Systematic Review of the Psychopathological Symptomatology and Neuropsychological Disorders of Chronic Primary Musculoskeletal Pain
Abstract
1. Introduction
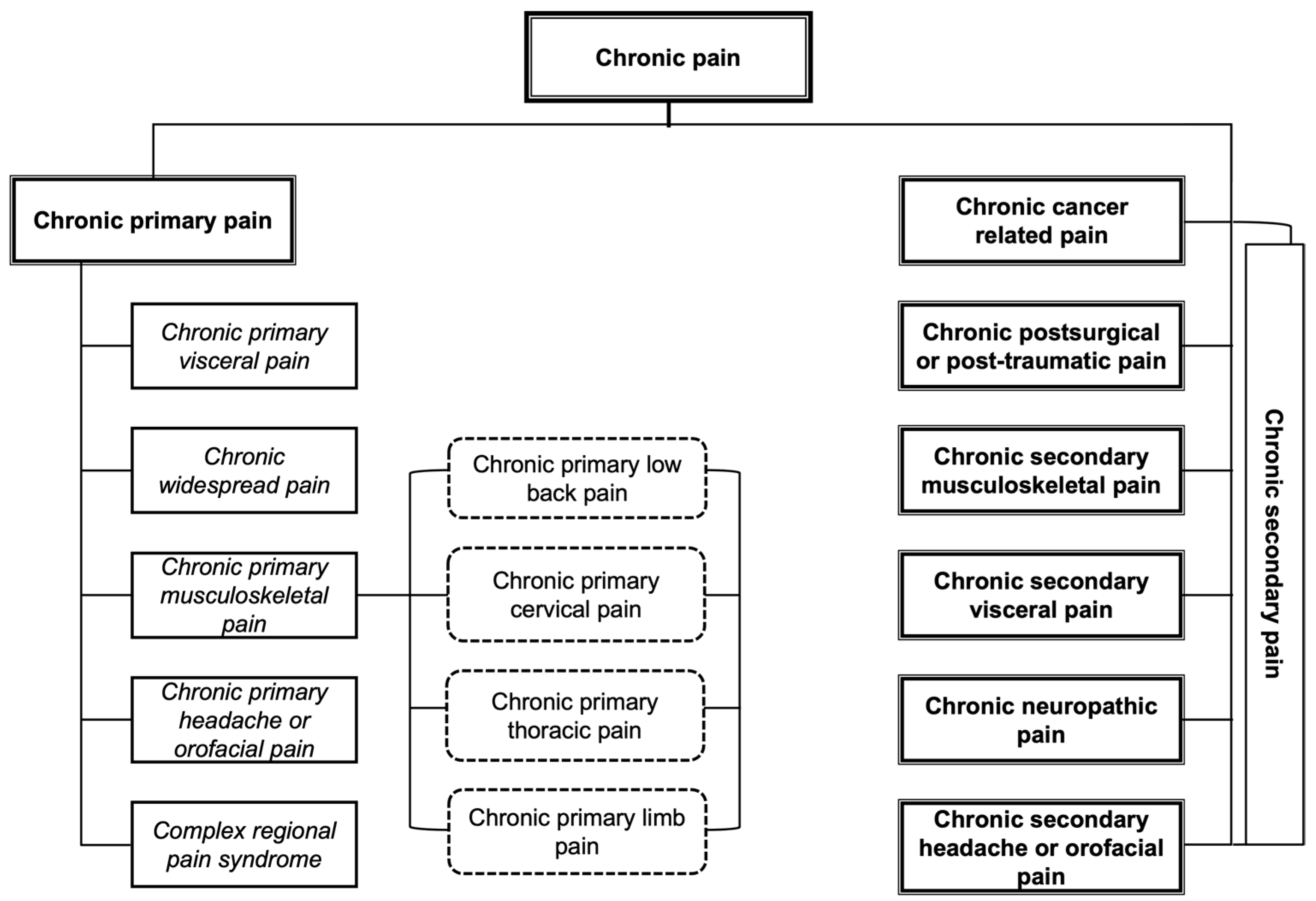
- (A)
- Chronic primary pain.
- (B)
- Located in the muscles, bones, joints, or tendons.
- (C)
- Associated with significant emotional distress and/or functional disability.
- (D)
- The diagnosis is applicable independently of the biological or psychological factors, except when another diagnosis explains the symptoms more exactly.
