteopathic palpatory diagnostic tests of the lumbar spine: Improvements from consensus training. J. Am. Osteopath. Assoc. 2005, 105, 465–473. [Google Scholar] [PubMed]
- Hill, C.N.; Romero, M.; Rogers, M.; Queen, R.M.; Brolinson, P.G. Effect of osteopathic manipulation on gait asymmetry. J. Osteopath. Med. 2022, 122, 85–94. [Google Scholar] [CrossRef]
- Krekoukias, G.; Sakellari, V.; Anastasiadi, E.; Gioftsos, G.; Dimitriadis, Z.; Soultanis, K.; Gelalis, I.D. Gait Kinetic and Kinematic Changes in Chronic Low Back Pain Patients and the Effect of Manual Therapy: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 3593. [Google Scholar] [CrossRef]
- Ward, J.S.; Coats, J.; Sorrels, K.; Walters, M.; Williams, T. Pilot study of the impact sacroiliac joint manipulation has on walking kinematics using motion analysis technology. J. Chiropr. Med. 2013, 12, 143–152. [Google Scholar] [CrossRef]
- Ward, J.; Sorrels, K.; Coats, J.; Pourmoghaddam, A.; Deleon, C.; Daigneault, P. Pilot study of the impact that bilateral sacroiliac joint manipulation using a drop table technique has on gait parameters in asymptomatic individuals with a leg length inequality. J. Can. Chiropr. Assoc. 2014, 58, 85–95. [Google Scholar]
- Cury, A.C.; Pinto, R.Z.; Madaleno, F.O.; Resende, R.A. Do older adults present altered pelvic and trunk movement pattern during gait? A systematic review with meta-analysis and GRADE recommendations. Braz. J. Phys. Ther. 2021, 25, 484–499. [Google Scholar] [CrossRef]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef]
- Shishov, N.; Gimmon, Y.; Rashed, H.; Kurz, I.; Riemer, R.; Shapiro, A.; Debi, R.; Melzer, I. Old adult fallers display reduced flexibility of arm and trunk movements when challenged with different walking speeds. Gait Posture 2017, 52, 280–286. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. 2019, 24, e1743. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, C.J.C.; Meijer, O.G.; Wuisman, P.I.J.M.; van Dieën, J.H.; Levin, M.F.; Beek, P.J. Pelvis-Thorax Coordination in the Transverse Plane During Walking in Persons with Nonspecific Low Back Pain. Spine 2002, 27, E92–E99. [Google Scholar] [CrossRef]
- Cimolin, V.; Galli, M.; Tenore, N.; Albertini, G.; Crivellini, M. Gait strategy of uninvolved limb in children with spastic hemiplegia. Eura Medicophys. 2007, 43, 303–310. [Google Scholar] [PubMed]
- Bigoni, M.; Cimolin, V.; Vismara, L.; Tarantino, A.G.; Clerici, D.; Baudo, S.; Galli, M.; Mauro, A. Relationship between gait profile score and clinical assessments of gait in post-stroke patients. J. Rehabil. Med. 2021, 53, jrm00192. [Google Scholar] [CrossRef]