2. Materials and Methods
2.1. Selection Criteria
2.2. Search Strategy
2.3. Quality Assessment and Risk of Bias
2.4. Data Extraction and Analysis
3. Results
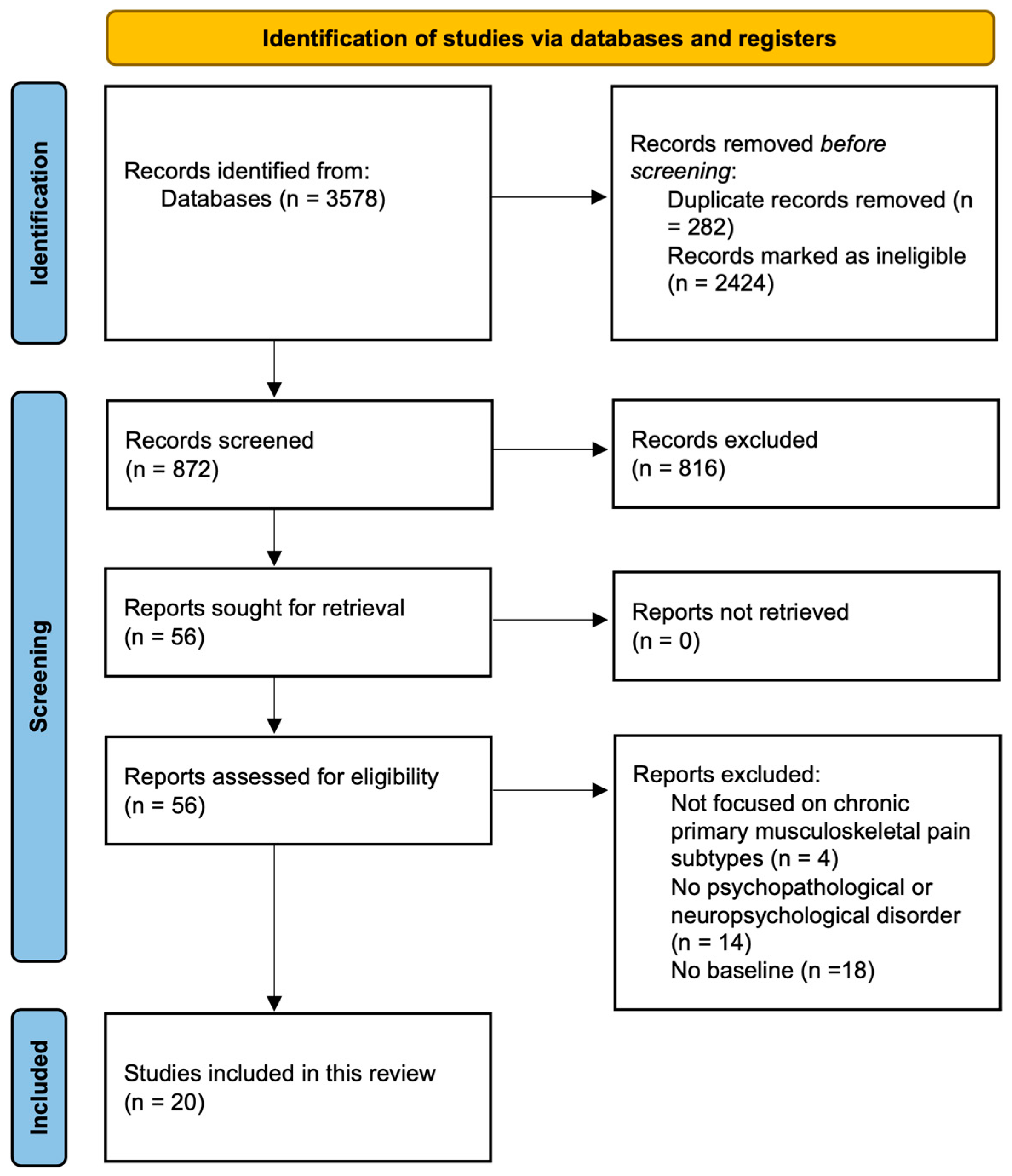
3.1. Selection of the Studies and Their Characteristics
3.2. Study Design, Subtypes of CPMP, and Existence of a Control Group and/or Additional Groups
3.3. Number, Gender, and Age of the Participants
3.4. Instruments of Evaluation and Symptoms and/or Psychopathological and Neuropsychological Dysfunctions of CPMP
3.5. Results of the Evaluation of CPMP, Psychopathological Symptomatology, and Neuropsychological Dysfunctions
3.5.1. Chronic Primary Musculoskeletal Pain
3.5.2. Psychopathological Symptomatology
3.5.3. Neuropsychological Dysfunctions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velly, A.M.; Mohit, S. Epidemiology of Pain and Relation to Psychiatric Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 159–167. [Google Scholar] [CrossRef]
- Laisné, F.; Lecomte, C.; Corbière, M. Biopsychosocial Predictors of Prognosis in Musculoskeletal Disorders: A Systematic Review of the Literature (Corrected and Republished). Disabil. Rehabil. 2012, 34, 1912–1941. [Google Scholar] [CrossRef] [PubMed]
- Meints, S.M.; Edwards, R.R. Evaluating Psychosocial Contributions to Chronic Pain Outcomes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 168–182. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.N.; Kohberg, M.; Juul-Kristensen, B.; Herborg, L.G.; Søgaard, K.; Roessler, K.K. Psychosocial Aspects of Everyday Life with Chronic Musculoskeletal Pain: A Systematic Review. Scand. J. Pain 2014, 5, 131–148. [Google Scholar] [CrossRef]
- Crofford, L.J. Psychological Aspects of Chronic Musculoskeletal Pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 147–155. [Google Scholar] [CrossRef]
- Mazza, S.; Frot, M.; Rey, A.E. A Comprehensive Literature Review of Chronic Pain and Memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Primary Pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef]
- World Health Organization. MG30 Chronic Pain—International Classification of Diseases, 11th ed.; 2018; Available online: https://icd.who.int/browse/2024-01/mms/en#1581976053 (accessed on 10 March 2023).