- Vismara, L.; Cimolin, V.; Menegoni, F.; Zaina, F.; Galli, M.; Negrini, S.; Villa, V.; Capodaglio, P. Osteopathic manipulative treatment in obese patients with chronic low back pain: A pilot study. Man. Ther. 2012, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Grecco, L.A.; Duarte, N.D.; Mendonça, M.E.; Cimolin, V.; Galli, M.; Fregni, F.; Oliveira, C.S. Transcranial direct current stimulation during treadmill training in children with cerebral palsy: A randomized controlled double-blind clinical trial. Res. Dev. Disabil. 2014, 35, 2840–2848. [Google Scholar] [CrossRef]
- Hecht, G.G.; Van Rysselberghe, N.L.; Young, J.L.; Gardner, M.J. Gait Analysis in Orthopaedic Surgery: History, Limitations, and Future Directions. J. Am. Acad. Orthop. Surg. 2022, 30, e1366–e1373. [Google Scholar] [CrossRef]
- Stecco, C.; Schleip, R. A fascia and the fascial system. J. Bodyw. Mov. Ther. 2016, 20, 139–140. [Google Scholar] [CrossRef]
- Wilke, J.; Schleip, R.; Yucesoy, C.A.; Banzer, W. Not merely a protective packing organ? A review of fascia and its force transmission capacity. J. Appl. Physiol. 2018, 124, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Zügel, M.; Maganaris, C.N.; Wilke, J.; Jurkat-Rott, K.; Klingler, W.; Wearing, S.C.; Findley, T.; Barbe, M.F.; Steinacker, J.M.; Vleeming, A. Fascial tissue research in sports medicine: From molecules to tissue adaptation, injury and diagnostics: Consensus statement. Br. J. Sports Med. 2018, 52, 1497. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Rodriguez, V.; Fede, C.; Pirri, C.; Petrelli, L.; Loro-Ferrer, J.F.; Rodriguez-Ruiz, D.; De Caro, R.; Stecco, C. Fascial Innervation: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5674. [Google Scholar] [CrossRef] [PubMed]
- Pischinger, A. The system of basic regulation. Zahnarztl Mitt. 1977, 67, 1255–1256. [Google Scholar]
- Benias, P.C.; Wells, R.G.; Sackey-Aboagye, B.; Klavan, H.; Reidy, J.; Buonocore, D.; Miranda, M.; Kornacki, S.; Wayne, M.; Carr-Locke, D.L. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci. Rep. 2018, 8, 4947. [Google Scholar] [CrossRef]
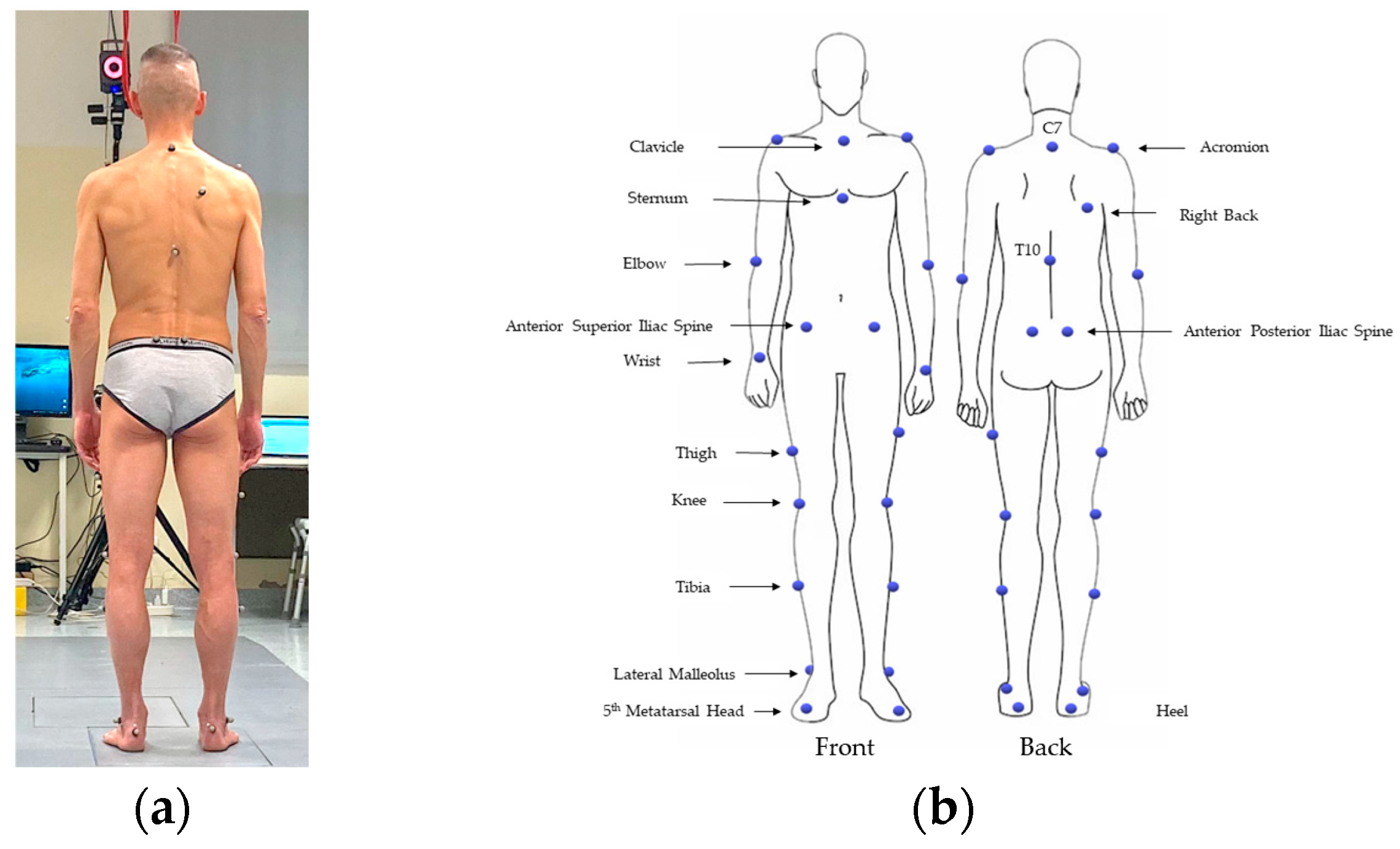
| Variable Name | Mean | Sta.Dev. | Min–Max |
|---|---|---|---|
| Age—years | 42.5 | 10.4 | 27–55 |
| Height—cm | 169 | 6.53 | 157–180 |
| Weight—Kg | 65.9 | 9.90 | 49–85 |
| BMI—Kg/m2 | 22.8 | 4.03 | 17.7–32.4 |
| Stride length (right)—m | 1.40 | 0.13 | 1.09–1.69 |