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Cheung, C.W.; Choi, S.W.; Wong, S.S.C.; Lee, Y.; Irwin, M.G. Changes in Prevalence, Outcomes, and Help-Seeking Behavior of Chronic Pain in an Aging Population Over the Last Decade. Pain Pract. 2017, 17, 643–654. [Google Scholar] [CrossRef]
- van Hecke, O.; Torrance, N.; Smith, B.H. Chronic Pain Epidemiology and Its Clinical Relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zajacova, A.; Grol-Prokopczyk, H.; Zimmer, Z. Pain Trends among American Adults, 2002–2018: Patterns, Disparities, and Correlates. Demography 2021, 58, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Eisenberg, E.; O’Brien, T. The Individual and Societal Burden of Chronic Pain in Europe: The Case for Strategic Prioritisation and Action to Improve Knowledge and Availability of Appropriate Care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of Chronic Pain among Adults in the United States. PAIN 2022, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; Tarantino, U. Chronic Pain in Musculoskeletal Diseases: Do You Know Your Enemy? J. Clin. Med. 2022, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Demircioğlu, A.; Özkal, Ö.; Dağ, O. Multiple Factors Affecting Health-Related Quality of Life in Women With Chronic Multisite Musculoskeletal Pain: A Cross-Sectional Study in Ankara, Turkey. Eval. Health Prof. 2022, 45, 115–125. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Chronic Primary Musculoskeletal Pain: A New Concept of Nonstructural Regional Pain. Pain Rep. 2022, 7, e1024. [Google Scholar] [CrossRef] [PubMed]
- Perrot, S.; Cohen, M.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. IASP Taskforce for the Classification of Chronic Pain The IASP Classification of Chronic Pain for ICD-11: Chronic Secondary Musculoskeletal Pain. Pain 2019, 160, 77–82. [Google Scholar] [CrossRef]
- Bułdyś, K.; Górnicki, T.; Kałka, D.; Szuster, E.; Biernikiewicz, M.; Markuszewski, L.; Sobieszczańska, M. What Do We Know about Nociplastic Pain? Healthcare 2023, 11, 1794. [Google Scholar] [CrossRef]
- Clauw, D.J.; Essex, M.N.; Pitman, V.; Jones, K.D. Reframing Chronic Pain as a Disease, Not a Symptom: Rationale and Implications for Pain Management. Postgrad. Med. 2019, 131, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic Pain: Towards an Understanding of Prevalent Pain Conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsayed, A.; Deer, T.R. Different Types of Pain. In Pain: A Review Guide; Abd-Elsayed, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 15–16. ISBN 978-3-319-99124-5. [Google Scholar]
- Prescott, S.A.; Ratté, S. Chapter 23—Somatosensation and Pain. In Conn’s Translational Neuroscience; Conn, P.M., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 517–539. ISBN 978-0-12-802381-5. [Google Scholar]
- Zhuang, J.; Mei, H.; Fang, F.; Ma, X. What Is New in Classification, Diagnosis and Management of Chronic Musculoskeletal Pain: A Narrative Review. Front. Pain Res. 2022, 3, 937004. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 January 2023).
- Brown, M.R.; Personius, K.E.; Langan, J. Participants with Mildly-Disabling Chronic Neck Pain Perform Differently during Explicit Compared to Implicit Motor Learning of a Reaching Task. PLoS ONE 2022, 17, e0266508. [Google Scholar] [CrossRef]
- Brunner, E.; Dankaerts, W.; Meichtry, A.; O’Sullivan, K.; Probst, M. Physical Therapists’ Ability to Identify Psychological Factors and Their Self-Reported Competence to Manage Chronic Low Back Pain. Phys. Ther. 2018, 98, 471–479. [Google Scholar] [CrossRef]
- Clark, J.R.; Nijs, J.; Yeowell, G.; Holmes, P.; Goodwin, P.C. Trait Sensitivity, Anxiety, and Personality Are Predictive of Central Sensitization Symptoms in Patients with Chronic Low Back Pain. Pain Pract. 2019, 19, 800–810. [Google Scholar] [CrossRef]
- Coppieters, I.; Cagnie, B.; De Pauw, R.; Meeus, M.; Timmers, I. Enhanced Amygdala-Frontal Operculum Functional Connectivity during Rest in Women with Chronic Neck Pain: Associations with Impaired Conditioned Pain Modulation. Neuroimage Clin. 2021, 30, 102638. [Google Scholar] [CrossRef]
- Day, M.A.; Matthews, N.; Newman, A.; Mattingley, J.B.; Jensen, M.P. An Evaluation of the Behavioral Inhibition and Behavioral Activation System (BIS-BAS) Model of Pain. Rehabil. Psychol. 2019, 64, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Oka, H.; Katsuhira, J.; Tonosu, J.; Kasahara, S.; Tanaka, S.; Matsudaira, K. Association between Somatic Symptom Burden and Health-Related Quality of Life in People with Chronic Low Back Pain. PLoS ONE 2018, 13, e0193208. [Google Scholar] [CrossRef]
- Ho, K.K.N.; Simic, M.; Cvancarova Småstuen, M.; de Barros Pinheiro, M.; Ferreira, P.H.; Bakke Johnsen, M.; Heuch, I.; Grotle, M.; Zwart, J.A.; Nilsen, K.B. The Association between Insomnia, c-Reactive Protein, and Chronic Low Back Pain: Cross-Sectional Analysis of the HUNT Study, Norway. Scand. J. Pain 2019, 19, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, S.; Niwa, S.-I.; Matsudaira, K.; Sato, N.; Oka, H.; Fujii, T.; Konno, S.-I.; Kikuchi, S.-I.; Yamada, Y. High Attention-Deficit/Hyperactivity Disorder Scale Scores Among Patients with Persistent Chronic Nonspecific Low Back Pain. Pain Physician 2021, 24, E299–E307. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mawla, I.; Kong, J.; Lee, J.; Gerber, J.; Ortiz, A.; Kim, H.; Chan, S.-T.; Loggia, M.L.; Wasan, A.D.; et al. Somatotopically Specific Primary Somatosensory Connectivity to Salience and Default Mode Networks Encodes Clinical Pain. Pain 2019, 160, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, X.; Qiu, Q.; Zhan, H.; Wu, W. Changes in Empathy in Patients With Chronic Low Back Pain: A Structural-Functional Magnetic Resonance Imaging Study. Front. Hum. Neurosci. 2020, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.P.C.; Mendoza, C.; Barone, M.; Rocha, R.S.; Dias Dos Santos, R.; Hazime, F.A. Reduction in Pain Inhibitory Modulation and Cognitive-Behavioral Changes in Patients With Chronic Low Back Pain: A Case-Control Study. Pain Manag. Nurs. 2021, 22, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Hampel, P. Long-Term Effects of Rehabilitation and Prevention of Further Chronification of Pain among Patients with Non-Specific Low Back Pain. J. Back Musculoskelet. Rehabil. 2022, 35, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Pakpour, A.H.; Yaghoubidoust, M.; Campbell, P. Persistent and Developing Sleep Problems: A Prospective Cohort Study on the Relationship to Poor Outcome in Patients Attending a Pain Clinic with Chronic Low Back Pain. Pain Pract. 2018, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.C.; Lluka, L.J.; Smith, M.T.; Haslam, C.; Moore, B.; O’Callaghan, J.; Strong, J. Effects of Long-Term Opioid Analgesics on Cognitive Performance and Plasma Cytokine Concentrations in Patients with Chronic Low Back Pain: A Cross-Sectional Pilot Study. Pain Rep. 2018, 3, e669. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.H.; Garey, L.; Raines, A.M.; Allan, N.P.; Schmidt, N.B.; Zvolensky, M.J. Anxiety Sensitivity and Opioid Use Motives among Adults with Chronic Low Back Pain. Exp. Clin. Psychopharmacol. 2022, 30, 23–30. [Google Scholar] [CrossRef]
- Rumble, D.D.; O’Neal, K.; Overstreet, D.S.; Penn, T.M.; Jackson, P.; Aroke, E.N.; Sims, A.M.; King, A.L.; Hasan, F.N.; Quinn, T.L.; et al. Sleep and Neighborhood Socioeconomic Status: A Micro Longitudinal Study of Chronic Low-Back Pain and Pain-Free Individuals. J. Behav. Med. 2021, 44, 811–821. [Google Scholar] [CrossRef]
- Shen, W.; Tu, Y.; Gollub, R.L.; Ortiz, A.; Napadow, V.; Yu, S.; Wilson, G.; Park, J.; Lang, C.; Jung, M.; et al. Visual Network Alterations in Brain Functional Connectivity in Chronic Low Back Pain: A Resting State Functional Connectivity and Machine Learning Study. Neuroimage Clin. 2019, 22, 101775. [Google Scholar] [CrossRef] [PubMed]
- Skarpsno, E.S.; Mork, P.J.; Nilsen, T.I.L.; Nordstoga, A.L. Influence of Sleep Problems and Co-Occurring Musculoskeletal Pain on Long-Term Prognosis of Chronic Low Back Pain: The HUNT Study. J. Epidemiol. Community Health 2020, 74, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Tabira, T.; Maruta, M.; Matsudaira, K.; Matsuo, T.; Hasegawa, T.; Sagari, A.; Han, G.; Takahashi, H.; Tayama, J. Relationship Between Attention Bias and Psychological Index in Individuals With Chronic Low Back Pain: A Preliminary Event-Related Potential Study. Front. Hum. Neurosci. 2020, 14, 561726. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.K.; Gentili, A.; Coffey-Vega, K.; Morone, N.; Rossi, M.; Perera, S. Biopsychosocial Profiles and Functional Correlates in Older Adults with Chronic Low Back Pain: A Preliminary Study. Pain Med. 2019, 20, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Dydyk, A.M.; Conermann, T. Chronic Pain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gauthier, K.; Dulong, C.; Argáez, C. Multidisciplinary Treatment Programs for Patients with Chronic Non-Malignant Pain: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines—An Update; CADTH Rapid Response Reports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019; pp. 1–27. [Google Scholar]
- Scheidegger, A.; Gómez Penedo, J.M.; Blättler, L.T.; Aybek, S.; Bischoff, N.; grosse Holtforth, M. Improvements in Pain Coping Predict Treatment Success among Patients with Chronic Primary Pain. J. Psychosom. Res. 2023, 168, 111208. [Google Scholar] [CrossRef]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An Overview of Treatment Approaches for Chronic Pain Management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef]
- Semmons, J. The Role of Physiotherapy in the Management of Chronic Pain. Anaesth. Intensive Care Med. 2019, 20, 440–442. [Google Scholar] [CrossRef]
- Korwisi, B.; Barke, A.; Kharko, A.; Bruhin, C.; Locher, C.; Koechlin, H. Not Really Nice: A Commentary on the Recent Version of NICE Guidelines [NG193: Chronic Pain (Primary and Secondary) in over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain] by the Pain Net. PAIN Rep. 2021, 6, e961. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.H.; Colvin, L.A.; Donaldson-Bruce, A.; Birt, A. Drugs for Chronic Pain: We Still Need Them. Br. J. Gen. Pract. 2021, 71, 172. [Google Scholar] [CrossRef]