| Stride length (left)—m | 1.39 | 0.14 | 1.11–1.59 |
| Step length (right)—m | 0.71 | 0.07 | 0.58–0.84 |
| Step length (left)—m | 0.69 | 0.08 | 0.53–0.75 |
| Mean gait speed—m/s | 1.37 | 0.20 | 1.09–1.61 |
| Cadence mean—steps/min | 117 | 10.4 | 103–133 |
| Double support (right)—s | 0.19 | 0.06 | 0.09–0.36 |
| Double support (left)—s | 0.19 | 0.05 | 0.10–0.29 |
| Foot off (right)—% of stance | 59.6 | 2.59 | 54.4–64.2 |
| Foot off (left)—% of stance | 60.2 | 1.98 | 56.7–64.7 |
| Variable | Overall % of Agreement | Fleiss’s k | 95% CI |
|---|---|---|---|
| Cranio-cervical region | 85.2 | 0.78 | 0.62–0.94 |
| Cervico-dorsal region | 75.3 | 0.63 | 0.44–0.82 |
| Dorso-lumbar region | 79.0 | 0.69 | 0.50–0.87 |
| Lumbo-pelvic region | 75.3 | 0.63 | 0.44–0.82 |
| Right lower limb | 79.1 | 0.69 | 0.50–0.87 |
| Left lower limb | 69.1 | 0.54 | 0.33–0.74 |
| Variable | Overall % of Agreement | Fleiss’s k | 95% CI |
|---|---|---|---|
| Cranio-cervical region | 62.9 | 0.51 | 0.34–0.68 |
| Cervico-dorsal region | 60.4 | 0.47 | 0.29–0.66 |
| Dorso-lumbar region | 72.8 | 0.64 | 0.47–0.81 |
| Lumbo-pelvic region | 62.9 | 0.51 | 0.34–0.68 |
| Right lower limb | 66.7 | 0.56 | 0.38–0.74 |
| Left lower limb | 70.3 | 0.60 | 0.42–0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vismara, L.; Bergna, A.; Tarantino, A.G.; Dal Farra, F.; Buffone, F.; Vendramin, D.; Cimolin, V.; Cerfoglio, S.; Pradotto, L.G.; Mauro, A. Reliability and Validity of the Variability Model Testing Procedure for Somatic Dysfunction Assessment: A Comparison with Gait Analysis Parameters in Healthy Subjects. Healthcare 2024, 12, 175. https://doi.org/10.3390/healthcare12020175
Vismara L, Bergna A, Tarantino AG, Dal Farra F, Buffone F, Vendramin D, Cimolin V, Cerfoglio S, Pradotto LG, Mauro A. Reliability and Validity of the Variability Model Testing Procedure for Somatic Dysfunction Assessment: A Comparison with Gait Analysis Parameters in Healthy Subjects. Healthcare. 2024; 12(2):175. https://doi.org/10.3390/healthcare12020175
Chicago/Turabian StyleVismara, Luca, Andrea Bergna, Andrea Gianmaria Tarantino, Fulvio Dal Farra, Francesca Buffone, Davide Vendramin, Veronica Cimolin, Serena Cerfoglio, Luca Guglielmo Pradotto, and Alessandro Mauro. 2024. "Reliability and Validity of the Variability Model Testing Procedure for Somatic Dysfunction Assessment: A Comparison with Gait Analysis Parameters in Healthy Subjects" Healthcare 12, no. 2: 175. https://doi.org/10.3390/healthcare12020175