- Blanco, S.; Sanromán, L.; Pérez-Calvo, S.; Velasco, L.; Peñacoba, C. Olfactory and Cognitive Functioning in Patients with Fibromyalgia. Psychol. Health Med. 2019, 24, 530–541. [Google Scholar] [CrossRef]
- Loftus, N.; Dobbin, N.; Crampton, J.S. The Effects of a Group Exercise and Education Programme on Symptoms and Physical Fitness in Patients with Fibromyalgia: A Prospective Observational Cohort Study. Disabil. Rehabil. 2022, 44, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.K.; Nanda, S.; Arya, S.; Kumar, U.; Sharma, R.; Kumaran, S.S.; Bhatia, R. Correlating Cognition and Cortical Excitability with Pain in Fibromyalgia: A Case Control Study. Adv. Rheumatol. 2021, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- Trucharte, A.; Leon, L.; Castillo-Parra, G.; Magán, I.; Freites, D.; Redondo, M. Emotional Regulation Processes: Influence on Pain and Disability in Fibromyalgia Patients. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S123), 40–46. [Google Scholar] [PubMed]
- Zitko, P.; Bilbeny, N.; Balmaceda, C.; Abbott, T.; Carcamo, C.; Espinoza, M. Prevalence, Burden of Disease, and Lost in Health State Utilities Attributable to Chronic Musculoskeletal Disorders and Pain in Chile. BMC Public Health 2021, 21, 937. [Google Scholar] [CrossRef]
- Bigler, E.D. Structural Image Analysis of the Brain in Neuropsychology Using Magnetic Resonance Imaging (MRI) Techniques. Neuropsychol. Rev. 2015, 25, 224–249. [Google Scholar] [CrossRef]
| Author | Publication Year | Country | Design | Subtypes | |
|---|---|---|---|---|---|
| 1. | Brown et al. [29] | 2022 | The USA | Cross-sectional study | Chronic primary cervical pain (CPCP) |
| 2. | Brunner et al. [30] | 2018 | Germany | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 3. | Clark et al. [31] | 2019 | New Zealand | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 4. | Coppieters et al. [32] | 2021 | Belgium | Cross-sectional study | Chronic primary cervical pain (CPCP) |
| 5. | Day et al. [33] | 2019 | Australia | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 6. | Fujii et al. [34] | 2018 | Japan | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 7. | Ho et al. [35] | 2019 | Norway | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 8. | Kasahara et al. [36] | 2021 | Japan | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 9. | Kim et al. [37] | 2019 | The USA | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 10. | Ma et al. [38] | 2020 | China | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 11. | Moreira et al. [39] | 2021 | Brazil | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 12. | Neumann and Hampel [40] | 2022 | Germany | Longitudinal study | Chronic primary low back pain (CPLBP) |
| 13. | Pakpour et al. [41] | 2018 | Iran | Longitudinal study | Chronic primary low back pain (CPLBP) |
| 14. | Richards et al. [42] | 2018 | Australia | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 15. | Rogers et al. [43] | 2022 | The USA | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 16. | Rumble et al. [44] | 2021 | The USA | Longitudinal study | Chronic primary low back pain (CPLBP) |
| 17. | Shen et al. [45] | 2019 | The USA | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 18. | Skarpsno et al. [46] | 2020 | Norway | Longitudinal study | Chronic primary low back pain (CPLBP) |
| 19. | Tabira et al. [47] | 2020 | Japan | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| 20. | Weiner et al. [48] | 2019 | The USA | Cross-sectional study | Chronic primary low back pain (CPLBP) |
| Authors | Sample | Gender and Age | Instruments | Results |
|---|---|---|---|---|
| Brunner et al. [30] | n = 49 | Men = 24 (49%) Women = 25 (51%) Average age = 47.08 | NRS RMDQ SBT 4DSQ TSK | The sample with CPLBP showed the following correlations:
|
| Clark et al. [31] | n = 165 | Men = 39 (23.6%) Women = 126 (76.4%) Average age = 45 | CSI AASP STAI MCSDS | The sample with CPLBP presented different sensory profiles as a result of combining the neurological thresholds to sensory stimuli with the adaptive behavioural response to sensory stimuli. The sensory profiles presented in the sample were one or more of the following: high trait sensory sensitivity (low threshold, passive response; 55%, n = 91), sensation avoidance (low threshold, active response; 44%, n = 72), low registration (high threshold, passive response; 36%, n = 60), and low trait sensation-seeking (high threshold, active response; 38%, n = 62). The proportions of the types of personality in the sample were defensive high anxious (45%; n = 75), high anxious (25%; n = 43), and repressor (25%; n = 41). The sample with CPLBP presented the following correlations:
|
| Day et al. [33] | n = 69 | Men = 33 (48%) Women = 36 (52%) Average age = 51 | NRS PCS SOPA PROMIS SHS NRP–Avoidance scale CPAQ-8 BFI EEG | The sample with CPLBP showed the following correlations:
|
| Fujii et al. [34] | n = 3100 | Men = 1617 (52.2%) Women = 1483 (47.8%) Average age = 44.5 | SSS-8 PHQ-2 EQ-5D | The sample with CPLBP showed the following correlations:
|
| Ho et al. [35] | n = 30,669
| Men = 14,006 (46%) Women = 16,663 (54%) Average age = 52.2 | Clinical diagnoses from the HUNT Study 3 (2006–2008) | In the entire sample, 6.1% (n = 1871) of the participants presented insomnia and 2.4% insomnia and CPLBP (n = 719). The logistic regression model showed evidence that insomnia (OR = 2.46; p < 0.001), age (OR = 1.01; p < 0.001), physical activity (OR = 0.88; p < 0.001), depression (borderline: OR = 1.66; p < 0.001/possible: OR = 2.40; p < 0.001), and anxiety (borderline: OR = 1.73; p < 0.001/possible: OR = 2.48; p < 0.001) were all significantly associated with the presence of CPLBP. In the multiple logistic regression model, which included the said factors, those with insomnia presented almost double the probabilities of suffering from CPLBP as compared with those who did not suffer from insomnia (OR = 1.99; p < 0.0001). |
| Kasahara et al. [36] | n = 60 | Men = 29 (48.3%) Women = 31 (51.7%) Average age = 54.9 | CAARS CAARS-S CAARS–O NRS | Within the sample suffering from CPLBP, 48.3% (n = 29) obtained positive scores on CAARS-S and 60% (n = 36) obtained positive scores on CAARS-O. Overall, 76.6% (n = 46) obtained positive scores on CAARS-S or CAARS-O, and 31.1% (n = 19) obtained positive scores on both. The results obtained were compared with those obtained by the general population in another study in which the same instruments were administered. On both the CAARS-S and the CAARS-O scales, the group with CPLBP had significantly higher scores on the subscales of Inattention/Memory Problems (p < 0.05; p < 0.05), Hyperactivity/Restlessness (p < 0.001; p < 0.05), Problems with Self-Concept (p < 0.05; p < 0.001), DSM-IV Inattentive Symptoms (p < 0.005; p < 0.05), DSM-IV Hyperactive–Impulsive Symptoms (p < 0.005; NS), DSM-IV ADHD Symptoms Total (p < 0.001; NS), and the ADHD Index (p < 0.001; p < 0.005) with respect to the general population. Similarly, evidence of the following correlations was found:
|
| Moreira et al. [39] | n = 36
| Men = 10 (26.6%) Women = 28 (77.7%) Average age = 24.2 | CPM Protocol NRS SF-MPQ TSK BDI VAS PCS RMDQ | The group with CPLBP presented levels of intensity of pain (p < 0.01), symptoms of depression (p < 0.05), and symptoms of anxiety (p < 0.01) that were significantly higher than those of the control group. Furthermore, they present significantly higher levels of pain and mood symptoms (p < 0.01; p < 0.01), fear of movement and physical activity (p < 0.05), and pain catastrophizing (p < 0.05) with respect to the control group. The group with CPLBP showed a significantly lower pain pressure threshold (13.5%) with respect to the control group, as well as a considerable exacerbation of cognitive–behavioural changes. |
| Neumann and Hampel. [40] | n = 526 | Men = 94 (17.9%) Women = 432 (82.1%) Average age = 53.2 | MPSS CES-D HADS Mini-SCL SF-12 DSF | The sample with CPLBP was classified into three progressive states of pain depending on the pain’s level of chronification as follows: state of pain I (n = 126), state of pain II (n = 270), and state of pain III (n = 130). Among these three states there existed statistically significant differences in the duration of pain (p < 0.05), the location of pain (p < 0.01), and the average intensity of pain (p < 0.01); the differences were greater as the chronification of pain advanced. Similarly, there were statistically significant differences in the presence of depressive symptoms among the different groups (p < 0.01); the differences were greater as the chronification of pain advanced. As for the state of health, there were statistically significant differences for physical health (p < 0.01) but not for mental health. |
| Pakpour et al. [41] | n = 761 | Men = 414 (55.4%) Women = 347 (44,6%) Average age = 41.15 | VAS PSQI HADS | At the start of the study, 48% (n = 365) of the sample with CPLBP stated that they had problems sleeping in the previous month, increasing to a total of 67.6% (n = 514) after six months of monitoring. Regarding the intensity of pain, 38.3% of the sample presented severe levels of pain after six months of monitoring. The logistical regression model presented the following results:
|
| Rogers et al. [43] | n = 294 | Men = 92 (31.1%) Women = 202 (68.9%) Average age = 45.8 | BPI ODSIS SSASI OPMM COMM | The sample with CPLBP declared having suffered, on average, for 4.30 years, with an average intensity of 6.54/10. Regarding the state of abusive consumption of opioids, 56.7% (n = 167) classified themselves as abusive consumers. In addition, the following correlations were found:
|
| Rumble et al. [44] | n = 117
| Men = 54 (46.2%) Women = 63 (53.8%) Average age = 43.4 | GCPS CES-D The STOP-BANG Home sleep monitoring through the Actiwatch2 Sleep diaries | The group with CPLBP presented levels of pain intensity (p < 0.01), depressive symptoms (p < 0.01), wake after sleep onset (p < 0.01), and time spent in bed (p < 0.05) significantly higher than those of the control group. In addition, the group with CPLBP presented quality of sleep (p < 0.01) and of refreshed sleep (p < 0.01) significantly below that of the control group.
|
| Skarpsno et al. [46] | n = 6200 | Men = 2488 (40%) Women = 3712 (60%) Average age = 49.7 | Information obtained from the HUNT Study 2 (1995–1997) and the HUNT Study 3 (2006–2008) | Within the sample suffering from CPLBP, the women (RR = 0.65) and men (RR = 0.81) who frequently/always experienced insomnia had lower probabilities of recuperating from CPLBP as compared with those who did not suffer from insomnia. The probability of recuperating from CPLBP was inversely associated with the number of symptoms of insomnia in women (one symptom: RR = 0.81; two: RR = 0.68; three: RR = 0.60) and, to a lesser extent, in men (one symptom: RR = 0.99; two: RR = 0.84; three: RR = 0.82). Both women and men with CPLBP had a lower probability of recuperation (RR = 0.46; 0.67) if they stated that they always/often suffered from insomnia in comparison with those who rarely/never suffered from insomnia (RR = 0.68; RR = 0.81) |
| Weiner et al. [48] | n = 47 | Men = 41 (87.2%) Women = 6 (12.8%) Average age = 68 | NRS RMDQ PHQ-9 GAD-7 The Insomnia Severity Index CSQ FABQ | Within the sample suffering from CPLBP, 83% (n = 39) presented factors that contribute to pain in the central nervous system. Concretely, 38.3% (n = 18) presented moderate levels of anxiety and depression, 63.8% (n = 30) moderate levels of insomnia, and 63.8% (n = 30) a catastrophizing cognition with respect to CPLBP and a maladaptive coping style with respect to physical activity. The presence of anxiety and depression (p < 0.05), insomnia (p < 0.01), and maladaptive coping strategies (p < 0.05) were significantly associated with more intense levels of pain. |
| Authors | Sample | Gender and Age | Instruments | Results |
|---|---|---|---|---|
| Brown et al. [29] | n = 38
| Men =13 (34.2%) Women = 25 (65.8%) Average age = 24.6 | Demographic questionnaire NDI NRS Digital cognitive assessments developed by Cogstate: attention/reaction time, verbal working memory, and working memory | The group with CPCP presented a light level of pain intensity, as well as low levels of disability associated with neck pain. The group with CPCP did not present statistically significant differences in the tests of verbal working memory: duration (ms) (p = 0.726), verbal working memory: correct responses (p = 0.417), attention: speed (ms) (p = 0.426), attention: errors (p = 0.974), working memory: speed (ms) (p = 0.771), or in working memory: errors (p = 0.424) with respect to the control group. |
| Coppieters et al. [32] | n = 107
| Women = 107 (100%) Average age = 32.6 | VNRS-11 NDI CSI QST Digital pressure algometer fMRI | The group with CPCP and the group with neck pain as a consequence of a physical trauma presented statistically significant results in the intensity of neck pain (p < 0.001), disability related to neck pain (p < 0.001), and in the scores of the CSI, with respect to the control group. The results of the fMRI showed that the group with CPCP and the group with trauma pain presented better performance in resting state functional connectivity (rsFC) between the left amygdala (associated with the processing and regulation of affection and the processing of possible threats) and the left frontal operculum (the region in the ventrolateral frontal cortex associated with sensory discrimination of the processing of pain and the cognitive–affective implications of pain) in comparison with the control group (p < 0.001). The results were associated with a decrease in the endogenous inhibition of pain in the groups with CPCP and trauma pain and with a greater number of symptoms with self-reported central sensitivity in the CPCP group (p = 0.02). These associations implied a link between cognitive–affective and sensory modulations in CPCP. |
| Kim et al. [37] | n = 181
| Men = 80 (44.2%) Women = 101 (55.8%) Average age = 39.4 | BDI BPSD PROMIS PCS fMRI | The group with CPLBP presented significantly higher levels of depressive symptoms (p < 0.01), intensity of pain (p < 0.01), and pain catastrophizing (p < 0.01) with respect to the control group. The group with CPLBP presented a greater connectivity of the salience network with the pons, the cerebellum, and the primary somatosensory cortex (S1) to the nociceptive stimuli applied to the lower back with respect to the control group. In pain exacerbation manoeuvres, the group with CPLBP presented greater posterior connectivity of the primary somatosensory cortex with different cerebral regions of the salience network including the following: the anterior insular cortex, dorsolateral prefrontal cortex, and anterior temporoparietal junction. The increase in the intensity of low back pain following pain exacerbation manoeuvres presented a regular positive correlation with a greater connectivity between the somatosensory cortex (S1) and the left anterior insula (r = 0.36; p < 0.05). The salience network was closely linked to the ventral attention network and associated with the reallocation of attention resources towards outstanding stimuli, such as pain. Furthermore, the said connectivity was found to be strongly influenced by pain catastrophizing, |
| Ma et al. [38] | n = 46
| Men =17 (37%) Women = 29 (63%) Average age = 32 | VAS BES-A:
| The group with CPLBP presented significantly lower levels in cognitive empathy (p = 0.0015), emotional disengagement (p = 0.0017), and total scores on the BES-A scale (p = 0.005) with respect to the control group; however, there were no significant differences in emotional contagion (p = 0.119). The results of the fMRI in the group with CPLBP showed evidence of multiple abnormal pathways in the brain centred on the anterior insula. Within the group with CPLBP, the abnormal functional connection state of the left parietal lobe and the left dorsolateral prefrontal cortex led, as a result, to an incorrect allocation of attention resources. Although there was no existing correlation between the connectivity of either of these and the scores in emotional disengagement, the results were close to being statistically significant (r = 0.39; p = 0.058). The said results suggested that CPLBP was the main cause of the reduction in the attention resources towards external stimuli because of the reallocation towards internal self-regulation, this being the reason why they obtain low scores in emotional disengagement (self-protection regulation mechanism). |
| Richards et al. [42] | n = 60
| Men = 34 (56.7%) Women = 26 (43.3%) Average age = 60.6 | BPI DASS-21 PSEQ PCS Battery of cognitive tests:
| The groups with CPLBP with opiate consumption (OP) and without opiate consumption (NO) presented significantly higher levels of depressive symptoms (p < 0.001/p < 0.001), anxiety (p < 0.001/p < 0.001), and stress (p < 0.001/p < 0.001) with respect to the control group; however, there were no significant differences between the groups in these dimensions. Nor were there significant differences between the groups in the average scores of the intensity of chronic pain, the interference of pain in the activities of daily life, or pain catastrophizing. Regarding cognitive functions, the groups with CPLBP with and without consumption of opioids presented a significantly lower performance in memory (p < 0.01/p < 0.01) and attention (p < 0.01/p < 0.05) with respect to the control group. Regarding executive functions, the groups with CPLBP with and without the consumption of opioids presented significantly lower performance with respect to the control group, concretely in working memory (p < 0.05/p < 0.05). There were no significant differences between the groups in these dimensions. |
| Shen et al. [45] | n = 164
| Men = 69 (42%) Women = 95 (58%) Average age = 33.4 | Pain Bothersomeness Scale BDI-II fMRI | The group with CPLBP presented very low scores in depression. The analysis of the resting state functional connectivity (rsFC) showed evidence that when the group with CPLBP used the primary visual network (in charge of orienting the visual attention resources), there were significant increases in rsFC in the right postcentral (S1) and precentral (M1) gyri and decreases in rsFC in the left angular gyrus/lateral occipital cortex. The results showed a significant alteration in the rsFC of the visual networks in the group with CPLBP, which obtained better performance than the control group. The rsFC between the primary visual network and the primary somatosensory cortex (S1: a critical component of the nociceptive pathway) presented a regular negative correlation with the duration of the CPLBP (r = −0.24; p < 0.05). In addition, the rsFC of the visual network allowed the participants with CPLBP and those of the control group to be differentiated with an accuracy of 79.3% (p < 0.001). |
| Tabira et al. [47] | n = 13 | Men = 2 (15.4%) Women = 11 (84.6%) Average age = 70.3 | EEG Japanese version of:
| The sample with CPLBP presented the following correlations:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo-Martínez, A.; Barbosa-Torres, C.; Moreno-Manso, J.M.; Cantillo-Cordero, P.; García-Baamonde, M.E.; Díaz-Muñoz, C.L. Systematic Review of the Psychopathological Symptomatology and Neuropsychological Disorders of Chronic Primary Musculoskeletal Pain. Healthcare 2024, 12, 1465. https://doi.org/10.3390/healthcare12151465
Arévalo-Martínez A, Barbosa-Torres C, Moreno-Manso JM, Cantillo-Cordero P, García-Baamonde ME, Díaz-Muñoz CL. Systematic Review of the Psychopathological Symptomatology and Neuropsychological Disorders of Chronic Primary Musculoskeletal Pain. Healthcare. 2024; 12(15):1465. https://doi.org/10.3390/healthcare12151465
Chicago/Turabian StyleArévalo-Martínez, Alejandro, Carlos Barbosa-Torres, Juan Manuel Moreno-Manso, Pilar Cantillo-Cordero, María Elena García-Baamonde, and César Luis Díaz-Muñoz. 2024. "Systematic Review of the Psychopathological Symptomatology and Neuropsychological Disorders of Chronic Primary Musculoskeletal Pain" Healthcare 12, no. 15: 1465. https://doi.org/10.3390/healthcare12151465
APA StyleArévalo-Martínez, A., Barbosa-Torres, C., Moreno-Manso, J. M., Cantillo-Cordero, P., García-Baamonde, M. E., & Díaz-Muñoz, C. L. (2024). Systematic Review of the Psychopathological Symptomatology and Neuropsychological Disorders of Chronic Primary Musculoskeletal Pain. Healthcare, 12(15), 1465. https://doi.org/10.3390/healthcare12151465






